One of the main current difficulties that all intensivist is facing during this pandemic crisis is the lack of ventilators around the world. Some institutions have begun to use all resources available to face unprecedented ethical decisions in the developed countries such as direct palliative routes. Sharing a ventilator is technically possible and has been tested only in controlled, experimental models using test lungs or animals for brief periods.
In 2006 Greg Neyman and Charlene Babcock Irvin and Paladino1–2 described how a single ventilator may be quickly modified to ventilate four simulated adults for a limited time. However, in each instance, Branson, Rubinson, and others have cautioned against the use of this technique.3–5 As pointed out by six organization including the Society of Critical Care Medicine and the American Society of Anesthesiologists, there are significant technical challenges that must be overcome.6 Such a strategy should only be considered as an absolute last resort, judged against the alternatives of long term “hand bagging” or death.7 However, we do know that many institutions are evaluating this practice, and protocols are being developed and tested, and in some places, preliminarily implemented in major cities, such New York has been using it since almost the beginning. On March 24, 2020, The Food and Drug Administration (FDA), granted an Emergency Use Authorization for modifications of a host of ventilator-type devices to be used during the COVID-19 pandemic.8
The novel idea was not initially conceived to ventilate ARDS/COVID19 patients. In the last several past weeks, we modified and tested this system (“in vitro”) at King's College Hospital NHS Trust Foundation, to be able to ventilate two patients with a standard ICU ventilator.
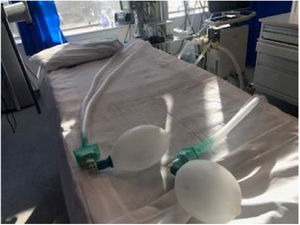
Two sets of standard ventilator tubing (Hudson) were connected to a single ventilator (tested in each model of a ventilator, Puritan-Bennett, 840 series and a Servo I Maquet) via two “T-tubes” (one on the patient inflow limb of the circuit, and one on the patient exhaust limb). Each “T-tube” was attached to a microfilter (total of four) to isolated both patients and the ventilator (Figure 1). Finally, a heat and moisture exchanger (HME) filter was placed for each patient to provide heating and humidification (Figure 2).
Two sets of standard ventilator tubing (Hudson) were connected to a single ventilator (tested in each model of ventilator, Puritan-Bennett, 840 series and a Servo I Maquet) via two T-tubes (one on the patient inflow limb of the circuit, and one on the patient exhaust limb). Each T-tube was attached to a microfilter (total of four) to isolated both patients and the ventilator. Especially if the circuit do not have in place non-return valves.
One of the clear advantages with pressure-control ventilation, it is that in the case of a change in the respiratory mechanics of one patient, the second is not affected and there is less dependence on ideal body weight, sex and the compliances of the lung. Also, with a flow/pressure sensor to measure the expiratory tidal volume (VTe), the inspiratory peak and mean airway pressures, with the capnography placed, at least in one patient, the monitoring and safety increase considerably. Other variants with extra features and potential better improvements have been also released recently by colleagues around the world, but we must declare that we have not tested these variations in our laboratory.
We are aware that there are no available randomised control studies to support this approach with full guarantees, however, in our current times where the professional is taking ethical decisions extremely difficult it may be considered as a good alternative as “compassionate treatment” or as a “bridge” for the time being.
Declarations sectionEthics approval and consent to participate: “Not applicable”.
Consent for publication: “Not applicable”.
Author ContributionsS. R. V was the main study “in vitro” researcher and who draft the manuscript.
FundingFinancial support, including any institutional departmental funds, was not sought for the study.
Conflict of interestsAll faculty and staff who are in a position to control or affect the content of this paper have declared that they have no competing financial /commercial interests at all.
Harvey Kelleway and Jonathan Saka technicians at King's College Hospital NHS Trust Foundation for their time and input.