Immunotherapy with chimeric antigen-specific receptor modified T cells, known as CAR-T, is emerging as a promising approach to hematological malignancies. In this regard, CAR-T against human cluster of differentiation (CD) 19 has demonstrated antitumor efficacy in application to B cell neoplasms resistant to conventional therapy. However, activation of the immune system induces severe and specific complications which can prove life-threatening. These include cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome (known as ICANS) - the latter being the subject of the present review. Although the physiopathological mechanisms underlying ICANS are not well known, a number of clinical and biological factors increase the risk of developing neurotoxicity associated to CAR-T therapy. Treatment is based on close monitoring, measures of support, anticonvulsivants, corticosteroids, and early admission to intensive care. The present study offers a comprehensive review of the available literature from a multidisciplinary perspective, including recommendations from intensivists, neurologists and hematologists dedicated to the care of critically ill adults.
La inmunoterapia con células T modificadas con receptor quimérico antígeno-específico (chimeric antigen receptor conocida como [CAR-T]) está emergiendo como un tratamiento prometedor para enfermedades hematológicas. Así, las CAR-T dirigidas contra el complejo de diferenciación (CD) 19 han demostrado gran eficacia antitumoral contra neoplasias de células B resistentes a terapias convencionales. Sin embargo, la activación dirigida de la respuesta inmunitaria desata en ciertos casos complicaciones específicas graves y potencialmente mortales. Entre ellas cabe destacar el síndrome de liberación de citoquinas y el síndrome de toxicidad neurológica asociado a la terapia con células inmuno-efectoras (Immune-effector cell associated neurotoxicity syndrome conocido como ICANS) siendo este último el objetivo de nuestra revisión. Aunque los mecanismos fisiopatológicos que conducen al ICANS son poco conocidos, existen factores clínicos y biológicos que aumentan el riesgo de desarrollo de neurotoxicidad asociada a terapia CAR-T. El tratamiento se basa en medidas de monitorización y soporte, tratamiento con anticonvulsivantes, corticosteroides e ingreso en los Servicios de Medicina Intensiva de forma precoz. Este artículo proporciona una revisión exhaustiva de la literatura disponible sobre el ICANS desde una perspectiva multidisciplinar, incluyendo recomendaciones de intensivistas, neurólogos y hematólogos formados en el cuidado de adultos críticamente enfermos.
Cancer is one of the leading causes of death worldwide.1 In Spain alone over 200 000 new cases of cancer are diagnosed each year. Of these, 10% have hematologic origin.2 Over the last few decades, several cancer specific therapies have been developed such as immunotherapy that has increased the survival rate of patients with onco-hematological disease.3,4 However, these therapies are no stranger to toxicities that, in turn, can threaten the patients’ lives.5 These onco-hematological emergencies have become a common thing now, and more and more patients require admission and management at the intensive care units (ICU).3,6,7
Recently, genetically modified T cell immunotherapy to express the chimeric antigen receptor (CAR-T) has proven clinical effective against B-cell lymphoid neoplasms in advanced stages of the disease.8,9 CARs are synthetic receptors for T-cell activation (CD3 zeta) plus combined CD28 or 4-1BB costimulation with a transmembrane domain, and an antigen-binding extracellular domain, a single chain fragment of an antibody, part of the variable region of immunoglobulins.8,10 The latter domain is the one that gives T-cells antigenic specificity. The process to redirect the immune system against tumor cells is based on extracting T lymphocytes from the patient and modifying them with a gene that endodes the CAR through retroviral or lentiviral transduction. The genetically modified lymphocytes are expanded and infused as immune therapy.8,10
There are currently 3 indications that have already been approved for the adult population. The first one is for refractory or relapsed B-cell acute lymphoblastic leukemia (B-ALL) after transplantation relapse or second or further relapses in patients <25 years; the second indication is to treat refractory or relapsed diffuse large B-cell lymphoma (DLBCL) after 2 or more lines of systemic therapy; the third indication is to treat primary mediastinal large B-cell lymphoma after 2 or more lines of systemic therapy.11–18
Ever since the first clinical trials on CAR-T cells targeting the CD19 antigen were conducted, their high rate of effectiveness was accompanied by significant and more severe toxicities compared to the ones described in other cellular therapies. The most common adverse event is the cytokine release syndrome (CRS) whose common clinical signs are fever, hypotension, and/or hypoxemia.18–20 The second most common complication is neurotoxicity although it was initially considered part of the CRS.
In order to clarify and manage the symptoms and neurological signs of patients treated with CAR-T cell therapy more accurately since 2018 the American Society for Blood and Marrow Transplantation (ASBMT) recognizes neurotoxicity as a separate clinical entity called immune effector cell-associated neurotoxicity syndrome (ICANS).20
The early detection of the signs and symptoms of ICANS, the early specific treatment of its complications, and the possibility of ICU admission are the standard recommendations established for the management of these patients.19,21,22 Therefore, the early assessment and follow-up of patients treated with CAR-T cells by a multidisciplinary team is essential.3,23
MethodsSearch of the most relevant articles was conducted using the Medline database (Pubmed) using the following keywords: immune effector cell associated neurotoxicity syndrome or CAR t-cell therapy. Additional keywords were hematologic malignancies or intensive care or critical care or critical illness. The references of the articles selected were used to identify additional studies. A total of 4 authors selected and accepted all papers by consensus.
Risk factors and pathophysiology for the development of the immune effector cell-associated neurotoxicity syndromeICANS is defined as a clinical disorder of the central nervous system (CNS) after treatment with immune therapy resulting in the activation or compromise of endogenous or infused T-cells and/or other immune effector cells.20 ICANS often occurs after clinical sign of the CRS, and is characterized by several neurological symptoms of variable intensity: confusion, linguistic disorder, dysarthria, mood swings, delirium, seizures, altered level of consciousness, and even brain swelling and death.20
To this date, the pathophysiology of the ICANS is not very well understood because there are few biomarkers available. Also, its clinical presentation is unspecific.24,25 These particularities are typical of syndromic entities (eg, adult respiratory distress syndrome or sepsis) treated at the ICU setting. The clinical criteria and risk factors associated with the development and severity of ICANS are age (younger patients), diagnosis of B-ALL, the high tumor load during the infusion, the high doses of CAR-T cells, and the fast spread of T-cells after infusion.24,26 Also, it has been reported that severe ICANS is often preceded by the CRS.26,27 In addition, there is a direct correlation between the severity of CRS and ICANS. On the other hand, analytical data on disseminated intravascular coagulation, high levels of C-reactive protein and ferritin, and high levels of proinflammatory cytokines including interleukin 6 (IL-6), interferon gamma (IFN-γ), and tumor necrosis factor alpha (TNF-α) have been associated with severe ICANS.24,26,28 Overall, this data suggests the development of some sort of inflammatory syndrome with capillary leak after the infusion of CAR-T cells.
From the pathophysiological point of view several factors triggering ICANS have been reported: the diffuse endothelial activation mediated by a high concentration of cytokines in peripheral blood (IL-6 and IFN-γ), which would also trigger disorders in the blood-brain barrier (BBB) confirmed by the detection of high levels of proteins and pleocytosis in the cerebrospinal fluid (CSF).24–26,29 In vitro studies have demonstrated that the pericytes of cerebral vascular endothelium are sensitive to the stimulation by proinflammatory cytokines, which would increase the patency of the BBB.26 Additionally, the local production of cytokines inside the CNS with significant increases of IL-8, IL-10, and the monocyte chemoattractant protein (MCP-1) in the CSF has been proposed as another mechanism associated with the appearance of severe ICANS.24,29 Santomasso et al.24 reported that during the production of severe neurotoxicity, high levels of IL-1, and IL-6 in the CSF have been reported suggestive of hyperproduction inside the CNS. Finally, the clinical signs of ICANS have been reported in CAR-T cells targeted against CD19, but neurotoxicity is not exclusive of this target. As a matter of fact, an in vivo model of neurotoxicity in macaques that infused CAR-T cells targeted against CD20 confirmed that, added to the high levels of proinflammatory cytokines reported, the diffuse infiltration of cerebral parenchyma by T-cells causes encephalitis, and symptoms of neurotoxicity.25
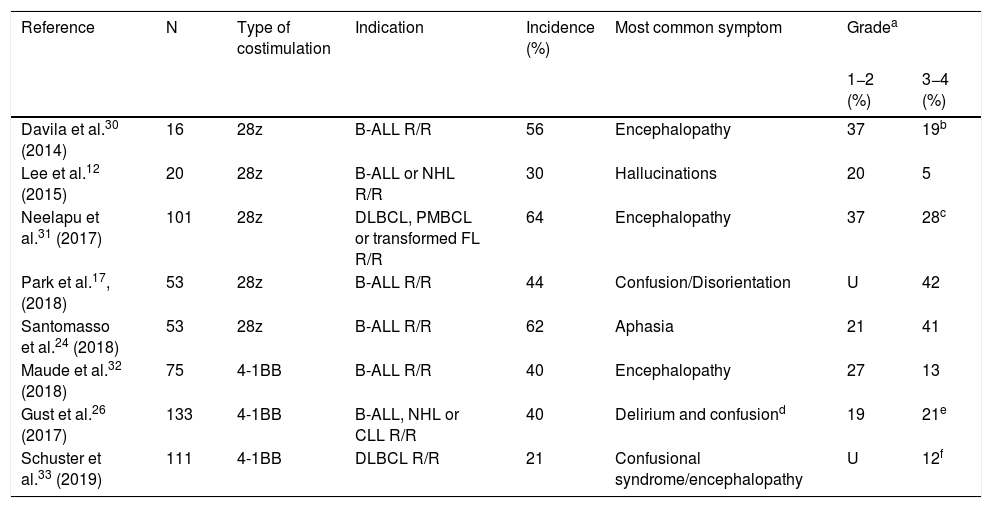
Clinical spectrum and diagnosis of the immune effector cell-associated neurotoxicity syndromeThe rate of neurotoxicity associated with CAR-T cell therapy sits at around 21%–64% of all infused patients12,17,24,26,30–33 (Table 1). A third of these can require support measures and even admission to the ICU setting.30,34,35 The median time for the appearance of the first neurological symptom after CAR-T cell infusion is 6 days (range, 1–34 days). It often develops simultaneously or right after the CRS symptoms have resolved.20,27,36 Symptoms last between 2 and 9 days, and they usually resolve within the first 3–4 weeks after infusion.20,26,27,31,33
Incidence and clinical characteristics of neurotoxicity due to T-CAR cell therapy.
| Reference | N | Type of costimulation | Indication | Incidence (%) | Most common symptom | Gradea | |
|---|---|---|---|---|---|---|---|
| 1−2 (%) | 3−4 (%) | ||||||
| Davila et al.30 (2014) | 16 | 28z | B-ALL R/R | 56 | Encephalopathy | 37 | 19b |
| Lee et al.12 (2015) | 20 | 28z | B-ALL or NHL R/R | 30 | Hallucinations | 20 | 5 |
| Neelapu et al.31 (2017) | 101 | 28z | DLBCL, PMBCL or transformed FL R/R | 64 | Encephalopathy | 37 | 28c |
| Park et al.17, (2018) | 53 | 28z | B-ALL R/R | 44 | Confusion/Disorientation | U | 42 |
| Santomasso et al.24 (2018) | 53 | 28z | B-ALL R/R | 62 | Aphasia | 21 | 41 |
| Maude et al.32 (2018) | 75 | 4-1BB | B-ALL R/R | 40 | Encephalopathy | 27 | 13 |
| Gust et al.26 (2017) | 133 | 4-1BB | B-ALL, NHL or CLL R/R | 40 | Delirium and confusiond | 19 | 21e |
| Schuster et al.33 (2019) | 111 | 4-1BB | DLBCL R/R | 21 | Confusional syndrome/encephalopathy | U | 12f |
B-ALL, B-cell acute lymphoblastic leukemia; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; NHL, non-Hodgking lymphoma; PMBCL, primary mediastinal B-cell lymphoma; R/R: relapsed or refractory; U, unreported.
The clinical expression of neurotoxicity is fairly large.20 Some neurological symptoms can occur, but they are somehow unspecific such as headache, tremor, myoclonies, asterixis or hallucinations. Also, these symptoms can occur in the CRS without associated neurological toxicity, which is why they have been excluded from the definition of ICANS. The frequency and severity of symptoms can be associated with the structure of the recipient. In this sense, patients treated with products whose CAR-T included the CD28-costimulator dominion had serious ICANS in around 40% of the cases while only 13%–21% of the events reported were serious in those cases with the 4-1BB-costimulator domain20 (Table 1).
Encephalopathy and aphasia are the most common and specific signs characterizing ICANS.20,27,30–32 Neelapu et al.,31 and Rubin et al.27 described that encephalopathy was the most common sign in patients with severe neurotoxicity. In contrast, Santomasso et al.24 studied 53 patients with refractory B-ALL treated with CAR-T cell therapy in whom expressive aphasia (34%)—especially regarding the naming of objects—was the most typical symptom of neurotoxicity. Aphasia developed in 95% of the patients with severe neurotoxicity.24 Expressive aphasia starts as a disorder to name objects, paraphasias, and hesitant language, and it can progress to global aphasia and/or mutism, characterized by an overall communication and naming difficulty. Symptoms can progress to seizures, stupor, and comma. Patients with global aphasia can seem awake, yet they are mute and akinetic.20 Davila et al.30 confirmed that some of the patients studied developed a gradual progression of confusion until they eventually showed aphasia, and 3 out of their 9 patients with neurotoxicity required intubation and invasive mechanical ventilation (IMV).
Diffuse brain swelling has been described as one of the most serious complications of CAR-T cell therapy.14,20,26,30,37 The beginning of this complication can be sudden, and premonitory clinical signs are scarce.20 In one of the first series published of patients with B-ALL treated with experimental CAR-T cell therapy 5 cases of brain swelling and death were reported.38 Also, Gust et al.26 reported 4 deaths, 2 of which were due to diffuse brain swelling in patients treated with CAR-T 19 therapy (4-1BB).
The frequency of seizures is variable and often occurs after the onset of aphasia.20 They are often generalized tonic-clonic seizures. However, non-convulsive seizures have been reported in up to 10% of the cases treated with CAR-T cell therapy.20,24 Gust et al.26 documented a rate of seizures of 8% (4/53 patients) associated with CAR-T anti-CD19 therapy costimulated with 4-1BB, and they only occurred in patients with a past medical history of seizures or in cases of severe neurotoxicity.
Levels of severity of the immune effector cell-associated neurotoxicity syndromeICANS shows a plethora of clinical signs that go from relatively mild disorders (grade 1) to extremely severe and potentially fatal clinical signs (grade 4) that require early diagnosis and procedures.20
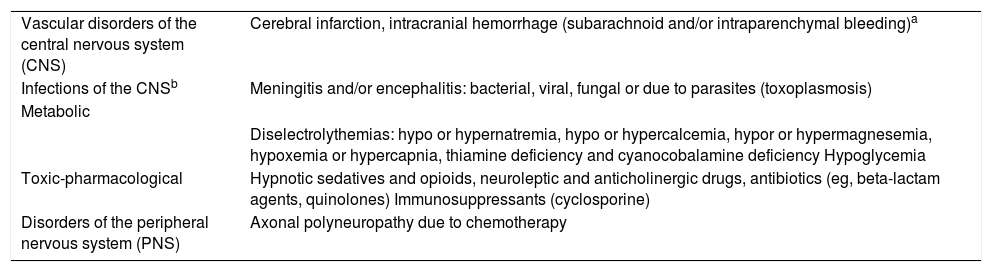
For the new classification of ICANS proposed by the ASBMT several unspecific neurological signs and symptoms like headache and confusion were discarded. The differential diagnosis of neurological symptoms should always been performed in these patients39 (Table 2). Intracranial hemorrhage and infections of the CNS have been excluded from the definition of ICANS.20
Differential diagnosis of ICANS.
| Vascular disorders of the central nervous system (CNS) | Cerebral infarction, intracranial hemorrhage (subarachnoid and/or intraparenchymal bleeding)a |
| Infections of the CNSb | Meningitis and/or encephalitis: bacterial, viral, fungal or due to parasites (toxoplasmosis) |
| Metabolic | |
| Diselectrolythemias: hypo or hypernatremia, hypo or hypercalcemia, hypor or hypermagnesemia, hypoxemia or hypercapnia, thiamine deficiency and cyanocobalamine deficiency Hypoglycemia | |
| Toxic-pharmacological | Hypnotic sedatives and opioids, neuroleptic and anticholinergic drugs, antibiotics (eg, beta-lactam agents, quinolones) Immunosuppressants (cyclosporine) |
| Disorders of the peripheral nervous system (PNS) | Axonal polyneuropathy due to chemotherapy |
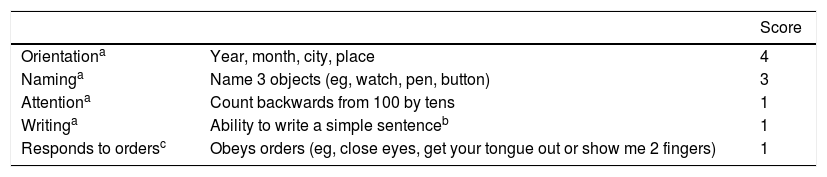
In the first place, for the proper assessment of the ICANS, the patient’s degree of encephalopathy needs to be measured using a scoring system called immune effector cell-associated encephalopathy (ICE) score (Table 3). This score goes from 1 to 10 being 10 the lack of disorder and 0 a significantly lowered level consciousness.20 Unlike the older CARTOX-10 score,22 the current ICE score includes yet another element (response to orders) to assess the signs of aphasia often seen in patients who develop severe neurological toxicity.24
Encephalopathy associated with immune-effector cells (ICE score).
| Score | ||
|---|---|---|
| Orientationa | Year, month, city, place | 4 |
| Naminga | Name 3 objects (eg, watch, pen, button) | 3 |
| Attentiona | Count backwards from 100 by tens | 1 |
| Writinga | Ability to write a simple sentenceb | 1 |
| Responds to ordersc | Obeys orders (eg, close eyes, get your tongue out or show me 2 fingers) | 1 |
CARTOX, CAR-T cell-therapy-associated toxicity; ICE score, immune effector cell-associated encephalopathy score.
The ICE score includes responses to verbal orders not present in the CARTOX-10 score including better linguistic assessment.55
The ICE score is a useful and easy-to-use tool both for the early assessment and detection of subtle neurological changes. However, in intubated patients or on drug sedation and invasive mechanical ventilation, encephalopathy cannot be assessed properly with the ICE score. We know that traditional sedation strategies do not offer any advantages compared to non-sedation strategies40 since the use of these substances (benzodiazepines in particular) should be minimized and individualized.41–43 On the other hand, several tools may be useful to assess patients in this setting. One of them would be the CAM-ICU tool44 or the ICDSC tool.45 Both are useful to assess confusion and delirium in the critically ill patient. However, they have not been validated in this context. For all this, it is important that the neurologist explores every single sign and symptom individually.
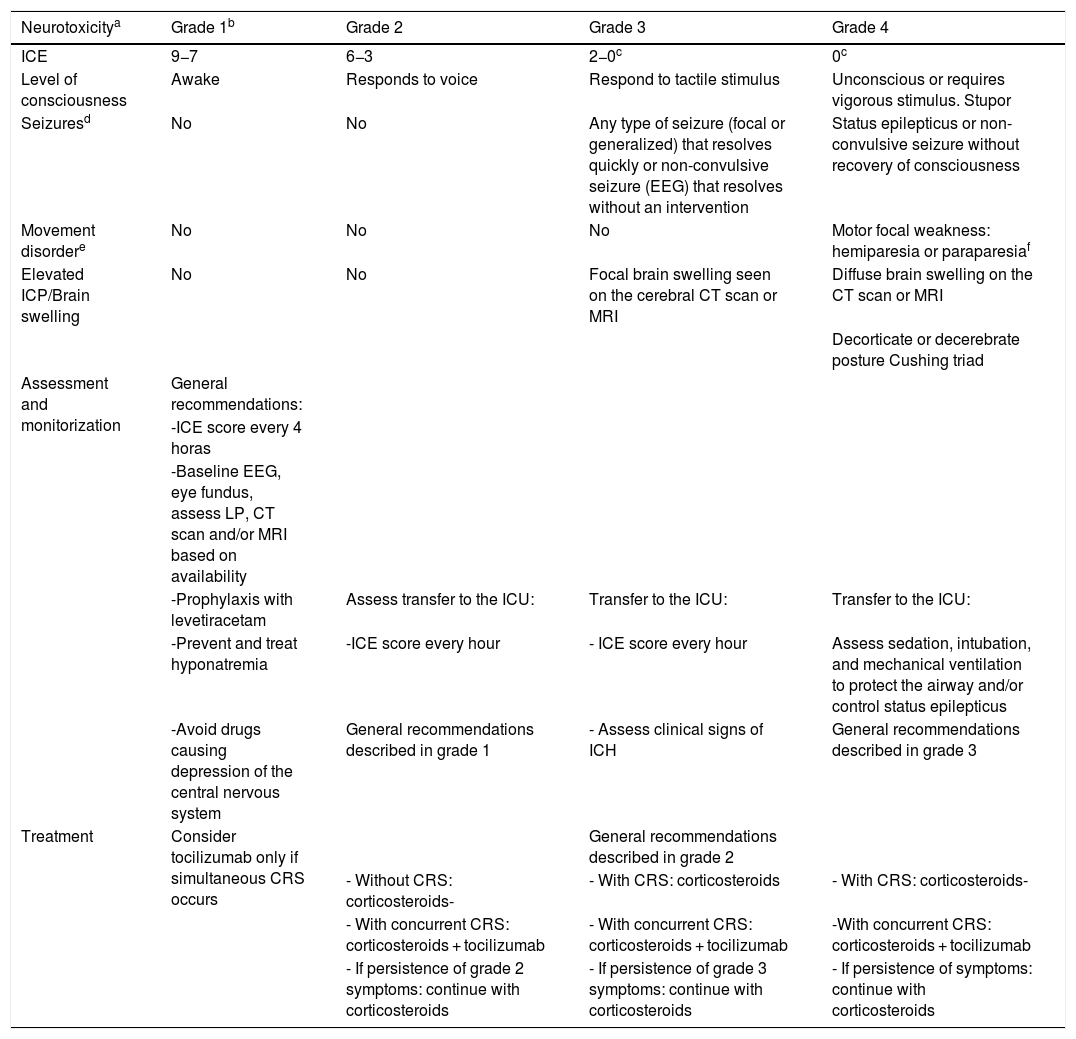
Once encephalopathy has been assessed, 4 more elements are also studied: the level of consciousness, motor symptoms, seizures, and signs of elevated intracranial pressure and/or brain swelling. The classification of the severity of ICANS should observe all the variables described grouped based on consensus from the ASBMT.20Table 4 details the ICANS severity score.
Levels of ICANS severity based on the ASBMT expert consensus.
| Neurotoxicitya | Grade 1b | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| ICE | 9−7 | 6−3 | 2−0c | 0c |
| Level of consciousness | Awake | Responds to voice | Respond to tactile stimulus | Unconscious or requires vigorous stimulus. Stupor |
| Seizuresd | No | No | Any type of seizure (focal or generalized) that resolves quickly or non-convulsive seizure (EEG) that resolves without an intervention | Status epilepticus or non-convulsive seizure without recovery of consciousness |
| Movement disordere | No | No | No | Motor focal weakness: hemiparesia or paraparesiaf |
| Elevated ICP/Brain swelling | No | No | Focal brain swelling seen on the cerebral CT scan or MRI | Diffuse brain swelling on the CT scan or MRI |
| Decorticate or decerebrate posture Cushing triad | ||||
| Assessment and monitorization | General recommendations: | |||
| -ICE score every 4 horas | ||||
| -Baseline EEG, eye fundus, assess LP, CT scan and/or MRI based on availability | ||||
| -Prophylaxis with levetiracetam | Assess transfer to the ICU: | Transfer to the ICU: | Transfer to the ICU: | |
| -Prevent and treat hyponatremia | -ICE score every hour | - ICE score every hour | Assess sedation, intubation, and mechanical ventilation to protect the airway and/or control status epilepticus | |
| -Avoid drugs causing depression of the central nervous system | General recommendations described in grade 1 | - Assess clinical signs of ICH | General recommendations described in grade 3 | |
| Treatment | Consider tocilizumab only if simultaneous CRS occurs | General recommendations described in grade 2 | ||
| - Without CRS: corticosteroids- | - With CRS: corticosteroids | - With CRS: corticosteroids- | ||
| - With concurrent CRS: corticosteroids + tocilizumab | - With concurrent CRS: corticosteroids + tocilizumab | -With concurrent CRS: corticosteroids + tocilizumab | ||
| - If persistence of grade 2 symptoms: continue with corticosteroids | - If persistence of grade 3 symptoms: continue with corticosteroids | - If persistence of symptoms: continue with corticosteroids |
ASBMT, American Society for Blood and Marrow Transplantation; CRS, cytokine release syndrome; CT, computed tomography; EEG, electroencephalogram; ICANS, immune-effector cell-associated neurotoxicity syndrome; ICE score, immune effector cell-associated encephalopathy score; ICP, intracranial pressure; ICU, intensive care unit; LP, lumbar puncture; MRI, magnetic resonance imaging.
A patient with an ICE score of 0 can be categorized as grade 3 if the patient is awake, but with global aphasia. However, he should be categorized as grade 4 if the patient is unconscious.
Supplementary tests should always be run to perform the differential diagnosis of ICANS (Table 2).
NeuroimagingNeuroimaging modalities including computed tomography (CT) scan and magnetic resonance imaging (MRI) are necessary in patients with neurotoxicity to confirm or discard diffuse brain swelling, the most serious complication of ICANS.14,26,37 The patient’s clinical status often conditions the selection of the neuroimaging modality that will be used. The MRI is preferred over the CT scan. However, when dealing with mild degrees of the disease the CT scan and the MRI barely detect any significant changes.13,14,20,26,30,46 These diagnostic imaging modalities can also be used to rule out the more frequent cerebrovascular events that are not associated with ICANS.27,47,48
ElectroencephalogramSeizures and status epilepticus are serious clinical signs of neurotoxicity. The most common electroencephalographic pattern is frontal intermittent rhythmic delta activity and slow activity present in diffuse cerebral swelling.49 However, a frontal intermittent rhythmic delta activity or a slowing pattern of the trace with low-voltage waves can occur in other entities like toxic-metabolic encephalopathies, CNS infections or sedation with CNS depressor drugs.50 Rubin et al.27 studied 36 patients with neurotoxicity due to CAR-T cell therapy who underwent an electroencephalogram (EEG); 77% of the patients showed slowing into the theta-delta frequency ranges, 2 patients showed generalized slow asynchronous activity and 33% a pattern of focal deceleration. The continuous EEG can reveal periodic episodes of discharges associated with periods of worsening ICANS.51 However, the continuous EEG is not readily available in many centers.
Intracranial pressure and cerebrospinal fluid analysisIt is advisable to perform a lumbar puncture if the patient’s clinical situation allows it including biochemistry, cytology, and microbiological studies (viral and bacterial) of the CSF. In the CSF, cellular count and proteins can be elevated. CAR-T cells can be found in the CSF both in patients with neurotoxicity and without any neurological symptoms.26,29
The consensus of ASBMT recommends performing a lumbar puncture to assess the opening pressure and papilledema of clinically and analytically stable patients. However, these measures can be difficult to implement in critically ill patients in the routine clinical practice since the opening pressure can vary with age, position, arterial pressure, IMV, and pharmacological sedation.52 Monitoring the signs of intracranial hypertension through transcranial Doppler ultrasound can be useful.34,53,54 However, the correlation between the severity of ICANS and the increased flow seen on the Doppler ultrasound has not been confirmed yet.27
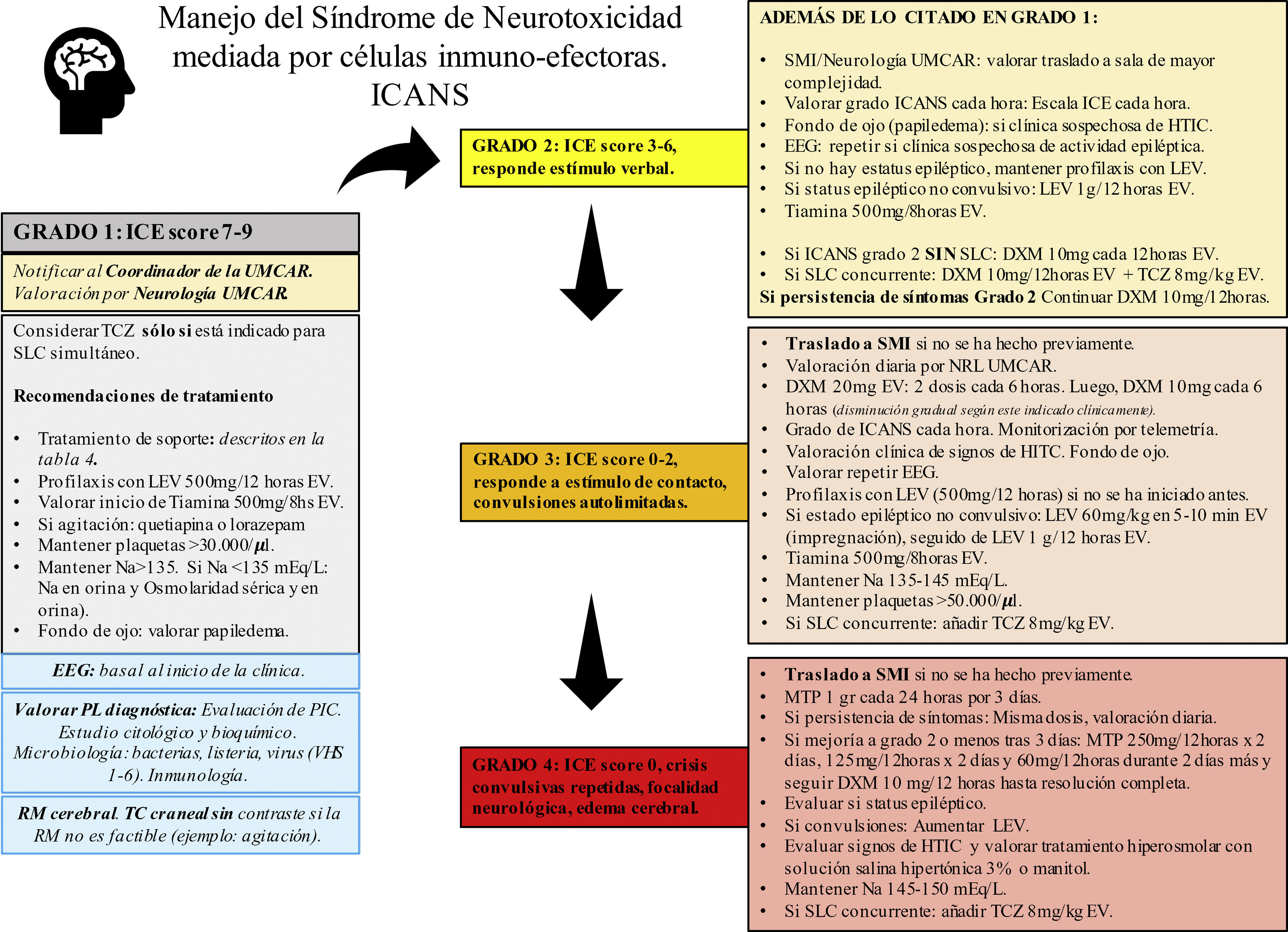
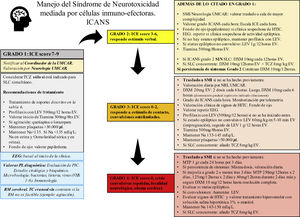
Management of the immune effector cell-associated neurotoxicity syndromeThere is not such a thing as a specific treatment of ICANS.20 As a rule of thumb, strict monitoring and the routine assessment of the patient by a multidisciplinary team including hematologists/oncologists, neurologists, and intensivists are required.19,34 The management of neurotoxicity depends on the degree of ICANS of every patient (Fig. 1).
Recommendations for the management of patients based on the ASBMT.
ASBMT, American Society for Blood and Marrow Transplantation; CARMU, CAR multidisciplinary unit; CRS, cytokine release syndrome; CT, computed tomography; DXM, dexamethasone; EEG, electroencephalogram; ICANS, immune effector cell-associated neurotoxicity syndrome; ICE score, immune effector cell-associated encephalopathy score; ICH, intracranial hypertension; ICU, intensive care unit; LEV, levetiracetam; LP, lumbar puncture; MRI, magnetic resonance imaging; MTP, methylprednisolone; Na, sodium, mEq/L; NLG, neurologist; TCZ, tocilizumab.
Corticosteroids are at the basis of the management of ICANS. Patients with grade 1–3 ICANS are often treated with dexamethasone while patients with grade 4 ICANS are usually treated with high doses of methylprednisolone20,26,34,55,56 (Fig. 1). Given the lymphocytic and anti-inflammatory effect of corticoids there is uncertainty surrounding the idea of whether these drugs can reduce the effectiveness of CAR-T cells. However, recent data suggests that the early administration and/or short cycles of steroids are associated with the resolution of neurological toxicities without detriment to the antitumor response.57,58 In this case, corticoids should be kept until neurotoxicity improves or is completely gone. The doses and cycles used in our center are fully explained in Fig. 1. Patients should be closely monitored to detect recurring neurotoxicity symptom while the dose of corticosteroids is being reduced.
Anti-interleukin receptorsTocilizumab, a humanized monoclonal antibody against the IL-6 receptor is not indicated for the treatment of ICANS.20 It is recommended for patients with simultaneous clinical signs of ICANS and CRS only.20,36 One of the reasons why ICANS does not respond to tocilizumab is that this drug does not reach significant levels in CSF.59 Also, some authors suggest that blocking the IL-6 receptor can increase IL-6 in the CSF thus worsening neurotoxicity.60,61 In a rat mode, Norelli et al. confirmed that blocking the IL-6 receptor with tocilizumab prevented CRS. On the contrary, tocilizumab did not stop the development of neurotoxicity. The same authors observed that the administration of an IL-1 receptor antagonist (anakinra) was associated with an improved CRS and neurotoxicity.28 A stage II clinical trial is currently in the pipeline to assess the potential utility of anakinra to prevent ICANS in patients with CAR-T cell therapy DLBCL receptors (NCT04205838).
Anticonvulsant drugsThe role of anticonvulsant drugs in the prophylaxis of patients on CAR-T cell therapy is controversial.10 Some centers start prophylactic anticonvulsant therapy on the same day of CAR-T cell infusion, especially if CAR-T cells with CD28-costimulator dominion are infused. In other centers, prophylaxis should be implemented when the first symptoms of neurotoxicity occur.21,34,55 At our center, the use of anticonvulsant prophylaxis in the presence of any signs of neurological toxicity is advised. Also, this prophylaxis should be kept until day +14, and its slow and gradual withdrawal agreed with the neurology team, and based on the characteristic of each patients (Fig. 1). All types of seizures and status epilepticus should be treated with benzodiazepines and antiepileptic drugs following the clinical practice guidelines of each center.23,34,47
Hyperosmolar therapyPatients with diffuse brain swelling should be treated with high doses of corticosteroids that should be followed by measures to reduce intracranial pressure with mannitol and/or a hypertonic saline solution.34 The use of neurosurgical measures such as external ventricular drainage and decompressive craniectomy, though rare, have been described in the medical literature.55 There is no evidence that invasive neurological monitorization is useful. The best way to prevent brain swelling is to detect and treat ICANS early on since one it is established it can be lethal.10
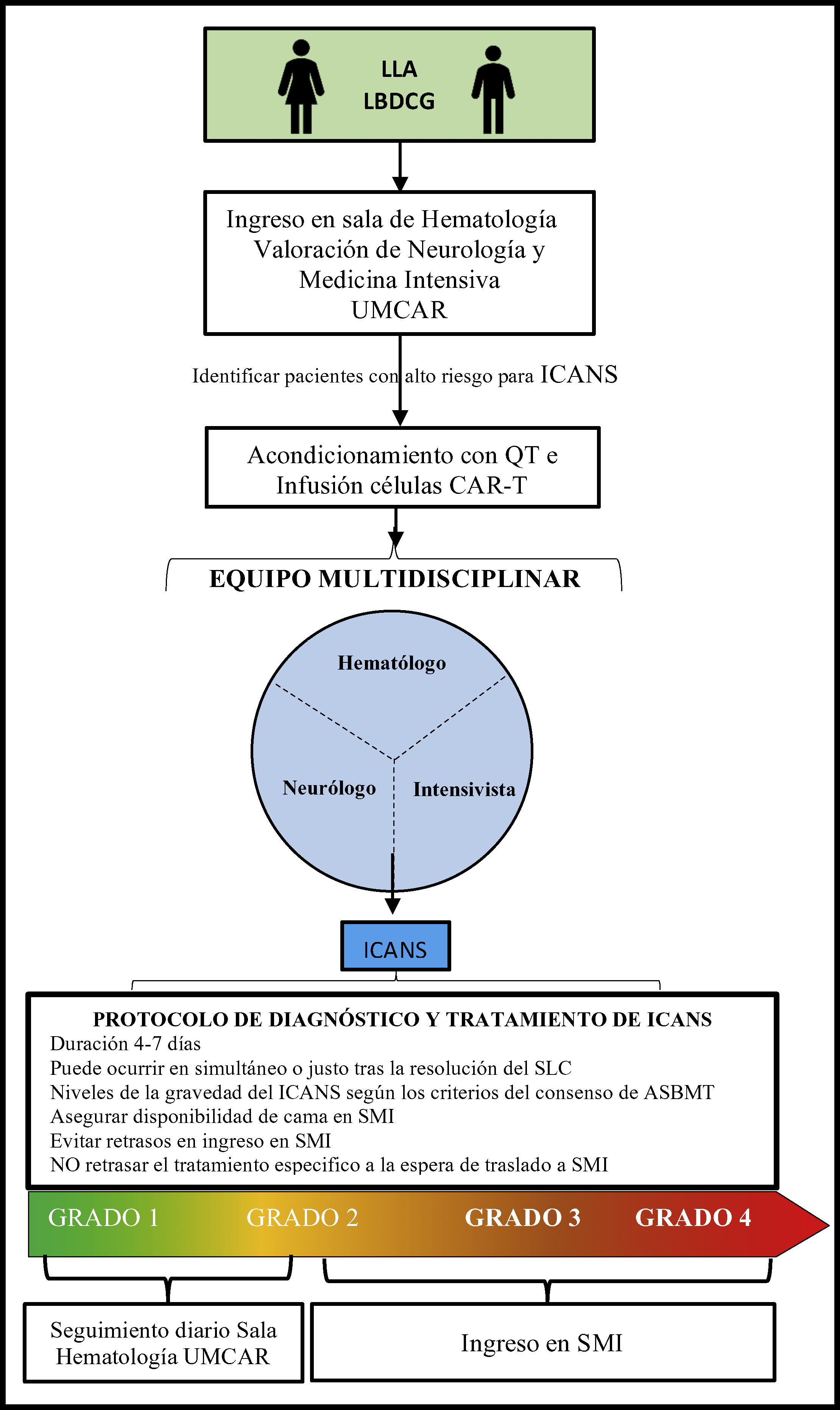
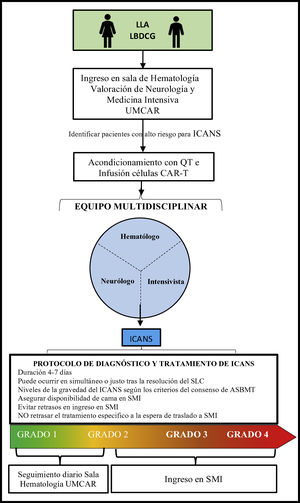
Specific considerations for the management of patients on T-cell therapy modified with antigen-specific chimeric receptor in the intensive care settingOncological patients who require ICU admission are often patients with high morbidity and mortality rates.6,62 Intensivists need to be aware that patients treated with CAR-T cell therapy can potentially need sedation, intubation, and IMV for the protection of respiratory airways, as well as ventilatory and oxygenation control, especially in cases of grade 3 or 4 ICANS.56 In our own experience the routine and combined visit of intensivist plus another specialist facilitates optimal and early clinical approaches and decision-making processes (Fig. 2). Similarly, the management of a serious complication or in the presence of multiorgan failure should be handled by the intensivists since this type of complication can be life-threatening for the patients.63
Process of admission and follow-up for patients on CAR-T cell therapy.
ASBMT, American Society for Blood and Marrow Transplantation; B-ALL, B-cell acute lymphoblastic leukemia; CAR, chimeric antigen receptor; CARMU, CAR multidisciplinary unit; CRS, cytokine release syndrome; CT, chemotherapy; DLBCL, diffuse large B-cell lymphoma; ICANS, immune effector cell-associated neurotoxicity syndrome; ICU, intensive care unit.
To guarantee safe and quality care for the patients it is highly advisable to have several beds available in the intensive care unit64 or prevent the differed implementation of the necessary support measures and specific treatments that should be administered in a conventional hematology room as quickly as possible before the patient’s transfer to the ICU setting. This is associated with better survival rates in these patients.62,65,66
Once the patients have been admitted to the ICU, they should follow specific recommendations for the management of ICANS on top of the routine therapeutic management of any critically ill patient with multiorgan failure with close monitoring from hematologists and neurologists.
ConclusionsThe increased rate of cancer and survival has been accompanied by the arrival of new biological therapies, some of which cause serious and specific complications. These complications associated with the use of biological therapies are due to pathophysiological mechanisms that are still unknown. All this makes these patients a especially vulnerable subgroup of patients. An expert multidisciplinary team of hematologists/oncologists, neurologists, and intensivists working in close collaboration, both clinically and on the research level, are the only guarantee to provide excellent patient care.
Conflicts of interestNone reported.
We wish to thank Dr. Javier Briones Meijide from the Santa Creu i Sant Pau Hospital Clinical Hematology Unit, and Dr. Luís Querol Gutiérrez from the Santa Creu i Sant Pau Hospital Neurology Unit.
Please cite this article as: Suarez Montero JC, Caballero Gonzalez AC, Martín Aguilar L, Mancebo Cortés J. Síndrome de neurotoxicidad asociada a células inmunoefectoras: un enfoque terapéutico en el paciente crítico. Med Intensiva. 2022;46:201–212.