To improve critical patient safety in the prevention of venous thromboembolic disease, using failure mode and effects analysis as safety tool.
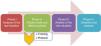
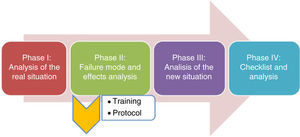
DesignA contemporaneous cohort study covering the period January 2014–March 2015 was made in 4 phases: (phase 1) prior to failure mode and effects analysis; (phase 2) conduction of mode analysis and implementation of the detected improvements; (phase 3) evaluation of outcomes, and (phase 4) post-checklist introduction impact.
SettingPatients admitted to the adult polyvalent ICU of a third-level hospital center.
PatientsA total of 196 patients, older than 18 years, without thromboembolic disease upon admission to the ICU and with no prior anticoagulant treatment.
InterventionsA series of interventions were implemented following mode analysis: training, and introduction of a protocol and checklist to increase preventive measures in relation to thromboembolic disease.
Variables of interestIndication and prescription of venous thrombosis prevention measures before and after introduction of the measures derived from the failure mode and effects analysis.
ResultsA total of 59, 97 and 40 patients were included in phase 1, 3 and 4, respectively, with an analysis of the percentage of subjects who received thromboprophylaxis. The failure mode and effects analysis was used to detect potential errors associated to a lack of training and protocols referred to thromboembolic disease. An awareness-enhancing campaign was developed, with staff training and the adoption of a protocol for the prevention of venous thromboembolic disease. The prescription of preventive measures increased in the phase 3 group (91.7 vs. 71.2%, p=0.001). In the post-checklist group, prophylaxis was prescribed in 97.5% of the patients, with an increase in the indication of dual prophylactic measures (4.7, 6.7 and 41%; p<0.05). There were no differences in complications rate associated to the increase in prophylactic measures.
ConclusionsThe failure mode and effects analysis allowed us to identify improvements in the prevention of thromboembolic disease in critical patients. We therefore consider that it may be a useful tool for improving patient safety in different processes.
Mejorar la seguridad del paciente crítico en la prevención de enfermedad tromboembólica venosa mediante metodología de la herramienta de seguridad del análisis modal de fallos y efectos.
DiseñoEstudio de cohortes con serie contemporánea de enero de 2014 a marzo de 2015 en 4 fases: fase 1) previa al análisis modal de fallos y efectos; fase 2) desarrollo del análisis modal e implementación de las mejoras detectadas; fase 3) evaluación de los resultados, y fase 4) impacto tras introducción post-checklist.
ÁmbitoPacientes hospitalizados en una UCI polivalente de adultos en un hospital de tercer nivel.
PacientesCiento noventa y seis pacientes hospitalizados en UCI, mayores de 18 años, sin enfermedad tromboembólica al ingreso y sin haber recibido tratamiento anticoagulante previamente.
IntervencionesTras el análisis modal, se implementó un paquete de intervenciones: formación, instauración de protocolo y checklist, para incrementar las medidas profilácticas de enfermedad tromboembólica.
Variables de interésIndicación y prescripción de medidas profilácticas de trombosis venosa antes y después de la implementación de medidas resultantes del análisis modal de fallos y efectos.
ResultadosEn la fase 1 se incluyeron 59 pacientes, 97 en la fase 3 y 40 en la fase 4, analizando el porcentaje de pacientes que recibieron tromboprofilaxis. Se desarrolló un análisis modal de fallos y efectos detectando errores potenciales, asociados a la ausencia de formación y de protocolos relacionados con la enfermedad tromboembólica. Se elaboró una campaña de sensibilización y formación del personal, así como la introducción del protocolo para la prevención de tromboembolismo venoso. La prescripción de medidas profilácticas aumentó en el grupo de la fase 3 (91,7 vs. 71,2%, p=0,001). En el grupo post-checklist, la profilaxis fue prescrita en el 97,5% de los pacientes, aumentado la indicación de la doble profilaxis (4,7, 6,7 y 41%; p<0,05). No hubo diferencias en la tasa de complicaciones asociadas al incremento de medidas profilácticas.
ConclusionesTras el análisis modal de fallos y efectos, se objetivaron mejoras en la prevención de enfermedad tromboembólica en el paciente crítico, por lo que consideramos que puede ser una herramienta útil para mejorar la seguridad de nuestros pacientes en diferentes procesos.
Deep venous thrombosis and pulmonary thromboembolism conform venous thromboembolic disease (VTD). Both are frequent and often silent complications that increase patient morbidity and potentially also mortality.1,2 The absence of thromboprophylaxis (TP) is estimated to be responsible for the appearance of VTD in up to 31% of all patients in Intensive Care Units (ICUs)3–pulmonary thromboembolism being regarded as the cause of death in 12–13% of such individuals.4
A Spanish multicenter study on the prevention of VTD found 82% of the patients in hospitals in Madrid to be receiving some form of TP. In turn, only 50% of the participating ICUs were seen to have a specific protocol for VTD prevention.5
Neither the administration of anticoagulant drugs nor the adoption of mechanical measures are without complications–bleeding and skin damage being the most frequent problems, respectively. The prescription of prophylaxis therefore should be based on careful weighing of the bleeding risk against the risk of thrombosis.
Since a delay in starting prophylactic measures beyond 24h after admission implies a poorer prognosis,6 the adoption of such measures is currently regarded as a quality indicator by our scientific society (SEMICYUC) in reference to patient safety (PS).7
The present study develops the different phases of a project for improvement (Fig. 1) with the general aim of increasing PS through the prevention of VTD in the Polyvalent ICU of the Department of Intensive Care Medicine of Doce de Octubre Hospital in Madrid (Spain). In parallel to the project, a safety structure has been established, based on tools for the detection and analysis of PS problems; a process for the incorporation of actions for improvement; and posterior evaluation of the outcomes, following the classical quality assessment scheme of Donabedian.8
Failure mode and effects analysis (FMEA) constitutes the basic reference of our study. This type of analysis is used to identify and evaluate potential failures of processes, their causes and possible effects.9 Although FMEA originated in the industrial sector, it is a very useful tool in application to healthcare processes.10,11 The analysis is structured into 5 phases and makes use of an interdisciplinary team to proactively evaluate a healthcare process. In this case we apply a process FMEA with the aim of discriminating and eliminating safety failures referred to prophylactic measures in VTD.
The aim of the final phase of the project is to evaluate the safety improvements referred to the prevention of VTD implemented in the Polyvalent ICU of the Department of Intensive Care Medicine following application of the actions for improvement defined by the FMEA.
Material and methodsA prospective study comprising four phases was carried out. We included all the patients over 18 years of age admitted to our ICU, excluding those with VTD at the time of admission or who were receiving prior anticoagulant treatment.
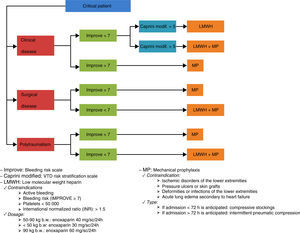
Phase I (January to April 2014): An evaluation was made of the real situation referred to the prevention of VTD in our ICU, based on the weekly documentation of demographic variables, reasons for admission, severity scores, bleeding risk factors using the bleeding risk scale of the IMPROVE cohort,12 thrombosis risk according to the scale of Caprini,13,14 and VTD prevention measures prescribed in our patients.
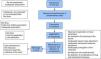
Phase II (May to July 2014): We analyzed the data collected by means of an FMEA,10 a quality analytical tool requiring the participation of all personnel members implicated in the process and which focuses on the identification, evaluation and prevention of the possible failures and effects that may appear in a given product or service. The analysis prioritizes potential failures according to risk, possibility of occurrence and possibility of detection. Based on these priorities, actions are defined with the aim of reducing the possibility of error/failure. Our FMEA resulted in a personnel sensitization campaign, a training measures packet for both the medical and nursing personnel, and the development of a VTD prevention protocol (Fig. 2).
This process FMEA was structured according to the following methodology:
- •
Area subject to analysis: implementation of VTD preventive measures in the Polyvalent ICU of Doce de Octubre Hospital.
- •
Selection of a team with experience in the treatments and the safety tool, with decision making capacity in the organization: Section Chief (Polyvalent ICU), Head of the Quality Unit, Nursing Supervisor, an expert staff physician, two residents in Intensive Care Medicine, two expert nurses, and a product specialist from the company supplying the technology.
- •
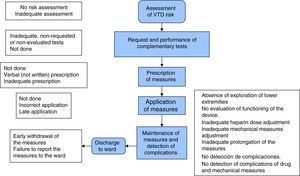
Development of the process flowchart (Fig. 3): graphic development of each of the sub-processes in sequential order, with consideration of the possible failures that may appear in each of them.
- •
Determination of potential failures, causes and effects of each of the activities of the process in an FMEA table.
- •
Analysis of each of the causes of failure according to their frequency, severity and possibility of detection. Numerical values (between 1 and 10) are assigned to the items based on a Likert scale.9
- •
Calculation of the risk priority number (RPN) from the product of the three values (frequency×severity×possibility of detection).
- •
Prioritization of the causes of failure according to their RPN.
- •
Proposal of actions for improvement designed to eliminate or mitigate those causes with the highest RPN.
- •
Development of control and evaluation indicators of the proposed actions.
- •
Evaluation of the effect of the proposed actions.
Phase III (August 2014 to January 2015): A cohort study was carried out following the methodology of the FMEA. We again collected the same variables on a weekly basis, with the same inclusion method as in phase i, establishing a comparison of the implementation rates of the prophylactic measures.
Phase IV (February to March 2015): A daily patient bedside checklist was introduced, based on a software application, with an evaluation of its impact added to the measures implemented in phase iii.
The absolute contraindications to drug prophylaxis were: the presence of active bleeding, a high bleeding risk as assessed by the IMPROVE scale (score≥7), and the single presence of a platelet count of <50,000/mm3 or INR>1.5. In turn, recent major bleeding or a decrease in hemoglobin values, requiring transfusion and without the objective possibility of ruling out active bleeding, were regarded as relative contraindications.
On the other hand, patients with vascular ischemic problems of the lower extremities, pressure ulcers (PUs) or skin grafts, deformities or infections of the lower extremities, acute lung edema secondary to congestive heart failure, or with edemas exceeding the size limits of the devices available in the Unit, were excluded from mechanical prophylaxis.
Ethical considerationsThe project was evaluated by the Clinical Research Ethics Committee of Doce de Octubre Hospital, which did not consider the obtainment of informed consent to be necessary.
Statistical analysisContinuous variables were reported as the mean and standard deviation, while qualitative variables were presented as absolute and relative frequencies. Where necessary, normal distribution of data was evaluated by histogram analysis and the Shapiro–Wilk test. Means were compared using the Student t-test or Wilcoxon test. Proportions were contrasted based on the chi-squared test and Fisher exact test. Statistical significance was accepted for p<0.05. The SPSS® version 22.0.0 statistical package was used throughout.
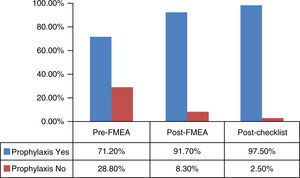
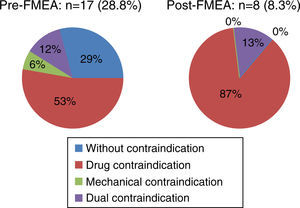
ResultsA total of 59 patients were enrolled in the study in the pre-FMEA period. The main epidemiological characteristics are shown in Table 1. Most of the patients (71.1% [42/59])(Fig. 4) received prophylaxis: 61.9% (26/42) in the form of drugs, 33.3% (14/42) in the form of mechanical measures, and 4.7% (2/42) in the form of both drugs and mechanical measures–criteria for such measures being met by 38.1% of the total subjects included in the group. All of the patients (26/26) that received pharmacological prophylaxis were administered enoxaparin. Mechanical prophylaxis in turn comprised intermittent pneumatic compression stockings (IPCS) in 64.3% (9/14) of the patients, while graded compression stockings were prescribed in 35.7% (5/14). A total of 28.8% (17/59) of the patients received no prophylaxis (Fig. 5). In turn, 29.4% (5/17) presented no contraindications, 52.9% (9/17) presented pharmacological contraindications, and 5.8% (1/17), presented mechanical contraindications. Only 11.6% (2/17) of the patients had contraindications for both drug and mechanical prophylaxis. The mean delay in starting the preventive measures was 28h.
General characteristics of the patients admitted during the pre-FMEA and post-FMEA periods.
| Patients | Pre-FMEA | Post-FMEA |
|---|---|---|
| n=59 | n=97 | |
| Disease of admission, % | Infectious 27.8 | Infectious 28.8 |
| Respiratory 19.4 | Respiratory 22.6 | |
| Neurological 19.4 | Neurological 20.6 | |
| Postsurgery 11.1 | Postsurgery 13.4 | |
| Toxic-metabolic 11.1 | Toxic-metabolic 9.2 | |
| Others 11.2 | Others 5.2 | |
| Age in years, mean±SD | 57.8±13.5 | 58.95±11.15 |
| Sex, n (%) | Males 37 (62.7) | Males 56 (57.7) |
| Females 22 (37.3) | Females 41 (42.2) | |
| APACHE score, mean±SD | 18.61±9.3 | 18.1±8.8 |
| SOFA score, mean±SD | 7.3±3.0 | 8.0±3.0 |
| Vasoactive drugs, % | 30.5 | 34 |
| Mechanical ventilation, % | 71.2 | 67.0 |
Without significant differences (p>0.05).
Following development and analysis of the FMEA, the main potential failures detected in relation to each of the sub-elements of the established flowchart and targeted for RPN intervention were: (1) absent or inadequate assessment of VTD risk; (2) absent or inadequate tests; (3) absent prescription of measures; (4) errors in application of the prescribed measures; and (5) defects in maintenance of the measures and the detection of complications. All these shortcomings were related to training deficiencies (RPN 486, 540, 729, 640 and 810, respectively) and the lack of related protocols (RPN 486, 486, 315, 0 and 810, respectively).
Following the analysis, the proposed actions for improvement were: the development of a protocol for the prevention of VTD including the indications, contraindications, dosage and risk factors referred to both the development of VTD and bleeding based on scales (Fig. 2). In turn, a VTD awareness campaign was established, targeted to the personnel and based on informative panels in the Unit and the conduction of training sessions for both the medical and nursing personnel. The project also contemplates the reinforcement of awareness through the a posteriori introduction of a daily checklist, if necessary.
In the post-FMEA period we included 97 patients with characteristic very similar to those of the first cohort (Table 1). The great majority of the patients (91.7% [89/97]) received prophylaxis (p<0.001) (Fig. 4): 77.5% (69/89) in the form of drugs, 15.7% (14/89) in the form of mechanical prophylaxis, and 6.7% (6/89) in the form of both drugs and mechanical prevention – criteria for such measures being met by 47.1% of the total subjects included in the group. All of the patients (69/69) that received pharmacological prophylaxis were administered enoxaparin. Mechanical prophylaxis in turn comprised IPCS in 60.0% (12/20) of the patients, while graded compression stockings were prescribed in 40.0% (8/20). Lastly, 7.2% (7/97) of the total patients (Fig. 5) presented a high bleeding risk but did not receive mechanical prophylaxis, and only 1.0% (1/97) presented contraindications for both types of measures. The mean delay in starting the preventive measures was 23h.
Following the introduction of a checklist (Fig. 4) based on a software application used at the patient bedside in the course of the daily visit, a total of 40 patients were included for analysis. A full 97.5% (39/40) of the patients received prophylactic measures: 46.1% in the form of drugs (18/39), 12.8% (5/39) in the form of mechanical prevention, and 41.0% (16/39) in the form of both types of preventive measures–criteria for such measures being met by all of the patients (p<0.001). Only one patient (2.5%) had dual contraindication for VTD preventive measures. All the subjects with an indication of drug prophylaxis were administered enoxaparin. In turn, of those who received mechanical prevention measures, 66.7% (14/21) received graded compression stockings and 33.3% (7/21) IPCS. The mean delay in starting the preventive measures was 23h.
Although the percentage of patients with VTD prevention measures increased post-checklist vs. post-FMEA, statistical significance was not reached (Fisher exact test; p=0.28).
No cases of severe bleeding were recorded in relation to heparin use. Only local abdominal wall hematomas were observed in 59.8% (79/139) of the patients treated with enoxaparin, with no abscessification in any case. Antibody determination through ELISA testing was requested in two patients with suspected heparin-induced thrombocytopenia, and although the results proved negative, prior temporary replacement of drug prophylaxis with mechanical prevention was decided.
The only complication associated to mechanical prevention was the presence of PUs, which manifested in 4 (2.0%) and 6 (3.0%) patients in the pre- and post-FMEA phases, respectively (p>0.05). Four of these patients presented grade i PU and two presented grade ii PU.
DiscussionAlthough there are few clinical trials on the use of thromboprophylaxis (TP) in critically ill patients, the ACCP 2012 guides recommend the use of TP with recommendation grade 2C,15 in the same way as the NICE guides.16 Our own scientific society (SEMICYUC) defines the implementation of such measures as an indicator of the quality of our work.7
Despite the above, initially 28.8% of our patients received no type of VTD preventive measures. Furthermore, little use was made of dual prophylaxis in very high risk patients. In view of these data, potential room for improvement was identified in our Unit. Recently, the PROF-ETEV multicenter study, carried out in Spanish critical patients, has evidenced the scant prescription of measures of this kind. In effect, they are not used in up to 19% of the patients; dual prophylaxis is infrequent (11%); and little use is made of specific protocols in our Units.17 Similar results have been obtained by a number of studies in other countries – thus again reflecting the low adherence to the clinical guides sometimes found in our setting.18
In our Department, all the patients requiring drug prophylaxis were administered low molecular weight heparin (LMWH)–specifically enoxaparin, adjusted to body weight (Fig. 2). Administration typically took place in the afternoon shift, in order to allow scheduled techniques or operations to be performed in the morning, without having to interrupt prophylaxis. No serious complications associated to the administration of LMWH were observed, and although subcutaneous cellular tissue hematomas were frequent, they were not clinically relevant. Anti-factor Xa antibody titers were determined in patients with morbid obesity or renal failure, in compliance with our protocol.
Mechanical prophylaxis is recommended in cases where drug prophylaxis is contraindicated according to the ACCP.15 Very few patients in our series were unable to receive mechanical prophylaxis. Intermittent pneumatic compression stockings (IPCS) were the most widely used option, in concordance with the observations of Garcia-Olivares et al.,17 though we possibly were unable to further increase their use because of limitations in the number of devices available in the Unit at the time. The frequency of superficial grade i and ii PUs was low, in coincidence with the findings of Knudson et al.19 All such ulcers were recorded with the IPCS systems, as a result of sizing problems, which were solved once all the possible stocking sizes became available. Furthermore, this situation served to organize training sessions for the nursing personnel, referred to the prevention and management of PUs in the ICU.
According to our protocol, postsurgery and polytraumatized patients without contraindications for drug prophylaxis and with a low bleeding risk (IMPROVE score <7) are candidates for dual prophylaxis, due to their high intrinsic risk of thrombosis. However, in addition to the aforementioned two conditions, clinical patients are required to present a high risk of thrombosis (Caprini score >5). The initially adopted post-FMEA measures were not sufficiently effective for TP prescription, and the daily checklist became the tool that allowed us to cover 100% of the patients with this indication.
Nurses cover management needs at the patient bedside, and are in charge of implementing and maintaining mechanical prophylaxis, as well as of providing skin damage preventive or treatment measures in the Unit. These professionals therefore were enrolled in our project, forming part of the research team. In this regard, teamwork and fluid communication between the medical and nursing personnel are essential not only for preventing risks associated to VTD but also for avoiding patient management errors with a view to improving patient safety.20–22 Safety is a dynamic process that validates the quality of the care provided. In this regard, the FMEA has been accepted as a useful tool allowing us to identify potential failures within a healthcare process, their consequences, and the measures required to minimize them from the analyses and perspectives of different healthcare professionals.23 The evaluation of our FMEA process allowed the elaboration of a specific VTD protocol, a personnel training program, and the development of a daily checklist that have improved the efficacy, safety and quality of the care received by our patients.18,24
We consider the existence of care protocols to be fundamental for improving PS, since they avoid variability in patient management and can even shorten admission to the ICU–as has already been demonstrated in a number of Units.25–27 In this regard, the introduction of our protocol and of the personnel training program allowed us to optimize the VTD preventive measure prescription rate, which increased from 72.8% to 97.5%.
The introduction of a checklist in routine clinical practice has resulted in favorable outcomes referred to early extubation,28 the prevention of catheter-related infections,29,30 and the reduction of omission errors.31 Indeed, and although with limitations, Weiss et al. even demonstrated a decrease in mortality and ICU stay.32 In our study the checklist not only resulted in increased dual prophylaxis prescription but also in increased knowledge and adherence to the protocol.
Limitations of failure mode and effects analysisThe main limitation of FMEA is its qualitative character. Shebl et al.33 found important discrepancies between two multidisciplinary teams that used FMEA referred to the use of two different antibiotics. They concluded that FMEA fails in apparent validity. These same investigators34 recommend that FMEA should not be used as a quantitative tool to prioritize, promote or study interventions in PS. Despite its limitations, however, this tool offers adequate methodology for standardization, analysis and schematization of the clinical process in the context of a multidisciplinary work group.35 We therefore consider it to be very useful for improving PS in different scenarios.
ConclusionsAfter implementing the improvement measures proposed by the FMEA, we have been able to optimize the prescription of prophylactic measures in VTD, reaching the quality standards established by our scientific society (SEMICYUC). We consider this safety tool to be very useful for improving different critical patient care processes.
On the other hand, protocolized dynamic management and the use of the daily checklist have served to optimize the efficacy and efficiency of prophylactic treatment in our patients.
Financial supportSupport for the development of research projects has been received from the Research Institute of Doce de Octubre Hospital, i+12, sponsored by AMGEN S.A.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Thanks are due to all the medical and nursing staff members of the Polyvalent ICU of Doce de Octubre Hospital for their active participation in the provision of quality care for our patients.
Please cite this article as: Viejo Moreno R, Sánchez-Izquierdo Riera JÁ, Molano Álvarez E, Barea Mendoza JA, Temprano Vázquez S, Díaz Castellano L, et al. Mejora en la seguridad de un proceso clínico utilizando el análisis modal de fallos y efectos: profilaxis de la enfermedad tromboembólica venosa en pacientes críticos. Med Intensiva. 2016;40:483–490.