To know the variability of transthoracic echocardiographic parameters that assess right ventricular systolic function by analyzing interobserver agreement in the early postoperative period of cardiovascular surgery.
Secondary objectiveTo assess the feasibility of these echocardiographic measurements.
DesignA cross-sectional study, double-blind pilot study was carried out from May 2011 to February 2013.
SettingCardiovascular postoperative critical care at the National Institute of Cardiology “Ignacio Chávez”, Mexico City, Mexico.
PatientsConsecutive, non-probabilistic sampling. Fifty-six patients were studied in the postoperative period of cardiac surgery.
InterventionThe first echocardiographic parameters were obtained between 6 and 8h after cardiac surgery, followed by blinded second measurements.
Main variablesTricuspid annular plane systolic excursion (TAPSE), tricuspid annular peak systolic velocity on tissue Doppler imaging (VSPAT), diameters and right ventricular outflow area, tract fractional shortening. The agreement was analyzed by the Bland–Altman method, and its magnitude was assessed by the intraclass correlation coefficient (95% confidence interval).
ResultsBoth observers evaluated TAPSE and VSPAT in 48 patients (92%). The average TAPSE was 11.68±4.53mm (range 4–27mm). Right ventricular systolic dysfunction was observed in 41 cases (85%) and normal TAPSE in 7 patients (15%). The average difference and its limits according to TAPSE were −0.917±2.95 (−6.821, 4.988), with a magnitude of 0.725 (0.552, 0.837); the tricuspid annular peak systolic velocity on tissue Doppler imaging was −0.001±0.015 (−0.031, 0.030), and its magnitude 0.825 (0.708, 0.898), respectively.
ConclusionsVSPAT and TAPSE were estimated by both observers in 92% of the patients, these parameters exhibiting the lowest interobserver variability.
Conocer la variabilidad interobservador de los parámetros ecocardiográficos transtorácicos que evalúan la función sistólica del ventrículo derecho en sujetos en el postoperatorio temprano de cirugía cardiaca.
Objetivo secundarioEvaluar la factibilidad en la medición de estos parámetros ecocardiográficos.
DiseñoPiloto, transversal, doble ciego. Mayo de 2011 a febrero de 2013.
ÁmbitoUnidad de Cuidados Intensivos Posquirúrgicos Cardiovasculares, Instituto Nacional de Cardiología «Ignacio Chávez», Ciudad de México (México).
PacientesMuestreo no probabilístico, consecutivo, se estudiaron 56 pacientes postoperados de cirugía cardiaca.
IntervenciónEntre 6 a 8 h después de la intervención de cirugía cardiaca se obtuvieron los parámetros ecocardiográficos. La segunda medición se realizó a posteriori, de forma cegada.
Variables de interésExcursión sistólica del plano valvular tricuspídeo (TAPSE), velocidad sistólica pico del anillo tricuspídeo medida por Doppler tisular (VSPAT), diámetros y fracción de acortamiento del tracto de salida del ventrículo derecho. La variabilidad interobservador y su magnitud se obtuvieron con el procedimiento de Bland-Altman y el coeficiente de correlación intraclase (intervalo de confianza del 95%).
ResultadosEl TAPSE y la VSPAT se pudieron estudiar por ambos observadores en 48 (92%) de los sujetos. El promedio del TAPSE fue 11,68±4,53mm con valor mínimo-máximo de 4 a 27mm. Se encontró disfunción sistólica del ventrículo derecho en 41 (85%) y TAPSE normal en 7 (15%) pacientes. La diferencia media y sus límites de acuerdo del TAPSE fueron -0,917±2,95 (–6,821; 4,988), y su magnitud de 0,725 (0,552; 0,837). Los valores de VSPAT fueron –0,001± 0,015 (–0,031; 0,030) con magnitud de 0,825 (0,708; 0,898) respectivas.
ConclusionesFue posible estimar el TAPSE y la VSPAT por parte de ambos observadores en el 92% de los sujetos. Estos índices ecocardiográficos tuvieron la menor variabilidad interobservador en sujetos postoperados de cirugía cardiaca.
The study of right ventricular systolic function (RVSF) is complicated due to the shape and position of the right ventricle (RV) in the thorax.1,2 Noninvasive evaluation can be made using echocardiography. In this regard, a recent publication3 has defined the echocardiographic parameters needed for reliable and objective evaluation, in contraposition to visual assessment, which although frequently used in clinical practice, is subjective and therefore influenced by inherent interobserver variability.4 These parameters have been studied in contexts other than the postoperative phase of heart surgery.
In contrast to transesophageal echocardiography (TEE), transthoracic echocardiography (TTE) is noninvasive and painless, and can be performed repeatedly. Furthermore, in the same way as TEE, it does not involve ionizing radiation and to date has not been shown to have any biological effects.5 This diagnostic technique is increasingly used in Intensive Care Units (ICUs),6 specifically in cardiovascular ICUs.
Heart surgery patients often experience alterations in RVSF,7–12 and such alterations have been identified as predictors of patient morbidity, severe hemodynamic changes and even death.12,13
Experience with the use of TTE in this patient population is very limited. To the best of our knowledge, the only study published to date on TTE and the postoperative phase of heart surgery14 defined the technique as a useful tool for guiding treatment.
In the presurgical context of mitral valve surgery,15 both tricuspid annular plane systolic excursion (TAPSE) and the tricuspid annular peak systolic velocity (TAPSV) measured by tissue Doppler have been the most reliable parameters for evaluating RVSF. In the late postoperative period (>6 months) of mitral valve surgery, RV function and systolic and diastolic volumes determined by three-dimensional echocardiography have been shown to be useful and reliable.16 In the postoperative phase of large artery transposition and atrial redirecting surgery, interobserver variation was found to be great, with an increasing tendency to overestimate the echocardiographic parameters of RVSF.17
The characteristics inherent to the RV and a poor acoustic window have made it difficult to obtain adequate images for analysis in the nonsurgical context.14–17 However, even in the presence of a good acoustic window, interobserver variability can be great if subjective methods are used for assessment.
In the postoperative period of cardiovascular surgery it is not clear whether mechanical ventilation (MV), overweight, and the presence of mediastinal and/or pleural drains can affect the acoustic window and thus the possibility of obtaining adequate echocardiographic images for analyzing RV function.
In view of the above, the present study was carried out to determine the feasibility of obtaining the echocardiographic indices related to RVSF, and to describe the interobserver variability of these parameters in individuals in the early postoperative phase of heart surgery.
Patients and methodsThe study was carried out in the Cardiovascular Postsurgery Intensive Care Unit of «Ignacio Chávez» National Cardiology Institute, in México D.F. (Mexico), between August 2012 and February 2013.
A cross-sectional, double-blind pilot study was designed, with consecutive non-probabilistic sampling. Patients between 18 and 75 years of age in the postoperative phase of myocardial revascularization and mitral, aortic or mitral-aortic valve replacement surgery in «Ignacio Chávez» National Cardiology Institute were included. All the echocardiographic data were acquired between 6 and 8h after surgery. The following exclusion criteria were applied: patients with active bleeding, packing measures due to mediastinal hemorrhage or hemodynamic instability (mean systemic blood pressure <60mmHg).
All the TTE parameters were obtained by the same investigators (SGOT and RJAA)–both cardiologists with formal training in echocardiography. The numerical values corresponding to the study variables were blinded between observers, and the order of data collection was established by simple randomization.
The parameters evaluated by TTE and related to RVSF3,15 were: TAPSE (mm), TAPSV (m/s), diastolic and systolic diameter of the RV (DDRV and SDRV, respectively)(mm), right ventricular diameter shortening fraction (RVDSF)(%), diastolic and systolic area of the RV (DARV, SARV)(cm2), right ventricular area shortening fraction (RVASF, %), diastolic and systolic diameter of the right ventricular outflow tract (DDRVOT and SDRVOT, respectively)(mm), right ventricular outflow tract shortening fraction (RVOTSF)(%), diastolic and systolic diameter of the left ventricle (DDLV and SDLV, respectively)(mm), and the right ventricle/left ventricle ratio (RV/LV).
The parameters were quantified according to the guides of the American Society of Echocardiography for evaluation of the right side of the heart3: TAPSE in apical plane four-chamber M-mode; TAPSV using apical plane four-chamber tissue Doppler with sample volume in the tricuspid ring; RV areas in apical plane four-chamber mode; RV diastolic and systolic diameters in apical plane four-chamber mode in the basal region of the RV; and RV outflow tract in the short parasternal axis at large vessel level.
In all cases we used the VIVID E9 cardiovascular ultrasound system with the M5S-D sectorial cardiac probe (GE Vingmed Ultrasound A/S, Strandpromenaden 45, N-3191 Norten, Norway) and 1.5–4.6MHz transducer. The images were acquired with tissue harmonics. No ultrasound contrast was used to improve visualization of the endocardial margins.
The patients signed the informed consent form before surgery, and the study was approved by the Ethics Committee of «Ignacio Chávez» National Cardiology Institute.
Both observers obtained the different study parameters after ensuring normalization of the hemodynamic parameters in the first 6–8h after surgery, with the patient in supine decubitus and without any therapeutic maneuvering capable of modifying the echocardiographic recordings. A third participant, unrelated to the measurements, entered the results in the database.
Statistical analysis: Numerical values were reported as the mean and standard deviation, while nominal variables were expressed as frequencies and percentages.
Interobserver variability was analyzed based on the mean difference and limits according to the Bland–Altman statistical procedure.18,19 Its magnitude was calculated with the intraclass correlation coefficient (ICC) and corresponding 95% confidence interval (95%CI).19,20 Almost perfect agreement was defined as ICC 0.81–1.0; substantial agreement as ICC 0.61–0.80; moderate agreement as ICC 0.41–0.6; regular agreement as ICC 0.21–0.4; slight agreement as 0.01–0.2; and poor agreement as ICC 0.0.20
ResultsA total of 56 post-heart surgery patients were enrolled, of which four were excluded due to the presence of extreme acoustic window conditions (no observer was able to perform the measurements). A total of 52 subjects where therefore finally analyzed, of which the majority (n=30; 58%) were males. The demographic, clinical and surgical characteristics are reported in Table 1. The study population was overweight and presented other comorbidities. The predominant type of procedure was valve surgery. Mediastinal drains were placed in all cases. Most patients had two pleural drains (left and right), and almost one-half (48%) were on MV during recording of the echocardiographic data.
Demographic, clinical and surgical characteristics of the patients.
| Males (%) | 30 (58) |
| Weight (kg) | 69.9±12.53 |
| Height (m) | 1.63±0.08 |
| BMI (kg/m2) | 26.22±3.66 |
| Diabetes mellitus (%) | 9 (17.3) |
| Arterial hypertension (%) | 14 (27) |
| CRVS (%) | 12 (23) |
| Valve surgery (VR, CRVS+VR, AoR) (%) | 39 (75) |
| Tumor resection (%) | 1 (2) |
| Mediastinal drain (%) | 52 (100) |
| 1 pleural drain (%) | 14 (27) |
| 2 pleural drains (%) | 38 (73) |
| Mechanical ventilation (%) | 25 (48) |
The figures express the mean±standard deviation or frequency (percentage).
CRVS: coronary revascularization surgery; CV: valve replacement; n (%); BMI: body mass index; AoR: aortic valve replacement.
The four subjects in which no echocardiographic measurements could be obtained were all subjected to MV, with mediastinal and pleural drains at the time of recording, and three were moreover overweight.
The mean TAPSE in the overall group of patients was 11.68±4.53mm (minimum 4mm and maximum 27mm). In 7 patients (15%) the data were in the normal range (TAPSE≥17mm), while 41 (85%) presented right ventricular systolic dysfunction (<17mm).21 Specifically, in these cases right ventricular systolic dysfunction proved mild (TAPSE≥12mm, <17mm) in 18 (37.5%), moderate (≥7mm, <12mm) in 19 (39.5%), and severe (<7mm) in four patients (8%).
In our study series the frequency of the evaluated echocardiographic variables proved inconstant for either of the two observers, as can be seen in Table 2. Only TAPSE and TAPSV could be determined in over 90% of the cases.
Number of subjects in which the echocardiographic parameters could be obtained according to each observer, both observers jointly, and the magnitude of interobserver variability with the corresponding 95% confidence interval.
| Measure | Observer 1 n (%) | Observer 2 n (%) | Both n (%) | ICC (95%CI) |
|---|---|---|---|---|
| TAPSV, m/s | 50 (96) | 48 (92) | 48 (92.3) | 0.825 (0.708, 0.898) |
| TAPSE, mm | 50 (96) | 48 (92) | 48 (92.3) | 0.725 (0.552, 0.837) |
| RV/LV | 36 (69) | 34 (65) | 31 (59.6) | 0.699 (0.467, 0.842) |
| DARV, cm2 | 47 (90) | 45 (86) | 45 (86.5) | 0.653 (0.447, 0.793) |
| SARV, cm2 | 47 (90) | 45 (86) | 45 (86.5) | 0.621 (0.405, 0.771) |
| RVASF, % | 47 (90) | 45 (86) | 45 (86.5) | 0.375 (0.085, 0.598) |
| DDRV, mm | 46 (88) | 45 (86) | 44 (84.6) | 0.593 (0.361, 0.756) |
| SDRV, mm | 46 (88) | 45 (86) | 44 (84.6) | 0.488 (0.227, 0.684) |
| FACD, % | 46 (88) | 45 (86) | 44 (84.6) | 0.388 (0.105, 0.613) |
| DDRVOT, mm | 26 (50) | 29 (56) | 25 (48) | 0.728 (0.470, 0.871) |
| SDRVOT, mm | 26 (50) | 29 (56) | 25 (48) | 0.622 (0.318, 0.812) |
| RVOTSF, % | 26 (50) | 29 (56) | 25 (48) | 0.467 (0.114, 0.720) |
DARV, diastolic area of the RV; SARV, systolic area of the RV; ICC (95%CI), intraclass correlation coefficient (95% confidence interval); DDRVOT, diastolic diameter of the right ventricular outflow tract; DDRV, diastolic diameter of the right ventricle (RV); DDLV, diastolic diameter of the left ventricle (LV); SDRVOT, systolic diameter of the right ventricular outflow tract; SDRV; systolic diameter of the RV; SDLV, systolic diameter of the LV; RVASF right ventricular area shortening fraction; RVDSF, right ventricular diameter shortening fraction; RVOTSF, right ventricular outflow tract shortening fraction; LVEF, left ventricular ejection fraction; TAPSE, tricuspid annular plane systolic excursion; RV/LV, right ventricle/left ventricle ratio; TAPSV, tricuspid annular peak systolic velocity measured by tissue Doppler ultrasound.
The respective ICC (95%CI) of these parameters are presented in Table 2. In turn, Table 3 presents the echocardiographic parameters exhibiting the greatest ICC (>0.61, corresponding to substantial agreement), the mean differences, limits of agreement and values corresponding to ICC (95%CI).
Means and standard deviations, mean differences and limits of agreement of the echocardiographic parameters with the best intraclass correlation coefficients obtained.
| Measure | Observer 1 (X¯)±SD | Observer 2 (X¯)±SD | Mean difference (X¯)±SD | Limits of agreement |
|---|---|---|---|---|
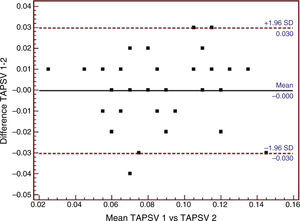
| TAPSV, m/s (n=48) | 0.84±0.02 | 0.85±0.025 | −0.001±0.015 | −0.031, 0.030 |
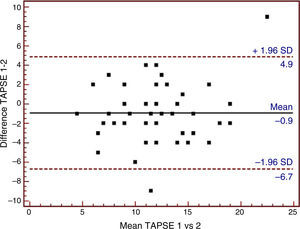
| TAPSE, mm (n=48) | 11.68±4.53 | 12.47±3.84 | −0.917±2.95 | −6.821, 4.988 |
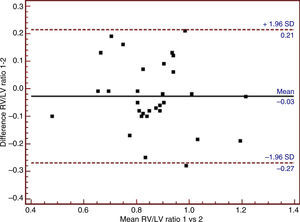
| RV/LV (n=31) | 0.86±0.15 | 0.86±0.16 | −0.028±0.12 | −0.276, 0.219 |
ICC (95%CI), intraclass correlation coefficient (95% confidence interval); TAPSE, tricuspid annular plane systolic excursion; RV/LV, right ventricle/left ventricle ratio; TAPSV, tricuspid annular peak systolic velocity measured by tissue Doppler ultrasound.
The Bland–Altman plots of the three echocardiographic parameters exhibiting substantial agreement (ICC>0.61) are shown in Figs. 1–3.
Bland–Altman analysis of tricuspid annular peak systolic velocity (TAPSV) using tissue Doppler ultrasound. SD: standard deviation; TAPSV 1: tricuspid annular peak systolic velocity obtained using tissue Doppler by observer 1; TAPSV 2: tricuspid annular peak systolic velocity obtained using tissue Doppler by observer 2.
Bland–Altman analysis of tricuspid annular plane systolic excursion (TAPSE) using tissue Doppler ultrasound. SD: standard deviation; TAPSE 1: tricuspid annular plane systolic excursion obtained using tissue Doppler by observer 1; TAPSE 2: tricuspid annular plane systolic excursion obtained using tissue Doppler by observer 2.
Tables 4 and 5 show the number of patients in which it was possible and not possible to study the different RVSF parameters stratified according to observer 1 or 2, the presence or absence of MV, and the presence or absence of overweight as established from the body mass index.
Number of patients in which it proved possible and impossible to study the different right ventricular systolic function parameters with respect to observer 1, the presence or absence of mechanical ventilation, and the presence or absence of overweight.
| Measure | Observer 1 (Yes) | Observer 1 (No) | ||||||
|---|---|---|---|---|---|---|---|---|
| MV (yes) | MV (no) | MV (yes) | MV (no) | |||||
| BMI≥25 | BMI<24.9 | BMI≥25 | BMI<24.9 | BMI≥25 | BMI<24.9 | BMI≥25 | BMI<24.9 | |
| TAPSV, m/s | 15 | 9 | 17 | 9 | 1 | 0 | 1 | 0 |
| TAPSE, mm | 15 | 9 | 17 | 9 | 1 | 0 | 1 | 0 |
| RV/LV | 10 | 5 | 14 | 7 | 6 | 4 | 4 | 2 |
| RVOTSF, % | 7 | 1 | 13 | 5 | 9 | 8 | 5 | 4 |
| RVASF, % | 13 | 8 | 17 | 9 | 3 | 1 | 1 | 0 |
| RVDSF, % | 13 | 7 | 17 | 9 | 3 | 2 | 1 | 0 |
| DARV, cm2 | 13 | 8 | 17 | 9 | 3 | 1 | 1 | 0 |
| SARV, cm2 | 13 | 8 | 17 | 9 | 3 | 1 | 1 | 0 |
| DDRV, mm | 13 | 7 | 17 | 9 | 3 | 2 | 1 | 0 |
| SDRV, mm | 13 | 7 | 17 | 9 | 3 | 2 | 1 | 0 |
| DDRVOT, mm | 7 | 1 | 13 | 5 | 9 | 8 | 5 | 4 |
| SDRVOT, mm | 7 | 1 | 13 | 5 | 9 | 8 | 5 | 4 |
DARV, diastolic area of the right ventricle (RV); SARV, systolic area of the RV; DDRVOT, diastolic diameter of the right ventricular outflow tract; DDRV, diastolic diameter of the RV; SDRVOT, systolic diameter of the right ventricular outflow tract; SDRV, systolic diameter of the RV; RVASF right ventricular area shortening fraction; RVDSF, right ventricular diameter shortening fraction; RVOTSF, right ventricular outflow tract shortening fraction; BMI, body mass index; Observer 1 (yes), the variables could be measured; Observer 1 (no), the variables could not be measured; TAPSE, tricuspid annular plane systolic excursion; RV/LV, right ventricle/left ventricle ratio; MV (yes), with mechanical ventilation; MV (no), without mechanical ventilation; TAPSV, tricuspid annular peak systolic velocity measured by tissue Doppler ultrasound.
Number of patients in which it proved possible and impossible to study the different right ventricular systolic function parameters with respect to observer 2, the presence or absence of mechanical ventilation, and the presence or absence of overweight.
| Measure | Observer 2 (yes) | Observer 2 (no) | ||||||
|---|---|---|---|---|---|---|---|---|
| MV (yes) | MV (no) | MV (yes) | MV (no) | |||||
| BMI≥25 | BMI<24.9 | BMI≥25 | BMI<24.9 | BMI≥25 | BMI<24.9 | BMI≥25 | BMI<24.9 | |
| TAPSV, m/s | 15 | 8 | 16 | 9 | 1 | 1 | 2 | 0 |
| TAPSE, mm | 15 | 8 | 16 | 9 | 1 | 1 | 2 | 0 |
| RV/LV | 8 | 5 | 14 | 7 | 8 | 4 | 4 | 2 |
| RVOTSF, % | 7 | 2 | 14 | 6 | 9 | 7 | 4 | 3 |
| RVASF, % | 13 | 7 | 16 | 9 | 3 | 2 | 2 | 0 |
| RVDSF, % | 13 | 7 | 16 | 9 | 3 | 2 | 2 | 0 |
| DARV, cm2 | 13 | 7 | 16 | 9 | 3 | 2 | 2 | 0 |
| SARV, cm2 | 13 | 7 | 16 | 9 | 3 | 2 | 2 | 0 |
| DDRV, mm | 13 | 7 | 16 | 9 | 3 | 2 | 2 | 0 |
| SDRV, mm | 13 | 7 | 16 | 9 | 3 | 2 | 2 | 0 |
| DDRVOT, mm | 7 | 2 | 14 | 6 | 9 | 7 | 4 | 3 |
| SDRVOT, mm | 7 | 2 | 14 | 6 | 9 | 7 | 4 | 3 |
DARV, diastolic area of the right ventricle (RV); SARV, systolic area of the RV; DDRVOT, diastolic diameter of the right ventricular outflow tract; DDRV, diastolic diameter of the RV; SDRVOT, systolic diameter of the right ventricular outflow tract; SDRV, systolic diameter of the RV; RVASF right ventricular area shortening fraction; RVDSF, right ventricular diameter shortening fraction; RVOTSF, right ventricular outflow tract shortening fraction; BMI, body mass index; Observer 2 (yes), the variables could be measured; Observer 2 (no), the variables could not be measured; TAPSE, tricuspid annular plane systolic excursion; RV/LV, right ventricle/left ventricle ratio; MV (yes), with mechanical ventilation; MV (no), without mechanical ventilation; TAPSV, tricuspid annular peak systolic velocity measured by tissue Doppler ultrasound.
The estimation of RVSF has become a need in presurgical and postsurgical patients, due to its importance in defining the prognosis of heart surgery.12,13
The limiting factors for the study of RVSF, such as the anatomical complexity of the ventricle and its location in the thorax, constitute a challenge for any study method. At present, cardiac magnetic resonance imaging is regarded as the gold standard for global assessment of systolic function.22 However, due to its limitations in relation to safety, technical factors, availability and time, the use of cardiac magnetic resonance imaging is not feasible in certain clinical scenarios such as for example the postoperative phase of cardiovascular surgery.
Thanks to its widespread availability and easy application in experienced hands, echocardiography is becoming the tool of choice for functional cardiac and hemodynamic assessment in the ICU.5,23
Our study population has a number of special features: serious clinical conditions with SDRV in the order of 85%; the need for MV; the presence of mediastinal and/or pleural drains; and excess body weight – all these being factors that can increase interobserver variability due to the lack of an adequate acoustic window.14–17
All of the patients had mediastinal drains, and the majority moreover also had two pleural drains (Table 1). Almost one-half were evaluated while on mechanical ventilation (with endotracheal tube), and the four subjects in which no measurements could be obtained were all on MV. On the other hand, the mean body mass index indicated overweight/obesity among the subjects. These characteristics appear to influence the increased estimated interobserver variability in this patient group.5,22
Table 2 shows that TAPSE and TAPSV could be studied in 90% of the patients, while the least measurable of the rest of the parameters were found to be RVOTSF and DDRVOT and SDRVOT.
Calculation of ICC revealed substantial agreement of TAPSV, TAPSE, DARV, SARV, RV/LV, DDRVOT and SDRVOT (Table 2). However, TAPSV and TAPSE demonstrated the best ICC values. Although the right ventricular diastolic and systolic area measurements presented ICC>0.61, they were not taken into account for the analysis because their corresponding shortening values (which determine systolic functional status, RVOTSF) showed the lowest ICC. The RV/LV ratio presented a ICC value of 0.69, and could be estimated in 65% of the patients; accordingly, it may be regarded as the third most reliable parameter in assessing RVSF.
Table 3 and Fig. 1 show the mean difference in TAPSV to be very small, in the same way as its limits of agreement. In contrast, TAPSE, with a mean difference of close to −1, showed broad limits of agreement (Table 3 and Fig. 2). In turn, the RV/LV ratio presented a mean difference of close to zero, with slightly broad limits of agreement, as can be seen in Table 3 and Fig. 3.
The results shown in Tables 4 and 5 suggest that MV and overweight affect the obtainment of adequate acoustic windows with TTE, and the association of both parameters appears to more deleterious for imaging quality than either parameter used separately – this being reflected in the increased interobserver variability.
The data of our study demonstrate the feasibility of obtaining echocardiographic indices such as TAPSV and TAPSE in this critical patient population, as well as the increased interobserver variability of the rest of the echocardiographic parameters studied. This interobserver variability is greater than in patients not subjected to heart surgery.13–17 However, such variability is no greater than that observed when estimating RVSF visually in other disease scenarios.4
Transesophageal echocardiography could be used in patients with a poor acoustic window.5 The use of three-dimensional echocardiography6 likewise could be an option specifically for calculating volumes or ejection fractions–though its use is limited by the fact that it requires good echocardiographic windows and longer times to analyze RVSF, and is moreover expensive to acquire and maintain in the ICU.
Our study indicates that in this group of postsurgery patients, TAPSV is the RVSF echocardiographic parameter with the least interobserver variability, followed by TAPSE – although its limit of agreement is greater–and the RV/LV ratio in third place, though the feasibility of obtaining this parameter limits its use.
Given the lesser interobserver variability and the large number of patients in which TAPSV and TAPSE can be obtained, the use of TTE on a first intention basis for the assessment of this concrete group of patients appears to be justified.
Study limitationsOur study has limitations, related mainly to the sample size involved. Nevertheless, the sample allowed us to observe the important interobserver variability of most of the parameters used to assess RVSF using TTE. The measurements were made by cardiologists trained in echocardiography, and hence the results might not be extrapolatable to those obtained by cardiologists without such training or by intensivists trained in echocardiography. Inclusion and comparison with the visual estimation of RVSF–perhaps the most widely used method in routine practice–was not carried out, since we aimed to eliminate the subjectiveness of observation. In this regard, we based our study on objective measurements of RVSF. Lastly, all the measurements were made with the patient in supine decubitus, and this may have limited the obtainment of better quality acoustic windows if patient positioning in left lateral decubitus had been possible.
ConclusionsOur study identifies TAPSV and TAPSE as the RVSF indices with the least interobserver variability and the greatest measurement feasibility in patients in the early postoperative phase of heart surgery.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Olmos-Temois SG, Santos-Martínez LE, Álvarez-Álvarez R, Gutiérrez-Delgado LG, Baranda-Tovar FM. Acuerdo interobservador de los parámetros ecocardiográficos que estiman la función sistólica del ventrículo derecho en el postoperatorio temprano de cirugía cardiaca. Med Intensiva. 2016;40:491–498.