The implementation of invasive mechanical ventilation (IMV) in critically ill patients involves two crucial moments: the total control phase, affected among other things by the use of analgesics and sedatives, and the transition phase to spontaneous ventilation, which seeks to shorten IMV times and where optimizing patient-ventilator interaction is one of the main challenges. Ineffective inspiratory efforts (IEE) arise when there is no coordination between patient effort and ventilator support. IIE are common in different ventilatory modes and are associated with worse clinical outcomes: dyspnea, increased sedation requirements, increased IMV days and longer intensive care unit (ICU) and hospital stay. These are manifested graphically as an abrupt decrease in expiratory flow, being more frequent during expiration. However, and taking into consideration that it is still unknown whether this association is causal or rather a marker of disease severity, recognizing the potential physiological consequences, reviewing diagnostic methods and implementing detection and treatment strategies that can limit them, seems reasonable.
La implementación de ventilación mecánica invasiva (VMI) en pacientes críticos implica dos momentos cruciales: la fase control total, afectada entre otras cosas por el uso de analgésicos y sedantes, y la fase de transición a la ventilación espontánea, que busca acortar los tiempos de VMI y en donde optimizar la interacción paciente-ventilador es uno de los principales retos. Los esfuerzos inspiratorios inefectivos (EII) surgen cuando no hay coordinación entre el esfuerzo del paciente y el soporte del ventilador. Los EII son comunes en diferentes modos ventilatorios y están asociados con peores resultados clínicos: disnea, aumento de los requerimientos de sedantes, aumento de días de VMI y mayor estancia en la unidad de cuidados intensivos (UCI) y en el hospital. Los mismos se manifiestan gráficamente como una disminución abrupta del flujo espiratorio, siendo más frecuentes durante la espiración. Si bien, y teniendo en consideración que aún se desconoce si esta asociación es causal o más bien un marcador de severidad de la enfermedad, reconocer las potenciales consecuencias fisiológicas, repasar los métodos de diagnóstico e implementar estrategias de detección y tratamiento que puedan limitarlos, parece razonable.
After resolving the reason for which a person required invasive mechanical ventilation (IMV)—after having passed the initial phase of IMV—the weaning process should begin as soon as possible. Here, during the weaning phase, the focus should be on shortening the duration of IMV; to achieve this, coordination and appropriate interaction between the patient and the ventilator are essential.1–3 When this does not occur, asynchronies arise.
In IMV, the term asynchrony refers to the lack of coordination between the patient’s inspiratory effort—determined by their ventilatory needs—and the support provided by the ventilator. There are different types of asynchronies that can be grouped in various ways4,5: they can occur during the inspiratory time, during transition from inspiration to expiration, and/or during the expiratory phase.6 The most prevalent type across all ventilatory modes and throughout the entire course of IMV are ineffective inspiratory efforts (IIEs) (approximately 68% up to 70% of the total).7,8 This refers to an inspiratory muscle effort that fails to activate the ventilator, which occurs when the patient's attempt to initiate a breath does not reach the threshold necessary to trigger the equipment and start a new respiratory cycle.6
The consequences, when analyzing asynchronies as a whole include a plethora of adverse effects, thus leading to misinterpretations and incorrect decisions, resulting in unfavorable clinical scenarios.3,9–12 However, when the focus is solely on IIEs, the results are contradictory.8
Therefore, the aim of this review is to describe the frequency of occurrence, diagnostic methods, and potential physiological consequences of IIEs. Additionally, clinical strategies for their management are proposed.
The search strategy used included the MeSH terms “Mechanical Ventilation” combined with the Boolean operator AND with “other terms”: “Asynchronies”; “ineffective triggering”; “patient ventilator asynchrony”; “patient ventilator interaction”; “dysynchrony”; “ineffective effort”) and with the NOT operator to exclude studies related to “animals OR non-invasive mechanical ventilation.” The search was conducted in the PubMed database, covering publications from 2000 through 2024, with the aim of incorporating recent and relevant studies. Thus, the search was detailed as follows: (((“respiration, artificial”[MeSH Terms] AND “Asynchronies”[Other Term] OR “ineffective triggering”[Other Term] OR “patient ventilator asynchrony”[Other Term] OR “patient ventilator interaction”[Other Term] OR “dysynchrony”[Other Term] OR “ineffective effort”[Other Term] AND (2000:2024[pdat]))) NOT (animals[Other Term])) NOT (non-invasive positive pressure ventilation[MeSH Terms]) AND (2000:2024[pdat]). In this way, 114 results were registered.
Initially, titles and abstracts were reviewed to identify studies focused on patient-ventilator asynchrony in invasive mechanical ventilation, with a special emphasis on ineffective effort. Articles involving non-invasive ventilation or animal studies were excluded, as the focus of the review is invasive ventilation in humans. Additionally, references from the selected articles were reviewed. After a first phase of critical reading, a total of 62 studies were included in the final review, including clinical trials, observational studies (cohorts and case-controls), and systematic reviews and meta-analyses. Criteria were left to the authors’ discretion regarding methodological quality and clinical relevance to make sure that the selected studies provided a solid analysis of ventilatory asynchronies and their clinical implications. Studies that did not meet the inclusion criteria, and those with significant methodological limitations or without robust quantitative data on asynchrony-related clinical outcomes were excluded (A search diagram is attached in Supplementary data 1).
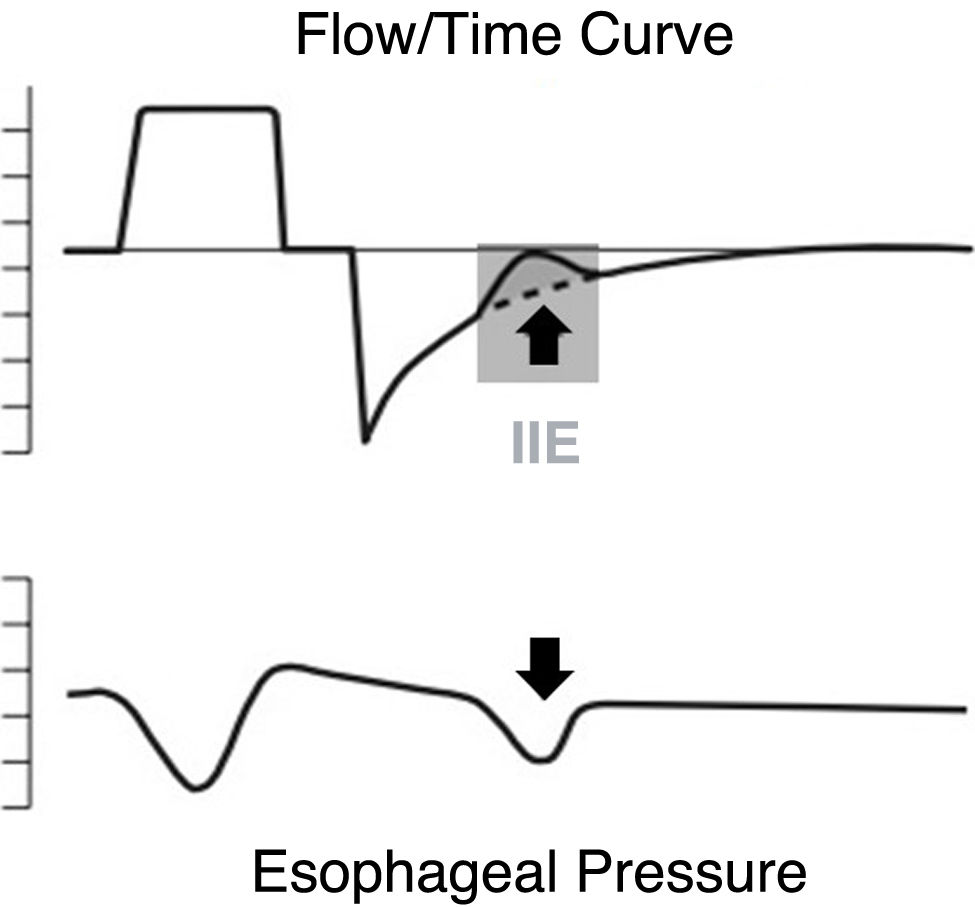
Ineffective inspiratory effortIneffective triggering refers to the inspiratory muscle effort that fails to activate the ventilator. Essentially, this occurs when the patient's attempt to initiate a breath does not reach the threshold necessary to trigger the equipment. Physiologically, this means that the ventilator does not detect the patient's inspiratory efforts, which are characterized by an increase in transdiaphragmatic pressure and/or in the electrical activity of the diaphragm.6 Graphically, IIEs manifest in the ventilator curves as a sudden decrease in expiratory flow (becoming less negative). They can occur in controlled or assisted modes, and while they may occur during the inspiratory phase, they are much more frequent during expiration.1 When this occurs during the expiratory phase, they reset the end-expiratory lung volume to a lower level, thereby reducing auto-PEEP.5,13
During IMV, the variable that initiates the ventilatory cycle depends on the ventilatory mode and the level of patient participation. In controlled modes, the variable that triggers inspiration is time, and a minimum respiratory rate (RR) is programmed into the ventilator. In assisted modes, the most widely used variables are instantaneous flow and pressure. The patient’s inspiratory effort accelerates the flow coming from the ventilator baseline and/or reduces pressure to a pre-established level (programmed trigger sensitivity), initiating inspiration. Although activation by flow is considered to require less effort for the patient,14 evidence shows minimal and non-significant differences between the two forms of activation.15
If the inspiratory effort begins before equilibrium in the system has been reached (i.e., before alveolar and airway pressures are equal), the initial part of the effort is consumed in achieving this (it must overcome the threshold elastic load). Furthermore, IIE occurs when the subsequent effort cannot overcome this and, therefore, reach the activation threshold16 (Fig. 1).
Prevalence of ineffective inspiratory effortsAsynchronies have been studied throughout the years generally as a whole, in the form of an index, and more recently as "clusters" (i.e., grouped instances of 30 or more IIEs for a 3-min period of IMV) among prolonged periods without events. This is due to the variety of factors that intervene in their development that can vary throughout the course of IMV.17,18
IIEs constitute one of the most common types of patient-ventilator asynchrony. They occur in most patient populations, across different ventilatory modes, and at different times of the day.15 An asynchrony index (AI) > 10% (more than 10% of all breaths in IMV with some type of asynchrony), and an IIE index > 10%, are considered severe and are associated with worse outcomes in patients: more days on IMV [(duration of IMV AI<10 vs AI>10: 7 vs 25 days respectively; p=0.005) (IMV>7 days AI<10 vs AI>10: 49% vs 87% respectively; p=0.01)].9 Similarly, the presence of, at least, 1 cluster event of IIE is an independent factor for unfavorable clinical outcomes: the occurrence of the event on day 1 was associated with a greater risk of remaining on mechanical ventilation for more than 8 days (OR, 6.4; 95%CI, 1.07–38.28; p=0.042) and with in-hospital mortality too (OR 20; 95 %CI, 2.28–175.23; p=0.007).17
In the largest study published to this date—which analyzed nearly 9 million breaths—the occurrence of IIE was associated with patients with airflow and pressure support mode obstruction (pressure control continuous spontaneous ventilation [PC-CSV]): IIE was significantly higher than during pressure control (pressure control continuous mandatory ventilation [PC-CMV]) and volume control (pressure control continuous spontaneous ventilation [VC-CMV]) modes, and was directly proportional to the level of pressure support used: higher assist pressure was associated with more chances of developing an AI and a severe IIE index, with worse clinical outcomes: a trend towards more days on IMV and higher mortality rates at the ICU and hospital settings [(ICU mortality AI<10 vs AI>10 23% vs 67% respectively; p=0.044) (hospital mortality AI<10 vs AI>14% vs 67% respectively; p=0.011)].7
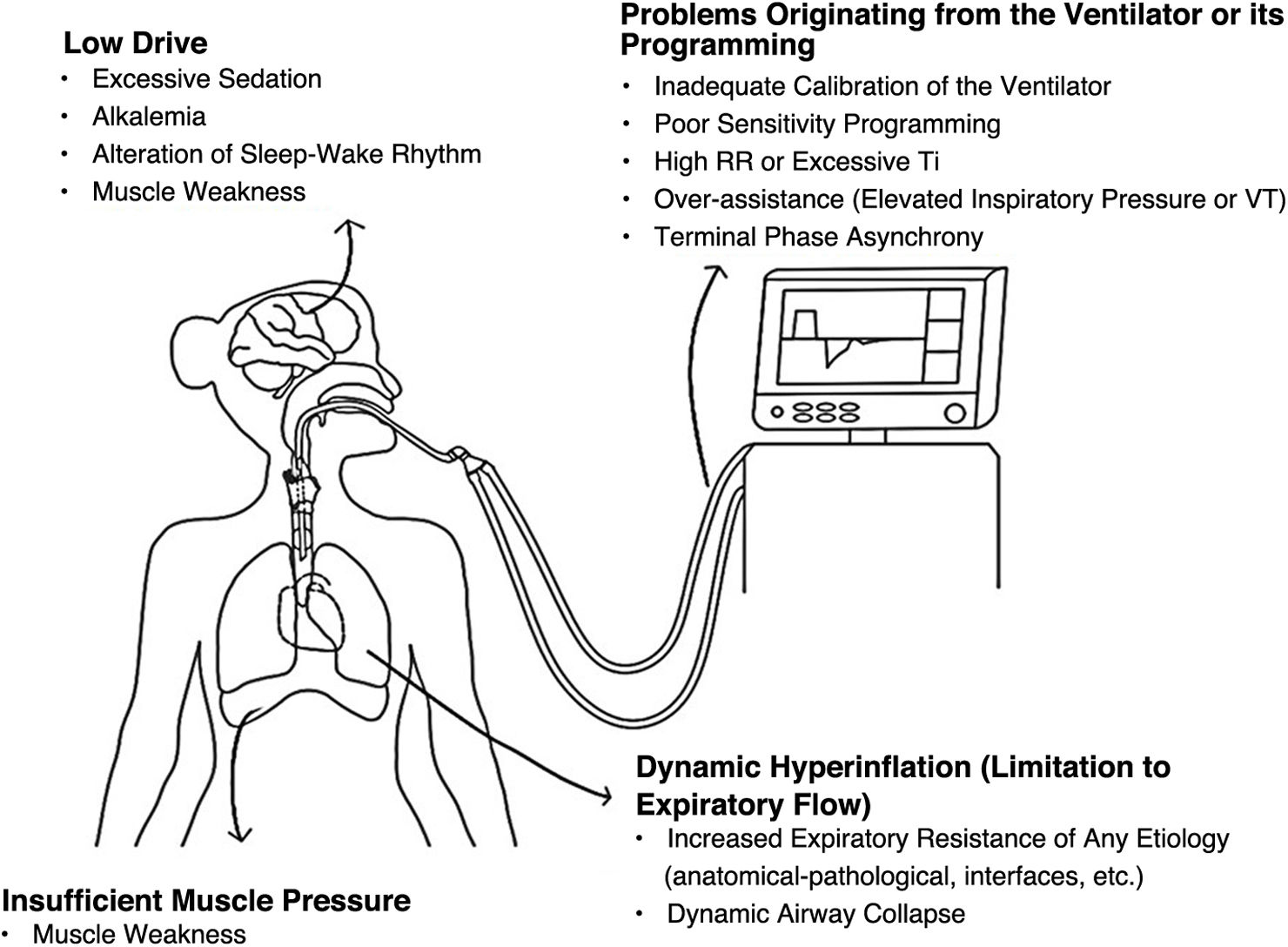
Pathophysiology of IIEsThe presence of IIE can be due to several causes (Fig. 2), some determined by predisposing factors—specific to patients—and others by triggering factors (determined by ventilatory programming and the patient's mechanical ventilation course): dynamic hyperinflation (DH) due to expiratory flow obstruction, dynamic airway collapse (primarily observed in obstructive patients), and weakness-related insufficient muscular pressure are predisposing factors. On the other hand, low drive (due to excessive analgesia/sedation) and the programming of ventilatory parameters that predispose to gaining large tidal volumes and insufficient expiratory time, among others, are triggering factors.
- •
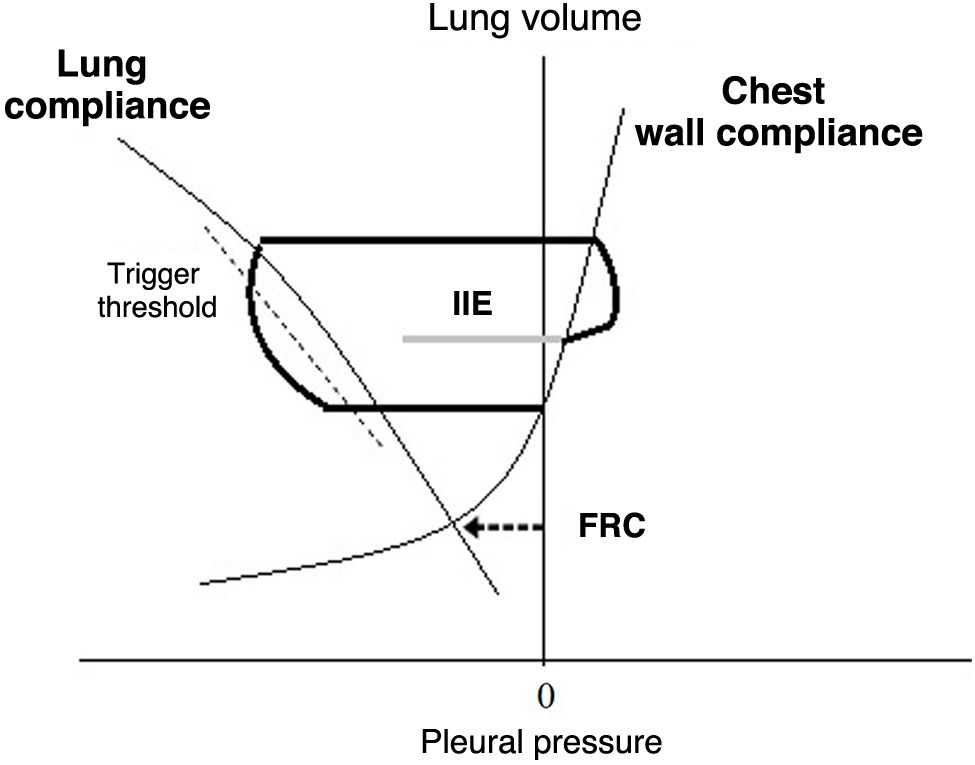
DH - expiratory flow limitation: in patients with prolonged expiratory time constants (who require extended expiratory times for pulmonary "emptying") and dynamic airway collapse, the system does not reach equilibrium at the end of expiration. IIE occurs if the patient begins to inspire at lung volumes above the end-expiratory lung volume (EELV; the lung volume at the end of expiration, which occurs when pressure at alveolar level is greater than that in the airway), and the effort is insufficient to overcome the elastic load imposed by trapping, equalize the pressures (at alveolar level and in the airway), and then reach the activation threshold. This can be visualized with a Campbell diagram, which allows for a graphical analysis of respiratory work (cycle by cycle) based on the relationship between pleural pressure (estimated via esophageal manometry), lung elastance, and thoracic elastance19 (Fig. 3).
- •
Low drive and insufficient muscular pressure: the decrease in muscular pressure may be associated with excessive sedation, altered sleep-wake rhythm, and/or muscular weakness.20,21 In this context, the patient’s inspiratory effort is less than that required to generate flow acceleration and/or decrease the airway pressure to pre-established levels determined by the "trigger" variable—programmed in the ventilator—to initiate the next inspiration.
- •
Ventilatory parameters: ventilatory programming can exacerbate the condition of air trapping and can even, by itself, generate auto-PEEP even in the absence of airway obstruction: high RRs, asynchronies during the transition between the inspiratory and expiratory phases (which predispose to greater tidal volumes at the end of inspiration, such as "double triggering"), and situations of over-assistance due to excessive inspiratory pressures/tidal volumes (in PC-CMV and VC-CMV, respectively) contribute directly to the development of IIE due to air trapping.18,22,23 The mechanism described for this situation is the same as in patients with auto-PEEP due to expiratory flow limitation and dynamic airway collapse.
Determining the amount of "ideal" work that respiratory muscles should perform during acute respiratory failure represents a challenging situation to resolve.24 In this context, ventilator-induced diaphragmatic dysfunction is likely the result of inappropriate ventilatory support. The term diaphragmatic myotrauma, which includes disuse atrophy, increased load due to insufficient assistance, excessive shortening, and eccentric contractions, occurs in 50 % of the patients on invasive mechanical ventilation (IMV).25–27 Most of these mechanisms have been reviewed in recent years and are associated with unfavorable outcomes.28 However, little is known about eccentric contractions (ECC) and their effect on diaphragm function.29 An ECC is, by definition, that in which a given muscle experiences activation and generates force while elongating its muscle fibers.30 One of the most common situations at the ICU setting where the diaphragm contracts during elongation is during IIE and "reverse trigger" asynchrony.31 During the latter, the patient's inspiratory efforts begin after passive insufflation (ventilator-initiated breathing, by time) and are primarily due to a reflex mechanism.32 Since this is fundamentally a reflex mechanism, it might not be strictly categorized as IIE per se. If so (depending on the size of the effort developed), it can be associated with beneficial adaptive changes that contribute to limiting muscular damage.33 Despite this, it is generally assumed that skeletal muscle injury is more pronounced after ECC, and that strength rapidly diminishes due to structural injuries and metabolic changes.34–37 However, conversely, in the context of a reflex mechanism, ECC may be associated (depending on the size of the inspiratory effort developed) with beneficial adaptive changes at muscle fiber level, which can limit and prevent the development of disuse atrophy.37 Evidence is scarce, and results are contradictory. Gea et al. evaluated the functional effects of repeated series of ECC on the diaphragm in a canine experimental model.38 They observed significant decreases in muscle contraction and the pressure generated by these. The authors argued that both metabolic and structural injuries occurred due to mechanical (rupture of sarcomeres and disruption of sarcolemmas) and biological factors: oxidative stress, local inflammatory response, glycogen depletion, and cytokine-mediated muscle damage.39,40 However, they added that ECC are not necessarily negative for the muscle and that they can promote, in certain situations, adaptive changes that generate larger fibers with more sarcomeres, providing benefits in terms of strength and resistance to future injuries.14,41
IIE and clinical outcomesAlthough clinical outcomes appear to be influenced by multiple factors and the way asynchronies are quantified—AI or presence of "clusters" seems decisive—patients with a higher number of IIE progress less favorably9,17,18 (Table 1). It remains to be elucidated whether this is merely a marker of disease severity or if IIE per se are associated with the development of such outcomes.13,29 There are physiological arguments that could support this causal relationship.20,44,45 However, the heterogeneity of the available evidence and some contradictory results do not allow for a conclusive answer to that question.
Prevalence of ineffective inspiratory efforts according to different authors.
| First author | No. of patients | Population and time of analysis | AI>10% (%) | Types of asynchronies included (%) | Risk factors | Results |
|---|---|---|---|---|---|---|
| Fabry14 | 11 | ICU – General Population; From the start of PC-CSV | Calculated AIa | IIE | Not specified | Not specified |
| N=9 (81%) | ||||||
| Chao42 | 171 | Weaning center – general population; weaning period | Not grouped by AI>10% | IIE | COPD – dynamic hyperinflation Muscle weakness | Only 13% were weaned off ventilation (vs 57% from the non-IIE group) and required more days on ventilation: 83 vs 33 days |
| Thille9 | 72 | ICU – general population; 30min after initiating all breaths | N=15 (24%) | All (85% IIE) | Alkalosis pH, trigger, excessive VT (VC-CMV) and pressure (PC-CSV) | Increased days on IMV, more patients on IMV>7 days, more tracheostomies |
| De Wit16 | 60 | ICU – general population; 10min within the first 24h of IMV | N=16 (26.6%) | IIE | Pressure trigger, higher intrinsic respiratory rate | More days on IMV, longer ICU and hospital stays, lower likelihood of discharge |
| Robinson43 | 35 | ICU – trauma (16% COPD); 30min within first 48h of invasive ventilation | N=9 (25.7%) | All (44% IIE) | Reduced respiratory drive, RR>10/min on VC-IMVb | No differences |
| Blanch7 | 50 | ICU – general population; 82.6% of total time for all patients on IMV | N=6 (12%) | All (IIE most common) | Spontaneous modes; increased patient control | Trend toward longer IMV course, increased ICU/hospital mortality |
| Vaporidi17 | 110 | ICU – general population; first 24h after starting spontaneous ventilation | N=13 (12%) | IIE | Sedation, sleep-wake cycle, and level of assistance | No differences |
| Clustersc | IIE | Sedation, sleep-wake cycle, and level of assistance | Increased risk of IMV>8 days | |||
| N=42 (38%); 30% of total AI<10% | Increased in-hospital mortality |
In the study by Thille et al.,9 as well as in others,17,18 IIE accounted for 85% of all asynchronies. Here, patients with high AI and ineffective efforts spent more days on mechanical ventilation and were less likely to be discharged. IIE were also the most common asynchrony in chronic critically ill patients outside the ICU setting: only 3 out of 19 patients who had a high index of IIE were able to wean off IMV42 (Table 1).
Based on the analysis of nearly 9 million breaths, Blanch et al.7 observed that those with an AI>10% had higher mortality rates at both the ICU and hospital settings, along with a trend towards longer IMV courses.
It seems that the way in which IIE are quantified influences the results. Based on the severity index of asynchronies proposed by Fabry and the one used by De Wit for IIE, most studies that dichotomized their population (AI-IIE>10% vs < 10%) revealed less favorable outcomes in those with a higher percentage of breaths with asynchronies.17,18 However, a few years ago, Vaporidi and Georgopoulos15 proposed and incorporated their concept of "clusters" (at least, one 3-min period on VM with 30 IIE or more) of IIE. The authors argued that the indices using a 10% cutoff do not derive from a representative sample. Moreover, they suggested that IIE tend to occur in "clusters," as the risk factors for the development of IIE (sedation, wakefulness states, level of assistance, and ventilatory drive) can vary during the IMV course.14 Thus, they argued that being nonlinear biological phenomena, assessments should be sporadic and dispersed. They designed a mathematical model to detect them and used the concept of "cluster" or event. They analyzed a total of 110 patients within the first 24h of assisted breathing for a total of 2931h and nearly 4.5 million breaths. After multivariate analysis, they found that the presence of the event on observation day 1 was associated with a significantly higher risk of IMV>8 days and in-hospital mortality and that, with greater power and duration of the event—at least 3min—more days on IMV starting from the index record. Conversely, when they divided the population according to the IIE index (>10% or <10%) and analyzed the results of patients with a severe index, they found no significant association with any outcome.
IIE and treatmentIIE can be due to multiple causes and result from a combination of predisposing and triggering factors, determined by ventilatory programming and pharmacological treatment.46 Controlling risk factors and implementing interventions aimed at reducing DH, avoiding over-assistance and excessive use of sedatives, in pursuit of appropriate patient-ventilator interaction, emerge as the suitable interventions that should be conducted.21,47 Moreover, reducing the ventilator sensitivity to triggering and programming external PEEP in specific situations could contribute to improving outcomes. Reducing support pressure has proven to be a very effective method for preventing IIE in Chao's study, and similarly, Nava et al. observed that lower levels of support pressure significantly decreased IIE without increasing the metabolic cost of breathing.22,48
The use of sedatives and neuromuscular blockers increases problems during the total and partial support phases.22 In this situation, proportional assist modes appear to offer significant benefits. Due to their physiological principles of operation (gas delivery that follows the patient’s respiratory pattern, in both amplitude and synchronization), both proportional assist ventilation (PAV+) and neurally adjusted ventilatory assist (NAVA) promote patient-ventilator synchronization (between neural and mechanical inspiratory times) and minimize the risk of over-assistance, as they reduce volume gain in each respiratory cycle.49,50 PAV+ (using flow and volume measurements and calculating compliance and resistance) determines the work pressure of each respiratory cycle and provides a specific percentage of assistance based on the "gain" programmed by the operator on the ventilator. Cycles are initiated by the patient's effort (which generates flow variations detected by the ventilator) and end with the cessation of such effort. Both pressure and flow vary with the patient’s effort, presenting 2 important conceptual advantages: flow and cycling synchronization and limitation of tidal volume (VT), as it depends solely on the size of the patient’s effort. NAVA requires placing a nasogastric tube with electrodes to detect diaphragm activity (EAdi, which stands for electrical activity of the diaphragm). The sensors send information on the onset, intensity, and cessation of efforts. In a similar way to PAV+ (and based on programming a specific gain in cmH2O/mV), NAVA delivers flow and pressure which are proportional to the electromyographic signal of the diaphragm. For this reason, it presents the same conceptual benefits as PAV+: synchronization in all phases of the respiratory cycle and improved variability in VT, driven by the patient’s effort.
In a crossover study, compared with PC-CSV no patient on NAVA—36% overall—had IIE>10%. Respiratory and clinical parameters were recorded, and multiple levels of assistance were used and randomly applied in both modes. At higher assistance levels, patients on PC-CSV had statistically significantly higher VT and lower respiratory efforts, measured as peak EAdi.51 In a different crossover study, compared with PC-CSV, NAVA significantly reduced the AI—median of 11.5% vs 24.3%—of patients undergoing weaning during a spontaneous breathing trial (SBT), without showing any differences in ventilatory parameters: VT and RR.52 In patients on IMV, without sedatives, and predominantly with COPD (a crossover study in which 2 levels of assistance were used in both PC-CSV and NAVA), the use of NAVA significantly decreased the incidence of IIE and improved patient-ventilator synchronization. At the highest level of assistance, compared with PC-CSV, NAVA statistically significantly eliminated IIE and reduced asynchrony at the onset of inspiration and during transition from inspiratory to expiratory phases.53 Similar results were found in studies that used PAV+. In a randomized study of 208 critically ill patients who had been on controlled IMV for, at least, 36h, proportional assisted ventilation significantly decreased the number of IIE vs PC-CSV,54 demonstrating a significant reduction in the percentage of AI (5.6% of the total in PAV+and 29% in PC-CSV). Patients were followed for 48h, except for those who met criteria to revert to controlled modes or were able to breathe without assistance. Although the proportion of patients who met the criteria for spontaneous breathing did not change across groups, the rate of failure—need to revert to controlled modes—was significantly lower in PAV+ vs PC-CSV (11.1% vs 22%, respectively).55 However, despite this, there is still no evidence associating these benefits—in terms of physiological effects—with better clinical outcomes.
As we have described, with conventional activation and cycling systems, multiple patients experience asynchronies, especially IIE. Software developed to address this problem (such as IE Sync™ by Puritan Bennett, IntelliCycle by Mindray, or IntelliSync) has proven effective in reducing the development of asynchronies compared to fixed criteria, without changes in respiratory patterns.56 This software has been designed to make sure that each patient’s breath is effective, without adding invasiveness to the method, as it do not require any catheters being inserted or additional sensors either. Instead, it uses the pressure and flow measurements that the ventilator is already taking to estimate changes in intrapleural pressure to recognize the start and end of a patient’s inspiratory effort. This non-invasive activation and cycling software can be particularly valuable to provide adequate synchronization, especially in patients with weak inspiratory efforts.57–59 In this situation, because the automatic adjustment system analyzes flow and pressure in the airway thousands of times per second—through real-time analysis of waveforms and slopes—the patient’s inspiratory effort does not need to mobilize flow to a predetermined value (for example, 1L/min). Here, the algorithm will activate the inspiratory phase of the ventilator as soon as it detects a sudden flow increase (or the derivative in the flow wave slope) reflecting the inspiratory effort. This technology can thereby reduce the delay in inspiratory triggering and the incidence of IIE.
The clinical diagnosis of asynchronies is cumbersome, prone to underdiagnosis, and even influenced by the evaluator’s level of experience.60 Various studies have shown that implementing specific training on the topic with interventions for the staff treating the patients improves outcomes.61 Furthermore, the difficulties in recognizing and treating asynchronies make this field highly suitable for intervention by artificial intelligence through algorithms capable of identifying changes to the ventilator curves in real time (primarily pressure and flow) and activating interventions that can have an impact on such changes. The study by Candelaria de Haro demonstrates that artificial intelligence—particularly recurrent neural networks—could be an excellent tool for identifying changes to airway pressure during VC-CMV with constant flow, allowing for the minimization of unrecognized periods of inadequate interaction between the patient and the ventilator.62
In light of the available evidence, IIE are associated with clinical outcomes that are often contradictory.8,11,14 From this, the question remains whether it is better to treat them (to eliminate them) or allow their presence to generate training for the diaphragm. Stratifying them (based on the presence or absence of "clusters"), identifying the phenotype, and fundamentally, measuring the effort during contractions is the best strategy. If this is not feasible in the routine clinical practice, identifying patients with risk factors for DH, avoiding excessive use of sedatives/analgesics/neuromuscular blockers, and promoting patient-ventilator synchronization using proportional assist modes during the weaning phase could be a viable option.
ConclusionsIIEs are one of the most frequent types of patient-ventilator asynchronies during mechanical ventilation. Beyond susceptible patients (with DH, due to expiratory flow obstruction in the context of dynamic airway compression), ventilatory over-assistance, muscle weakness, excessive sedation levels, and inadequate programming of inspiratory sensitivity could be the main mechanisms for their occurrence. Currently, there is evidence associating the high incidence of IIE with worse clinical outcomes. However, it is still to be elucidated whether this association is causal or merely a marker of disease severity. For now, implementing detection and treatment strategies that can limit them seems reasonable. Future research is still necessary to understand the potential role of eccentric muscle activation during this type of interaction.
Declaration of Generative AI and AI-assisted technologies in the writing processAdditionally, we declare that we have not used any type of artificial intelligence (AI) for the drafting of this manuscript or the images.
FundingNone declared.