Extracorporeal membrane oxygenation (ECMO) is a sophisticated therapy that has been associated to reduced mortality in patients with the most severe forms of Acute Respiratory Distress Syndrome (ARDS).1,2 It is recommended to be performed at high volume centers, in order to have the best possible results.3 With the aim of equally distributing healthcare resources, specialized “ECMO-Centers” should offer this therapy to patients admitted to other hospitals where ECMO cannot be done safely.
Early reports from China showed that ECMO use during the COVID-19 outbreak was anecdotal,4 nevertheless, up to now, more than 1900 COVID-19 patients have been treated with ECMO for COVID-19 around the world.5 As previously pointed out,6 ECMO is a finite resource and during the outbreak, there are limitations to providing ECMO, such as lack of material or limited staff available, which may restrict the access of patients to ECMO.
The aim of the study is to evaluate the feasibility, efficacy and safety of interhospital transport of patients on ECMO during the COVID-19 outbreak. It is a prospective observational study that compares transport related variables of all patients retrieved on ECMO between March 15th and April 30th 2020, with an historical cohort of patients transported on ECMO during the previous 6 months. Controls were randomly selected from the institution's ECMO patient registry, matching the number of patients included during the study period. Since ECMO patients are highly selected (age, days of mechanical ventilation, comorbidities, etc.), no matching by other variables was done. The study is performed in a tertiary ECMO center in Spain that receives referrals from 9 different hospitals in a region of 2.2 million inhabitants.
During the outbreak, contact with the ECMO center was performed using a centralized telephone number and online registration form, as usually done. Specific information about ECMO in COVID-19 patients was distributed among the network hospitals.
Indications and counter-indications for ECMO in these patients were obtained from the ELSO recommendations,7 individualizing them for every patient. A specific protocol for staff and equipment transportation was elaborated, which included two transport vehicles, and level 3 personal protective equipment (long-sleeved fluid-resistant surgical gown, fluid-resistant hood, FFP 3 mask, full face visor, safety glasses, 2 sets of long cuff gloves, closed shoes and boot covers) for every team member, in order to ensure patients and staff's safety. Data were obtained from the center's ECMO patient's registry. The study was approved by the institutional review board, and due to the observational nature of the study, informed consent was waived.
The primary outcome is successful transport with no major complications: vehicle failure, pump failure, oxygenator failure, console failure, air in circuit, ventilator failure, surgical site bleeding, hemodynamic instability and decrease in tidal volume. Secondary outcomes are: patients discarded due to lack of ECMO capacity, days on ECMO, survival at 28 days and survival to hospital discharge. Statistical analysis was performed using STATA v16.1 (Statacorp, Texas, USA). Comparisons were made using T-test, Wilcoxon Rank test and Fisher exact test as appropriate.
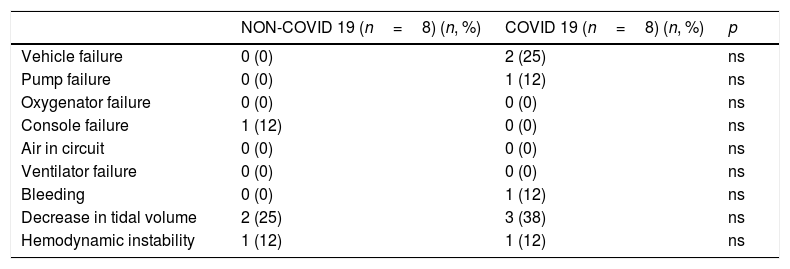
During the study period, 3614 confirmed COVID-19 patients were admitted to the region's hospitals. 289 of them required ICU admission. Out of them, 21 patients were evaluated for ECMO and 8 (38%) had final indication, all of which were successfully retrieved on ECMO. All transports were made by ground. Patients’ characteristics are summarized in Table 1. In the ECMO center, the number of ICU beds was extended from 27 to 61. During the same period, another 5 in-house ECMO therapies were performed. None of the considered patients were discarded for capacity reasons. No significant differences in major complications during transport with non-COVID-19 patients could be identified (Table 2). During two retrievals, one of the team members had intolerance to the personal protection equipment and needed to be substituted by another team member. None of the members of the team had symptoms or were tested positive in CRP for SARS-CoV-2 during the study period.
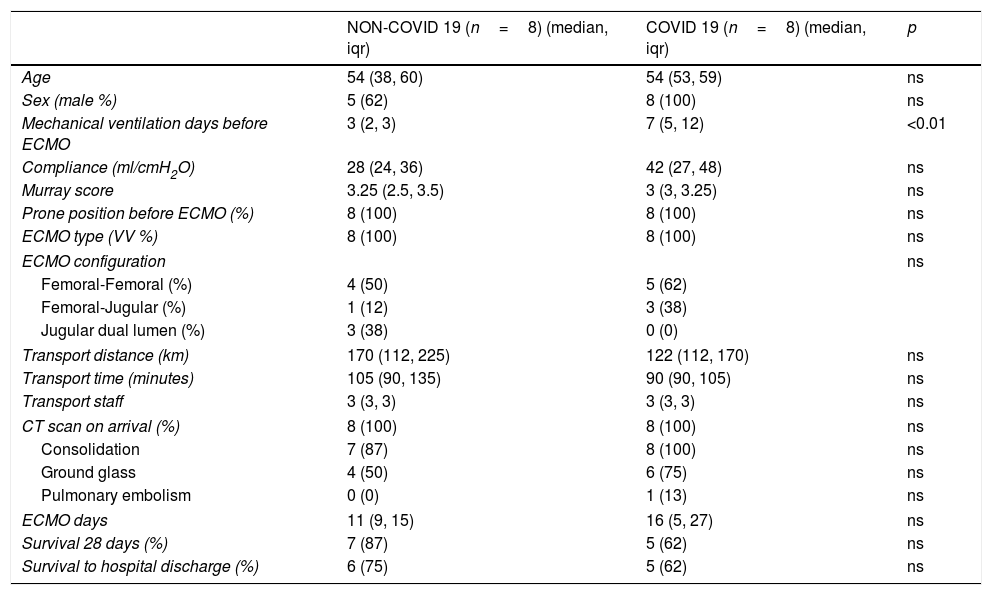
Characteristics of patients transported on ECMO.
| NON-COVID 19 (n=8) (median, iqr) | COVID 19 (n=8) (median, iqr) | p | |
|---|---|---|---|
| Age | 54 (38, 60) | 54 (53, 59) | ns |
| Sex (male %) | 5 (62) | 8 (100) | ns |
| Mechanical ventilation days before ECMO | 3 (2, 3) | 7 (5, 12) | <0.01 |
| Compliance (ml/cmH2O) | 28 (24, 36) | 42 (27, 48) | ns |
| Murray score | 3.25 (2.5, 3.5) | 3 (3, 3.25) | ns |
| Prone position before ECMO (%) | 8 (100) | 8 (100) | ns |
| ECMO type (VV %) | 8 (100) | 8 (100) | ns |
| ECMO configuration | ns | ||
| Femoral-Femoral (%) | 4 (50) | 5 (62) | |
| Femoral-Jugular (%) | 1 (12) | 3 (38) | |
| Jugular dual lumen (%) | 3 (38) | 0 (0) | |
| Transport distance (km) | 170 (112, 225) | 122 (112, 170) | ns |
| Transport time (minutes) | 105 (90, 135) | 90 (90, 105) | ns |
| Transport staff | 3 (3, 3) | 3 (3, 3) | ns |
| CT scan on arrival (%) | 8 (100) | 8 (100) | ns |
| Consolidation | 7 (87) | 8 (100) | ns |
| Ground glass | 4 (50) | 6 (75) | ns |
| Pulmonary embolism | 0 (0) | 1 (13) | ns |
| ECMO days | 11 (9, 15) | 16 (5, 27) | ns |
| Survival 28 days (%) | 7 (87) | 5 (62) | ns |
| Survival to hospital discharge (%) | 6 (75) | 5 (62) | ns |
ns (non-significant)=p>0.05. iqr=interquartile range.
Transport related complications of patients transported on ECMO.
| NON-COVID 19 (n=8) (n, %) | COVID 19 (n=8) (n, %) | p | |
|---|---|---|---|
| Vehicle failure | 0 (0) | 2 (25) | ns |
| Pump failure | 0 (0) | 1 (12) | ns |
| Oxygenator failure | 0 (0) | 0 (0) | ns |
| Console failure | 1 (12) | 0 (0) | ns |
| Air in circuit | 0 (0) | 0 (0) | ns |
| Ventilator failure | 0 (0) | 0 (0) | ns |
| Bleeding | 0 (0) | 1 (12) | ns |
| Decrease in tidal volume | 2 (25) | 3 (38) | ns |
| Hemodynamic instability | 1 (12) | 1 (12) | ns |
ns (non-significant)=p>0.05. Variables are defined in table S1 of the supplemental material.
The main finding of our study is that retrieval of COVID-19 patients on ECMO during the outbreak is feasible and safe in the setting of a previously well-established mobile ECMO program. To our knowledge this is the first study that directly compares interhospital transport of COVID-19 ECMO patients to a historical cohort. ECMO is a life-saving technique that might be useful for treatment of refractory respiratory failure in COVID-19 patients. Nevertheless, up to now, there are insufficient data about their outcomes. Our case series shows a similar survival rate between groups; however, the low number of patients makes data interpretation difficult. Upcoming reports from ELSO, EuroELSO and Critical Care Consortium will shed some light on this issue.
To be pointed out is that COVID-19 patients had more days of mechanical ventilation prior to the start of ECMO. We hypothesize this could be due to a slower evolution of the disease and/or an increase in super-infections due to immunomodulatory therapy used, when compared to other causes of ARDS, which may lead to refractory hypoxemia and/or hypercapnia in a posterior stage. However, late worsening of the respiratory function associated to ventilation induced lung injury is to be ruled out in COVID-19 patients aswell.
Despite the outbreak, all patients considered for ECMO could be retrieved, maintaining the standard of care established by the institution, following the recommendations for safe transport of COVID-19 patients.8 As previously pointed out, in patients under mechanical ventilation,9 the possibility of having the space, staff and stuff is a key factor for having good results in situations where the needs are close to exceed the attention capacity.
In conclusion, transport of COVID-19 patients on ECMO is feasible and safe in a regional ECMO center. More data are necessary to determine the efficacy of ECMO in COVID-19 patients.
Conflict of interestNone.