Patients with acute lung injury or acute respiratory distress syndrome (ARDS) admitted to the ICU present neuropsychological alterations, which in most cases extend beyond the acute phase and have an important adverse effect upon quality of life. The aim of this review is to deepen in the analysis of the complex interaction between lung and brain in critically ill patients subjected to mechanical ventilation. This update first describes the neuropsychological alterations occurring both during the acute phase of ICU stay and at discharge, followed by an analysis of lung–brain interactions during mechanical ventilation, and finally explores the etiology and mechanisms leading to the neurological disorders observed in these patients. The management of critical patients requires an integral approach focused on minimizing the deleterious effects over the short, middle or long term.
Los pacientes con lesión pulmonar aguda o síndrome de distrés respiratorio agudo (SDRA) ingresados en unidades de cuidados intensivos (UCI) presentan alteraciones neuropsicológicas que en la mayoría de los casos se extienden más allá de la fase aguda, acarreando importantes déficits en su calidad de vida. El objetivo de la presente puesta al día es profundizar en el análisis de la compleja interacción entre el pulmón y el cerebro en el enfermo crítico sometido a ventilación mecánica. Inicialmente se describen las alteraciones neuropsicológicas asociadas tanto durante la fase aguda de estancia en la UCI como al alta hospitalaria. En un segundo apartado se analiza la interacción pulmón-cerebro durante la ventilación mecánica y, finalmente, se explora la etiología y los mecanismos que dan lugar a las alteraciones neurológicas que se observan en estos pacientes. El manejo del paciente crítico requiere de una visión integradora enfocada a minimizar los efectos deletéreos a corto, medio o largo plazo.
The important advances that have occurred in the management of critically ill patients in the last decade have not only improved the survival rates, but have also underscored the need to reduce morbidity among such individuals. Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are associated to high morbidity rates.1,2 Among other problems, those patients who survive these conditions suffer neuropsychological alterations that extend beyond the acute phase and hospital stay, and have a strong negative impact upon quality of life that can persist over time. These neurocognitive sequelae represent not only a sociosanitary problem but also an economical problem, in view of the important resources that must be assigned to compensate the situations of dependency among the survivors. In this context, an analysis of the complex interaction among organs in the critical patient is essential in order to optimize the clinical management of these subjects and minimize the related complications.
The brain alterations that develop among critical patients are a consequence of both multiple organ dysfunctions derived from the underlying disease process and its management during admission to the intensive care unit (ICU).3 The present update focuses on the lung–brain axis with the purpose of determining which factors implicated in ALI and in its ventilatory management can give rise to the appearance of neuropsychological alterations. A review is made of the most recent literature referred to lung–brain communication in the non-neurological critical patient. A clinical description is first provided of the psychological and neuropsychological alterations observed in the critical patient, and which occur both during the acute phase of admission to the ICU and at hospital discharge. This in turn is followed by an analysis of the factors implicated in lung–brain interaction, offering an in-depth evaluation of both the etiology and mechanisms related to the disease and derived from its clinical management–placing special emphasis on the role of mechanical ventilation (MV). Lastly, we explore the possible signaling pathways and the molecular and histopathological bases of the described neurological alterations.
Neuropsychological alterations in the critical patientThe clinical and surgical management of patients admitted to the ICU is often associated to the presence of neurocognitive defects,1 even in patients without previous cognitive alterations. Moreover, it recently has been shown that although MV is an important life support measure for the critical patient, it is frequently related to the development of neuropsychological defects.1
Two types of neurocognitive alterations can be observed in critical patients subjected to MV, associated to both the acute phase and the chronic phase. Confusional syndrome is the neuropsychiatric condition most commonly observed during the acute phase of critical patient care.4,5 It is characterized by altered alertness, with a decrease in the capacity to focalize, maintain and/or shift attention, and changes in other cognitive functions and/or the development of perceptive alterations. Confusional syndrome usually manifests during a short period of time and exhibits a fluctuating course during the day. Among patients admitted to the ICU, confusional syndrome is one of the most frequent causes underlying the presence of cognitive impairment over the long term.6–8 A recent study in patients subjected to MV has shown that the duration of confusional syndrome in itself is an independent predictor of the cognitive defect to be expected 3 and 12 months after hospital discharge.9 The prevalence rate of confusional syndrome is about 50–70% in patients admitted to the ICU who are not subjected to MV, and increases to 60–80% in the case of patients subjected to MV.10–13
Interest in the study of the cognitive effects observed over the long term and associated to ICU stay is relatively recent. According to the latest review published by Hopkins and Jackson on this subject,14 there are currently only 10 cohorts with a total of 450 patients in which these neuropsychological deficits have been explored in critical patients following their stay in the ICU.15 The studies focused on patients with ARDS15,16 or ALI,17 and patients with respiratory failure,18 while only one study evaluated clinical patients in the ICU and two studies investigated all critical patients in the ICU.19,20 The data suggest that at least one-third of the patients in the ICU develop chronic neurocognitive deficits of a magnitude similar to those observed in mild and moderate dementia cases.21 This prevalence in turn reaches 80% in patients who develop ARDS during hospital admission.15
The neuropsychological alterations associated to patient stay in the ICU have been described in subjects with ARDS both at hospital discharge and two months after discharge,19 after 6 months and one year,16 two years,18 and even up to 6 years after the stay in the ICU. Such cognitive sequelae tend to improve during the first 6–12 months after hospital discharge,22 but then tend to remain stable and become chronic after the first year. It has been observed that these alterations affect 78% of the patients at the time of hospital discharge, 47% after two years, and 25% after 6 years following the stay in the ICU.1
The neuropsychological deficits that can be seen among survivors of the ICU are related to a broad range of cognitive domains. Hopkins and Jackson1 reported that in general, in the critical population in the ICU, memory defects are the most frequent problem, followed by defects in executive functions, information processing speed, and alterations in attentional capacity. The comparison of cognitive sequelae among different groups of patients is difficult, due to the methodological differences of the studies, discrepancies in the definition of cognitive sequelae, the variability of the administered neuropsychological tests, the duration of follow-up, the type of patient in the ICU, and the severity of the critical illness. Nevertheless, some authors have suggested a neuropsychological profile common to those patients who have been admitted to these units. Larson et al.22 reported poorer performance referred to general intellectual function, executive functions, information processing speed and verbal memory in patients with ARDS. These results in turn are consistent with those of previous studies in patients with ARDS and in survivors of clinical ICUs.8,21
In addition to cognitive alterations, mechanically ventilated patients can also manifest psychopathological symptoms such as anxiety and depression.23,24 The studies on this subject estimate a prevalence of depression and anxiety of 10–58% in patients of this kind. Hopkins et al.25 found that approximately 23% of the patients with ARDS had symptoms of anxiety one and two years after their stay in hospital, and that 16% of these same patients suffered depression one year after discharge – this percentage increasing to 23% after two years. The presence of depression and cognitive sequelae one year after hospital stay was found to be the best predictor of depression over the long term. The mentioned study suggested a possible relationship between neurocognition and affective disorders among ICU survivors. However, Larson et al. observed no influence on the part of emotional alterations upon the neurocognitive results obtained in ICU patients.22
In contrast to the above, the consequences of such chronic neurocognitive and psychopathological deficits have been widely studied. It is now clear that all these deficits have a strong impact upon the life of the patients and contribute to lessen their capacity to conduct activities of daily living, worsening the quality of life of both the patients and their families, generating an increase in medical costs associated to treatment, and complicating the return to work and the way of life before admission to the ICU.1,18,26 In a follow-up study of survivors of critical illnesses, close to 50% of the patients had been unable to return to work mainly because of the effects of cognitive defects 6 years after their stay in the ICU.17
Factors implicated in lung–brain interaction during mechanical ventilationAcute respiratory failure requiring MV is produced by a great variety of factors, including diseases inherent to the lungs, shock, the need for airway protection, or transient MV application after major surgery.27 The decrease in pleural pressure during spontaneous breathing is replaced by the application of a positive pressure generated by the ventilator in order to ensure tidal volume (TV) that is both effective for oxygenation and the elimination of carbon dioxide and comfortable for the patient. Although MV is an important life support tool for many critical patients, it is not without complications, and is intrinsically able to worsen or even produce new lung injuries, with possible spread to other organs (including the brain), and ultimately leading to multiorgan failure.
Years of research have led to a progressive decrease in mortality among these patients. At present, use is made of ventilation protocols that protect the lungs from damage associated to the excessive overdistension produced by high TV and alveolar pressure values, and at the same time minimize alveolar recruitment and derecruitment during each respiratory cycle.28,29 On the other hand, weaning from MV must involve efficient patient–ventilator interaction, since otherwise the patient suffers frequent disadaptation episodes and dyssynchrony, a prolongation of MV time, episodes of fear, anxiety, panic and confusional syndrome, a need to increase sedation and opioid use and, lastly, an increase in both mortality30,31 and neuropsychological sequelae.23,24 A recent study has shown the presence of dyspnea to be frequent, intense and associated to anxiety in critical patients subjected to MV.32 The intrinsic physiological and clinical relationship among anxiety, dyspnea and pain is a common denominator in patients admitted to the ICU, where physicians and nurses attempt to shorten stay and the use of MV, even though this implies a risk of exposing the patients to dyspneic stimuli for prolonged periods of time.
Good adaptation of the patient to MV implies adjustment of the ventilatory parameters in order to normalize gas exchange and afford patient comfort. This aspect is conditioned to precise analysis of the respiratory physiology, respiratory mechanics and metabolic requirements of the patient. Recent studies have shown that patients on MV often suffer asynchrony in their interaction with the ventilator (ventilation mode and parameters). Such asynchrony occurs throughout the respiratory cycle and is related to the inspiratory trigger, the duration of inspiration of the ventilator, the transition from inspiration to expiration, air trapping, and the presence of ineffective inspiratory effort throughout the respiratory cycle. A detailed analysis of these asynchronies, their causal mechanisms and treatment can be found in recent articles.33–37
The relationship between the different forms of patient asynchrony with the respirator, and its possible correlation to the development of dyspnea, neurocognitive alterations over both the short and middle term, and the presence of confusional syndrome during admission to the ICU, is not well known. Recent studies have shown that patient–ventilator asynchronies can manifest at the start of MV, related to the level of sedation and the start of weaning from MV.38 The presence of asynchronies has been associated to an increased duration of MV, a higher incidence of tracheostomy and a longer stay in the ICU, and has also been suggested to increase mortality.34,35 A recent study by our group, involving the recording of patient–ventilator asynchronies on a continuous basis throughout the duration of MV, showed asynchrony to occur at any time of the day or at any time during the stay of the patient.36 Consequently, and in the same way as dyspnea, the phenomenon may be greatly underestimated in Intensive Care Medicine.
The factors associated to the development of dyspnea and patient–ventilator asynchronies comprise high transpulmonary pressure ventilation, which can occur in both spontaneous and assisted respiration, air trapping secondary to limitation of expiratory airflow, and a decrease in lung volumes.32,39 Although these associations arise from basic physiology applied to patients with chronic respiratory diseases, they also can be extrapolated to patients subjected to MV with a high ventilatory stimulus at the start of severe respiratory failure, patients ventilated with excessive minute volumes, and patients who have to interact with a mechanical ventilator. It should be underscored that the dyspnea resulting from air hunger associated to hypercapnia is more intense than that resulting from elevated respiratory effort under conditions of normocapnia.40 Such air hunger activates the afferent pathways through chemo- or baroreceptors, with an increase in signal intensity in the magnetic resonance imaging scans of the brain areas related to the limbic system, and entails psychological and emotional disturbances and memory alterations.41
The neurophysiological model of response to the respiratory discomfort (dyspnea) that can manifest in the critically ill patient involves neural stimuli that reach the somatosensory cortex and are of different origins: (1) impulses from the brainstem and cortical motor centers; (2) afferent pathways from respiratory mechanoreceptors located in the respiratory muscles, lungs, airway and thorax; (3) a decrease in inhibitory reflexes toward the respiratory center from the vagus nerve and afferent pathways of the intercostal nerves secondary to protective ventilation limiting TV; and (4) activation of the limbic and paralimbic system, resulting in the development of a series of neuropsychological alterations and delirium in the critical patient subjected to MV.26,32,39,42
Etiology, signaling pathways and mechanisms implicated in lung–brain interaction during mechanical ventilationDifferent authors have described the development of lung complications in patients with acute brain damage, related to an increase in pulmonary susceptibility to the effects of MV43–45 and an increased risk of respiratory failure. These studies support the existence of a brain–lung communication axis46–48 that could be related to the release of catecholamines, neurokinins and neuropeptides.
Communication in the opposite sense, from peripheral organs and specifically from the lungs to the central nervous systems (CNS) has been less extensively investigated, despite the fact that the cognitive impairment seen in critical patients over the short or long term is an acute cerebral manifestation secondary to underlying physiopathological mechanisms that may originate at peripheral level.
The etiology underlying the neurocognitive deficits observed following the stay in the ICU is probably of a multifactorial nature.21 Different risk factors, such as hypoxemia,15 the use of sedatives and/or analgesics, hypotension,16 altered regulation of blood glucose levels, the duration of MV,49 the length of stay in the ICU, and the presence of confusional syndrome8,21 have all been associated to the neuropsychological deficits described in post-ICU patients. An increase in cytokines and inflammatory marker levels has also been related to an increased cognitive defect and, more specifically, to memory defects.50 For this reason, exploration of the signaling pathways involved in such communication constitutes one of the most innovating lines of research in this field.
There is a causal relationship between the changes in brain tissue oxygen consumption and cognitive impairment, and it should be taken into account that the ventilation strategy used can induce changes in regional blood flow and in brain oxygenation. In this context, Bickenbach et al.,51,52 in a porcine model of ALI, found the application of MV with low TV values to result in improved brain tissue oxygenation and metabolism, though they conducted no evaluation of the consequences at cognitive level. In relation to this issue, mention must be made of the relationship between hypoxia and hippocampal damage.53 The hippocampus is the brain structure most closely related to learning and memory capacity, and at the same time the hippocampal neurons are known to be particularly vulnerable to hypoxia.54,55 Not only hypoxia implies neuropathological alterations, there is also evidence that ARDS and ALI can induce structural alterations in the hippocampus, with the resulting secondary memory defects, independently of the degree of hypoxemia.56 Furthermore, other structural alterations of the brain have been described, including an enlarged ventricular size and generalized atrophy.57 All these structural alterations have been related to the long term neuropsychological deficits observed in post-ICU survivors.58
Many clinical and experimental studies have shown that MV intrinsically induces a biological response characterized by an infiltration of monocytes and macrophages, and the release of inflammatory mediators, which in turn can worsen lung damage and favor spread to other organs. Septic encephalopathy has been widely studied,59,60 and some authors have suggested that it may involve mechanisms similar in mechanistic and epidemiological terms to those found in the brain dysfunction observed in patients with ALI and ARDS (sepsis-like syndrome).61
Despite the above considerations, the intrinsic pathways through which inflammatory mediators can exert their harmful effects upon other organs, and specifically the lung–brain communication pathways, remain unknown. During MV, stimulation of the mechanoreceptors (baroreceptors/stretch receptors) or chemoreceptors located in the lungs generates information which reaches the CNS via different routes.62 The use of an inadequate ventilation strategy triggers the release of certain inflammatory mediators43 or metabolites into the bloodstream, and this in turn is detected by the CNS.62
The brain can respond by altering the permeability of the blood–brain barrier (BBB),46 or by modifying cerebral blood flow. These same stimuli can also produce neuronal alterations. In a first approach to this topic, Quilez et al.63 evaluated neuronal activation in a model of MV damage in rats. The authors analyzed the levels of the transcription factor of c-fos, a member of the family of early expression genes, and regarded as a fast neuronal activation marker, with a view to exploring early neuronal changes that may be associated with ALI induced by MV in different areas of the brain. The authors recorded no major differences in inflammatory response or in lung function between the groups ventilated with high versus low TV values. However, the animals ventilated with high tidal volumes showed a greater expression of c-fos in certain areas of the brain–suggesting a synergistic role on the part of high TV, and underscoring the potential importance of the ventilation strategy beyond its direct effect upon the lungs.63
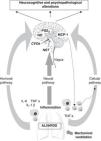
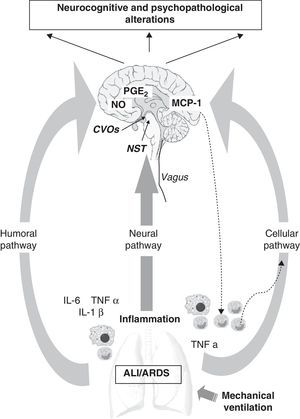
There are a number of communication pathways between the periphery and the CNS,64–66 and vice versa (Fig. 1). Firstly, mention should be made of the humoral pathway, through which inflammatory mediators, interleukin-6 (IL-6), tumor necrosis factor α (TNFα) and IL-1β, generated by the recruitment of peripheral monocytes or macrophages, may directly reach the brain. The BBB acts as a protective barrier, and is impermeable to a great number of molecules and to the cells of the immune system.66,67 Nevertheless, the mediators can reach the brain through the circumventricular organs or the choroid plexus,68–70 which are devoid of the mentioned barrier (Fig. 1). The lungs and brain share the same inflammatory mediators, and once these are released into the bloodstream they can also come into contact with the brain by interacting with specific CNS receptors. This first pathway also involves active transport mechanisms, as well as other mechanisms related to direct activation of the cerebral endothelium, and which lead to the release of prostaglandin E2 (PGE2) and nitric oxide (NO)(Fig. 1).
Communication pathways between the periphery (lungs) and the central nervous system (CNS) during mechanical ventilation. The CNS receives information from the periphery via three pathways: humoral, neural and cellular. (1) The recruitment of monocytes or macrophages in the lung increases the levels of inflammatory mediators (IL-6, TNFα, IL-1β), which are able to directly reach the CNS via the humoral pathway through the circumventricular organs (CVOs), without having to cross the blood–brain barrier (BBB). Other active transport mechanisms also intervene, leading to the release of PGE2 and nitric oxide (NO) at brain level. (2) The vagus nerve afferents reach the brain through the nucleus of the solitary tract (NST). (3) The cellular pathway is directly regulated by the release of TNFα in the lung, which in turn stimulates the release of MCP-1 in the brain. This can enhance the recruitment of activated monocytes both in the CNS and at peripheral level.
These same inflammatory mediators can stimulate the vagus nerve (neural pathway), whose afferents reach the brain at the level of the nucleus of the solitary tract (NST).70,71 Thus, the autonomic nervous system also should be considered in the context of such neuroimmune crosstalk, since the antiinflammatory cholinergic pathway72 may in turn control the systemic inflammatory response. With the purpose of increasing our knowledge of the role of this pathway, Dos Santos et al., in an experimental study,73 showed that while vagus nerve inhibition enhanced ALI, stimulation of the antiinflammatory cholinergic reflex exerted the opposite effect. These authors suggested vagal stimulation as a treatment alternative for ventilated patients with ALI/ARDS,72,73 though the effects at CNS level have not been explored to date.
Lastly, mention also must be made of a third pathway directly regulated by the release of TNFα (cellular pathway) at peripheral level, and which gives rise to the release of monocyte chemotactic protein-1 (MCP-1) in the brain.74 This in turn generates further recruitment of activated monocytes at both peripheral and brain level, thereby multiplying the response.
ConclusionsThe critical patient subjected to MV is exposed to possible lung and brain alterations beyond those inherent to the background disease process originally leading to admission to the ICU. The etiology of the neurocognitive deficits observed after the stay in the ICU is of a multifactorial nature. In the absence of more specific diagnostic methods, the professionals who care for such patients must be familiarized with the physiological aspects required to define treatment strategies capable of minimizing lung and brain damage additional to that associated with the background illness. Optimization of the ventilatory pattern is required, seeking efficiency of both ventilation and patient–ventilator interaction – thereby contributing to minimize the abovementioned neurocognitive alterations. The use of an integral approach to critical patient management will ensure that the interventions carried out will not have deleterious effects capable of producing sequelae over the short, middle or long term.
Financial supportFundació La Mataró (TV3)(exp. 112810), Neurocontent Project: IPT-300000-2010-030 (Ministry of Science and Innovation), MAPFRE Foundation, Parc Taulí Foundation.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: López-Aguilar J, Fernández-Gonzalo MS, Turon M, Quílez ME, Gómez-Simón V, Jódar MM, et al. Interacción pulmón-cerebro en el paciente ventilado mecánicamente. Med Intensiva. 2013;37:485–492.