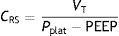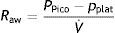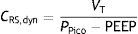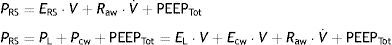Monitoring during mechanical ventilation allows the measurement of different parameters of respiratory mechanics. Accurate interpretation of these data can be useful for characterizing the situation of the different components of the respiratory system, and for guiding ventilator settings. In this review, we describe the basic concepts of respiratory mechanics, their interpretation, and their potential use in fine-tuning mechanical ventilation.
La monitorización durante la ventilación controlada permite la determinación de diferentes parámetros de mecánica respiratoria. La interpretación adecuada de estos datos puede ser de utilidad para conocer el estado de los diferentes componentes del sistema respiratorio del paciente, así como para guiar los ajustes del ventilador. A lo largo de esta revisión se describen los conceptos básicos de mecánica respiratoria, su interpretación y su potencial para el ajuste fino de los parámetros de ventilación mecánica.
A large percentage of critically ill patients require invasive mechanical ventilation (MV)–a technique that is often essential for patient survival, but which is not harmless or without risks. Growing concern about the so-called ventilator-associated lung injury (VALI) has led to attempts to develop ventilation strategies capable of reducing such injury and of avoiding its consequences at both pulmonary and systemic levels.1
Although the response to ventilation is ultimately of a biological nature, the triggering factor is mechanical.2 The application of a volume of gas to the respiratory system results in a more or less complex series of pressures and flows, depending on the components that come into play. In this sense, the response depends on whether ventilation is active or not, the characteristics of the airway, the lung parenchyma, the properties of the chest wall, and activation of the respiratory muscles. Monitorization of the ventilated patient is thus the end result of the interactions among all the above-mentioned elements.
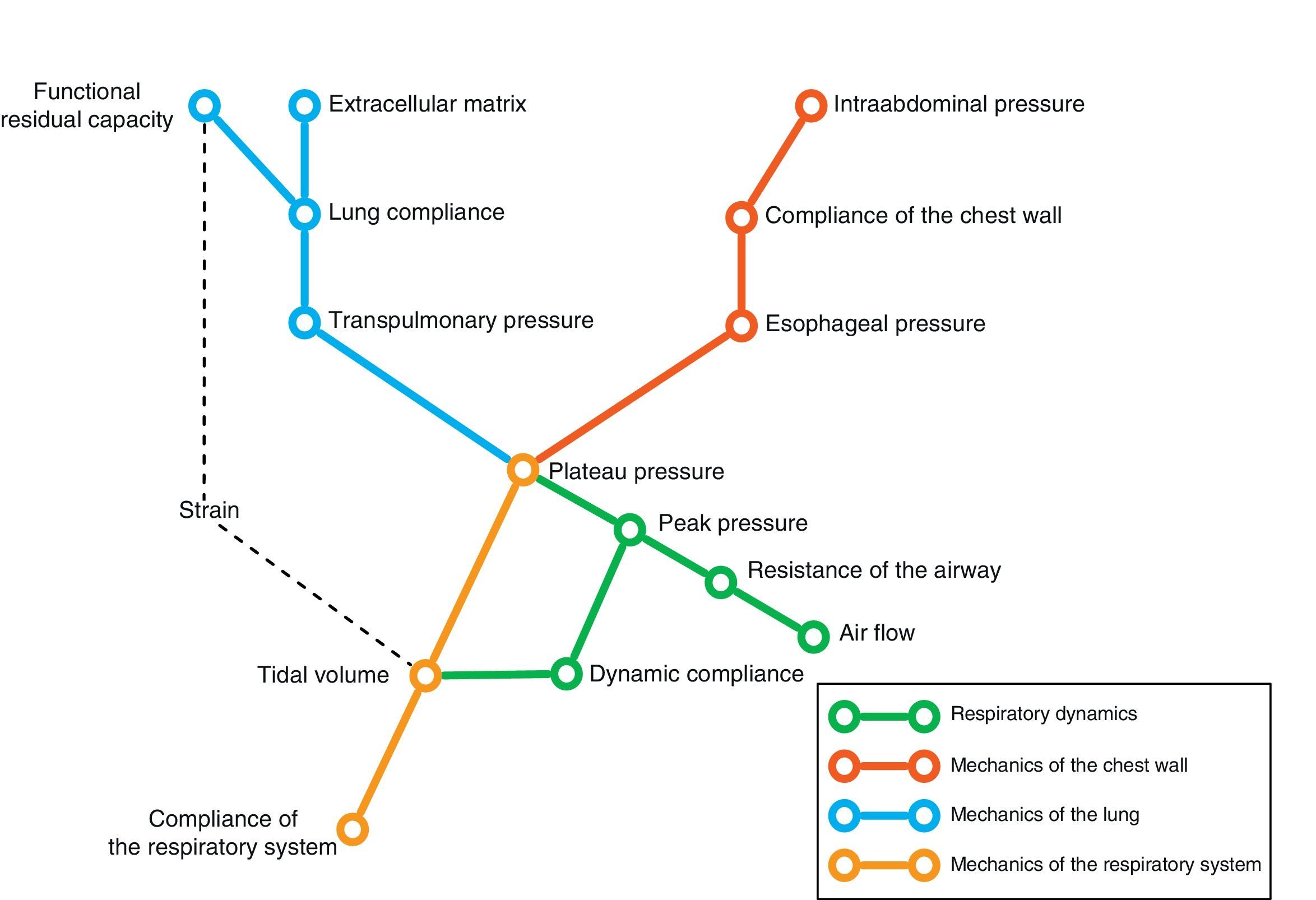
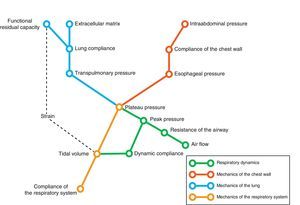
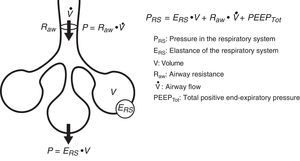
Conversely, we can try to deduce the condition of each of the elements intervening in respiratory mechanics from the end result reflected by monitorization. In the same way that a series of equations are needed to clarify certain aspects, we need different variables–sometimes measured under different conditions–in order to know the condition of each of the pieces in this puzzle. Fig. 1 schematically represents some of these variables and their main relationships.
In the end, we will need an analysis of different results to transform the data obtained into knowledge of relevance for patient management. The present review describes the main elements of ventilatory mechanics and their interactions, with the aim of establishing the necessary bases for correct interpretation of the data.
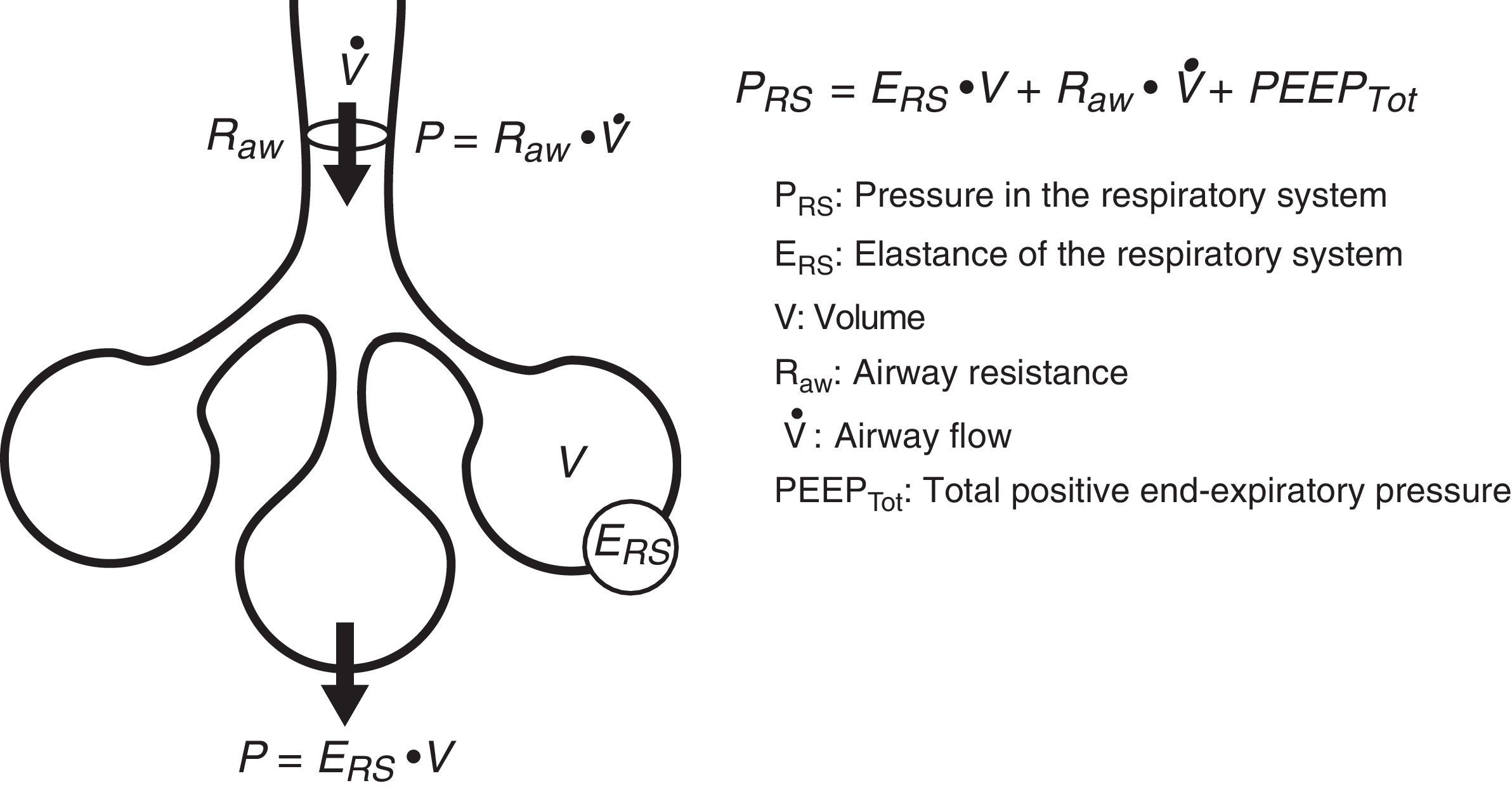
Equation of motionThe equation of motion refers to the relationship between the time course of one or more variables and the physical state of the system to which they belong. In application to our study, the equation of motion of the respiratory system refers to the relationship between the pressure in the system and the volume, flow and convective flow values.3 This equation and its components are shown in Fig. 2. The equation indicates that at each point in time, the pressure in the respiratory system has an elastic component needed for distension of the lung parenchyma, a resistive component needed for the air flow to advance against the resistances of the airway, and an inertial component due to the changes in the lung parenchyma caused by volume acceleration. It is accepted that at respiratory frequencies of under 1Hz (60rpm), the component due to the inertia of the system is negligible, and is therefore usually not taken into account.4
Based on the equation of motion, we can establish the conditions required to conduct an adequate study of respiratory mechanics. In order to facilitate interpretation of the data, the patient must not make any respiratory effort, as a result of which pressure due to muscle effort (Pmus)=0. If we obtain a pressure value under zero flow conditions (referred to as static conditions), the resistive component of the pressure is canceled. In this situation, we can calculate the compliance of the respiratory system, as will be explained further below. To this effect, we require inspiratory and expiratory pauses that cause the flow in the airway to be zero, with a view to measuring some of the mechanical parameters. Likewise, we can obtain measures under conditions of very low inspiratory flow rates (<9l/min) that cause the resistive component of the pressure to be negligible.5 In this case we speak of “quasistatic” conditions. Lastly, dynamic conditions are referred to when the air flow in the airway is not zero. One same parameter such as compliance can have very different meanings, depending on the conditions under which it has been obtained (static or dynamic).
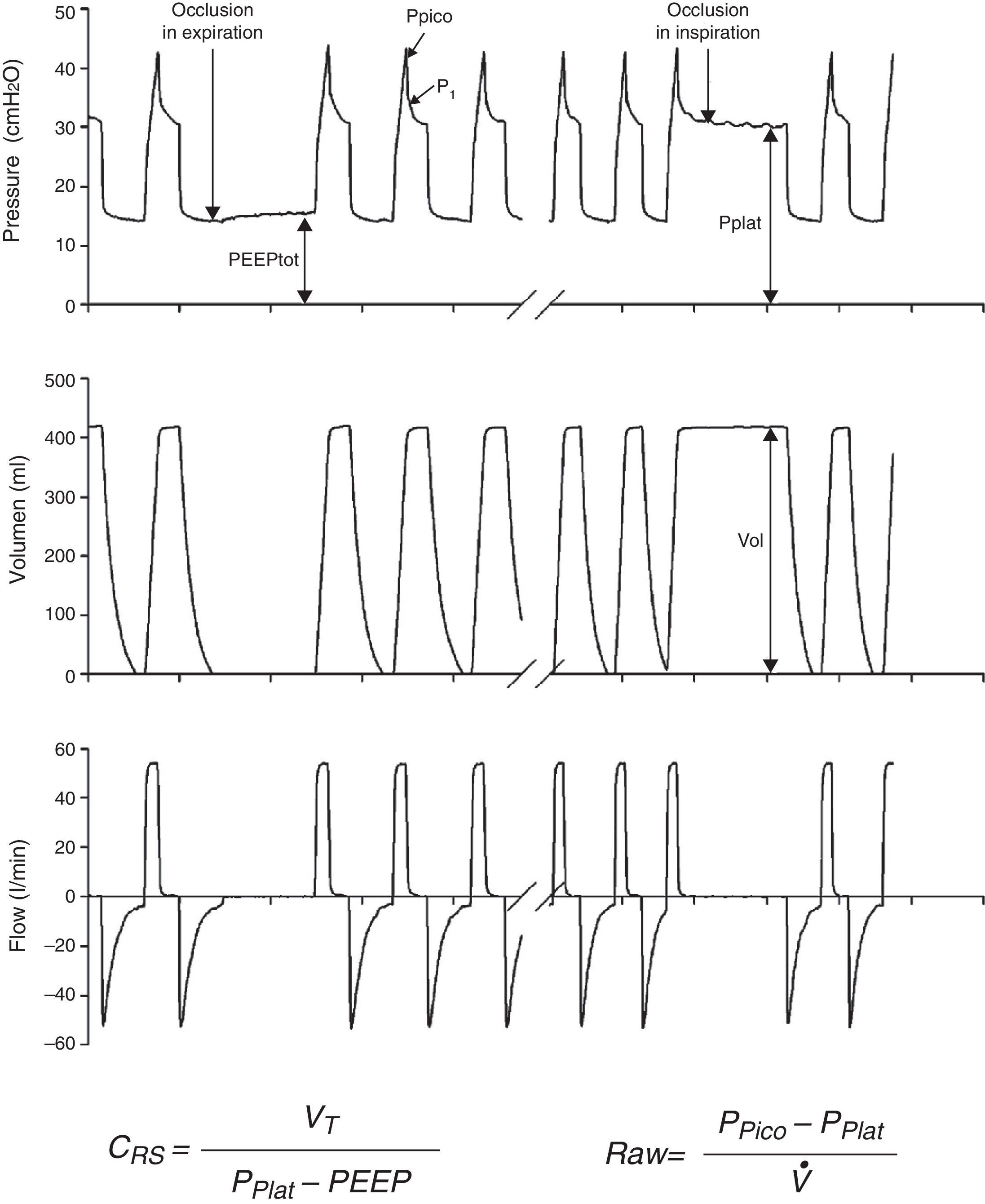
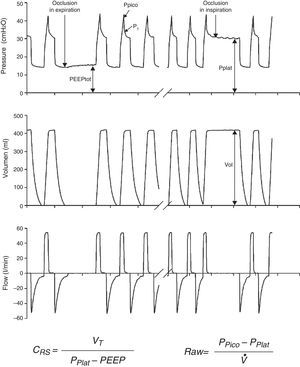
Measurements under static conditionsConsidering a volume-control ventilation mode and plotting the corresponding time-pressure curve, we observe a pressure drop immediately after closing of the inspiratory valve. During this inspiratory pause, before the expiratory valve opens, the flow stops and allows the volume of delivered air to be maintained and homogeneously distributed, thanks to the balance reached by the viscoelastic forces of the lung. The pressure reached at that point, under static conditions, is defined as the plateau pressure (Pplat), and reflects the elastic retraction pressure of the respiratory system. When equilibrium is reached in the airway pressures of the patient, Pplat is equivalent to the alveolar pressure (AP) (Fig. 3).
As a further development of this concept, the compliance of the respiratory system (CRS) can be defined as the relationship between pressure and volume, commonly calculated with the following formula:
while elastance is defined as the inverse of compliance (i.e., pressure per unit volume).The measurement of total positive end-expiratory pressure (PEEP) is also carried out under conditions of zero flow, at the end of expiration, performing a pause before the start of the next cycle. Although this parameter is easily determined, interpretation of the value obtained must be made taking into account the possible situations capable of producing it. Accordingly, in those patients with active expiration, a pressure gradient is generated between the alveoli and atmospheric air; we thus have a positive pressure at the end of inspiration, but which does not imply insufficient voiding of tidal volume. Rather, it reflects an increase in the chest retraction pressure as a result of the muscle effort. Therefore, the absence of expiratory effort is required for correct measurement of this parameter.
Compliance is a nonlinear variable inherent to the respiratory system that is modified as the conditions of both the lung parenchyma and chest change. Thus, the presence of atelectasis or of acute respiratory distress syndrome (ARDS) lessens total compliance of the respiratory system without affecting the elastic nature of the remaining healthy lung– thereby evidencing the dependency of compliance upon the ventilatable volume.6 However, the calculation of compliance with this formula is limited to a concrete state of the respiratory system. A more detailed study of the elastic properties of the system implies the determination of compliance at different levels of the curve (e.g., at different PEEP levels). Lastly, plotting of the pressure–volume curves affords more complete characterization of the mechanical properties of the respiratory system–compliance being represented by the slope of the curve at each point. In clinical practice, measurement of the static pressure–volume curves is tedious to say the least, and implies more or less risk for the patient, depending on the method used. Readers interested in this subject can consult recent reviews on this form of monitorization in particular.7,8
Measurements under dynamic conditionsOn again considering the equation of motion of the respiratory system, another variable that can be monitored is the resistance against air flow, which can be calculated as the ratio between the initial (proximal airway)–final pressure (alveolar) difference and the circulating air flow.
Although the lung tissue and chest structures offer some resistance, that exerted by the airway accounts for almost all the forces opposing air flow. The resistance of the airway is related to lung volume in that it decreases as the lung is insufflated and the airway tends to open.9 Consequently, resistance is generally less pronounced during inspiration, since expiration is characterized by the opposite tendency.
In the presence of laminar flow, with low velocities, the resistance of the airway varies in direct proportion to the viscosity of the gas and the length of the airway, and in inverse proportion to the radius of the airway raised to the fourth power. Thus, if the airway radius is halved, the circulating air flow faces a 16-fold increase in resistance (Poiseuille's law). Under normal conditions, the total airway cross-sectional area increases exponentially with the successive divisions of the tracheobronchial tree. The radius of the airway is the main determinant of resistance, since in principle the length of the airway and the viscosity of the gas do not change. In mechanically ventilated patients, the resistance generated by the endotracheal tube also must be taken into account.
Dynamic compliance10 is defined as the relationship between tidal volume and the maximum pressure reached in the respiratory system, as expressed by the following formula:
This parameter globally assesses the impact of the chest, lung parenchyma and airway resistance, yielding values between 10 and 20% less than those corresponding to static compliance. The values in turn are influenced by patient age and weight.11
Chest wall mechanicsFrom the mechanical perspective, the chest wall and lung parenchyma operate as a system connected in series, i.e., the pressures generated by both sub-systems are summed to contribute to the resulting final pressure. Accordingly, the above-represented equation of motion can be transformed into a formula that contemplates the contribution of each compartment to the final pressure:
where PL is the transpulmonary pressure, Pcw is the esophageal pressure, EL is lung elastance, V represents lung volume, Ecw is the elastance of the chest wall, V˙ is the airway flow, and PEEPTot is the total PEEP. It is generally admitted that the chest wall does not significantly contribute to generate resistive pressure in the respiratory system.Measurement of pleural pressure is required in order to monitor chest wall mechanics. The principal method used for measuring this pressure involves the placement of an esophageal catheter.12 The distal third of the esophagus lies in proximity to the pleural cavity, and it is assumed that the pressures recorded at this point are equivalent. The validity of this assumption is subject to debate, however. Pressure recordings in this zone are exposed to artifacts generated by the heart. On the contrary, it is difficult to offer an absolute value of pleural pressure, since there is a pressure gradient along the entire chest that influences the regional distension of the lung parenchyma. Despite these limitations, it is considered that the changes (relative values) in esophageal pressure are adequately correlated to the changes in pleural pressure.13 Lastly, although an acceptable correlation has been documented between the compliance of the chest wall and intraabdominal pressure,14 the validity of this correlation has recently been questioned.15
Based on these pressure recordings in a ventilated patient, we can calculate the transpulmonary pressure as the difference between the pressure in the airway and the esophageal pressure. This transpulmonary pressure is the true lung parenchyma distension pressure; it therefore seems reasonable to use this parameter for adjusting mechanical ventilation.16,17 By using the transpulmonary pressure and esophageal pressure values, we can calculate the compliance of the lung parenchyma and of the chest wall (expressed as the ratio between the tidal volume and each of the pressures). Since one same pressure in the airway can result in different transpulmonary pressures depending on the mechanical characteristics of the chest wall, it may be of interest to monitor the latter in order to determine the true stress to which the lung parenchyma is exposed.18 On the contrary, the presence of negative transpulmonary pressures at the end of expiration is indicative of a tendency toward collapse–fundamentally in dependent zones of the lung. Some authors have suggested that the PEEP values should be raised to make this end-expiratory transpulmonary pressure slightly positive.19
Estimation of lung parenchyma deformationAdvances in our knowledge of respiratory mechanics in the ventilated patient reflect the interest in using different parameters of respiratory mechanics as a guide for adjusting mechanical ventilation, and particularly for reducing ventilation-associated injury. However, as we have seen, interpretation of the information must be made in a concrete context, and a range of factors can influence each determination. As an example, an isolated plateau pressure value may have very different meanings depending on the compliance of the abdominal wall, the inspiratory effort of the patient, or the applied PEEP.
Improved characterization of the paradigms of ventilator-associated lung injury (VALI) has led to the identification of new injury mechanisms and the corresponding monitorization parameters. In this sense, the benefits of using PEEP together with the gradual reduction of tidal volume suggest that deformation of the lung parenchyma, not only the application of a pressure or volume, constitutes the cause of tissue damage.20 This concept whereby static stress (pressures and volumes in the absence of deformation) is better tolerated than dynamic stress (with tissue deformation) was developed from experimental models in cell cultures and animals,21,22 and opens perspectives for clinical application. The physiological parameters allowing us to quantify tissue deformation and its cost are stress and strain.23
Stress is the force required to deform a body. In our case, it refers to the force required to insufflate the lungs with tidal volume. From all the above-described concepts, it can be deduced that the equivalent of stress is transpulmonary pressure.
Strain is the magnitude of such deformation, expressed as a fraction of the starting (baseline) situation. Applied to respiratory mechanics, the magnitude of the deformation is the tidal volume. However, there is some controversy regarding what the starting situation should be. In its original proposal, the group led by Gattinoni made use of functional residual capacity (FRC),23 i.e., the volume of gas in the lung at the end of expiration at atmospheric pressure. Other authors have used the end-expiratory lung volume (EELV) in the presence of PEEP.24,25 The difference between the two measurements is given by the safety thresholds and adjustment of the calculations in the presence of PEEP (Table 1). In the first case, the increase in lung volume is regarded as “deformation”, and therefore would be summed to tidal volume. In the second case, the volume is summed to functional residual capacity. In other words, the addition of PEEP increases the strain when calculated with the first formula, and reduces it when calculated with the second formula. In fact, it should be understood that the application of PEEP produces both recruitment of previously non-aerated zones (thereby reducing strain, since the lung parenchyma available for ventilation is increased) and an increase in the volume of already aerated zones (which would increase strain). Thus, in order to correctly calculate the change in strain in response to PEEP, we again need to measure the recruited volume. The subject of alveolar recruitment falls beyond the scope of this work, though interested readers can consult a number of reviews on this topic.26,27
Clinical implicationsThe main aims of the monitorization of respiratory mechanics are to diagnose the state of respiratory function and to guide the adjustment of ventilation. No diagnostic technique alone is able to improve the patient prognosis if effective treatment does not accompany the diagnosis made. Therefore, in order to be of benefit for patients, the information obtained through monitorization, following adequate interpretation, must result in improvement of the treatment provided.
Regarding the diagnostic possibilities, an analysis of the recordings obtained from the ventilator allows us to detect air trapping and auto-PEEP, the presence of secretions in the endotracheal tube, or alterations in the interaction between the patient and the ventilator, as described in a recent monograph.28
However, the true impact of respiratory mechanics in the ventilated patient is closely related to its capacity to guide the adjustment of mechanical ventilation. The development of the concept of ventilator-associated or ventilator-induced lung injury has caused prevention of the latter to become a basic aim in patient management. In this sense, a current standard measure is to limit plateau pressure, particularly in patients with ARDS.29 As a marker of alveolar pressure, plateau pressure should be kept within certain safety limits in order to avoid damage secondary to overdistension. The current recommendation is to avoid values of over 28–32cmH2O.30
However, as has already been mentioned, plateau pressure can be elevated due to an abnormal decrease in chest wall compliance, and transpulmonary pressure is the true alveolar distension force. The use of this pressure as a guide for adjusting ventilation has been associated with good clinical results, despite high plateau pressures.19 Although there are no firm recommendations on the use of transpulmonary pressure (which implies the monitorization of esophageal pressure, with the already commented difficulties and limitations), it seems reasonable to adopt such an approach in patients with severe respiratory failure.16
Lastly, many studies in the literature propose an adjustment of PEEP according to the mechanical characteristics of the respiratory system. As early as 1976, the pioneering work of Suter correlated PEEP adjusted according to the point of best static compliance with improved oxygen transport in patients with ARDS.31 In a recent clinical trial, the adjustment of PEEP according to best compliance has been associated with improved oxygenation, a lesser incidence of organ failure, and a tendency toward lesser mortality.32
Adopting similar approaches, different groups have made use of dynamic compliance measures. PEEP resulting in increased dynamic compliance values has been associated by different authors to the prevention of alveolar collapse after recruitment33 and to optimum ventilation.34
Use has also been made of different inflexion points on the pressure–volume curve as a guide for adjusting PEEP.35,36 However, the few clinical trials37,38 that have used these strategies employed patients ventilated with high volumes as controls; as a result, it is impossible to know whether the beneficial effects were due to the fine-tuning of PEEP or simply to low tidal volume protection.
ConclusionsThe use of mechanical ventilation offers a good opportunity for carrying out studies of respiratory mechanics. Based on different techniques, maneuvers and calculations, we can determine the state of the respiratory system of the ventilated patient and apply treatments as a result. However, the measurements have their limitations, and the possible treatments are not without adverse effects. To date, there is no solid evidence that a concrete measure of respiratory mechanics is able to act as a clear guide in adjusting treatment. Therefore, although mechanics can help us to understand what is happening in the respiratory system of a ventilated patient, it is not possible to firmly propose a guide or strategy for ventilator adjustment based on such measures. Ventilatory mechanics should be interpreted by the clinician as an aid in the global context of the patient.
Conflict of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: García-Prieto E, Amado-Rodríguez L, Albaiceta GM, por el grupo de Insuficiencia Respiratoria Aguda de la SEMICYUC. Monitorización de la mecánica respiratoria en el paciente ventilado. Med Intensiva. 2014;38:49–55.