The high incidence of trauma, especially in elderly people anticoagulated with new oral anticoagulants (NOAs), has become a major challenge, particularly in critical situations with life-threatening bleeding. Under these circumstances, urgent NOA reversion becomes mandatory. Prothrombin complex has become a frequent indication in critical situations in which rapid reversal of anticoagulation is needed and where the use of fresh frozen plasma is limited. This study offers our point of view regarding the usefulness of NOAs, not only in the prevention of cardioembolic events but also as regards their emergent reversion in cases of severe bleeding associated to trauma.
La alta incidencia de los traumatismos, especialmente en aquella población mayor previamente anticoagulada con nuevos anticoagulantes orales (NACO), se ha convertido en un gran desafío, sobre todo en aquellas situaciones críticas en las que existe una hemorragia grave que pueda comprometer la vida del paciente. En estos casos se hace necesaria la aplicación urgente de medidas de reversión. El empleo de complejo protrombínico es una indicación cada vez más frecuente en estas situaciones de urgencia, en las que se necesita una reversión rápida de la anticoagulación y en las que el uso de plasma fresco congelado es limitado. El objetivo de este trabajo es dar nuestro punto de vista sobre la utilidad de los NACO, no solo en la prevención de enfermedades cardioembólicas, sino en su reversión emergente en aquellos casos de hemorragia grave asociada al trauma.
Traumatisms are a serious worldwide public health problem. Although the last World Health Organization (WHO) reports on the importance of trauma are separated by a period of almost 10 years, they continue to alert us to the magnitude and importance of the problem, and its possible future consequences (Document TDR/Gen/96.1; update 2008). It must be underscored that we are witnessing a change in the epidemiology of trauma, with an increase in the number of accidental falls among elderly people with comorbidities–a situation that results in increased mortality.1 This scenario points to the need to introduce primary and secondary prevention strategies referred to traumatisms in general, and indicates that care must be optimized in patients who suffer serious injuries.2
Bleeding is known to be one of the main causal factors of mortality associated to accidents (30–40% of all cases). However, a priori, bleeding produced by trauma should be regarded as a potentially reversible cause of mortality provided surgical damage control measures are adopted or coagulopathy associated to trauma is avoided.3,4 Nevertheless, if the patient is under the effects of some anticoagulant medication, the risk of bleeding increases–especially if anticoagulation cannot be quickly reverted.
Warfarin is prescribed in about 12.8% of the patients in the United States, mostly in elderly individuals over 65 years of age with chronic atrial fibrillation (AF), with the purpose of avoiding cardioembolic phenomena, among other problems.5 The development and increasingly widespread use of new oral anticoagulants (NOAs) imply a change in management strategy when patients who use these drugs suffer severe bleeding, independently of its spontaneous or traumatic origin.6,7 At present, the lack of effective means for reverting the effects of NOAs in non-scheduled situations such as after traffic accidents, casual falls or aggressions, where a “wait and see” approach is not possible, but constitutes the most critical issue regarding these new anticoagulant drugs. Although the half-life of all NOAs is about 12h, there are a number of situations such as those commented above in which we cannot wait for spontaneous reversion of their effects.6,7 In contrast, when classical anticoagulants are used, such as the vitamin K antagonists, their reversion according to the available time window is well known. Such a reversion or antagonization can be performed using vitamin K, fresh frozen plasma (FFP), or prothrombin complex concentrate (PCC).3
We obviously cannot forget that anticoagulation, in the context of AF, constitutes the main reversible cause of ischemic stroke.5 Since 1972, when Miller Fisher published a series of recommendations on the management of AF, many studies have attempted to define safer strategies for reducing the risk of embolic events.8,9 Between 1989 and 1993 a total of 6 clinical trials evaluated the usefulness of warfarin versus aspirin or placebo in preventing stroke in patients with arrhythmias of this kind. The data obtained from the global 2900 patients enrolled in these trials showed warfarin use to result in a decrease in cardioembolic phenomena.9 In this regard, we have established the main advantages and problems associated with the use of vitamin K antagonists. As positive aspects, mention must be made of their reasonable cost, good efficacy, the possibility of knowing their effects upon coagulation, the development of point of care devices that facilitate monitoring of treatment efficacy, dosing optimization through patient genetic studies, and the possibility of practically immediate reversion in the event of bleeding or the need to perform some urgent procedure.10 As inconveniences, the vitamin K antagonists take time in reaching optimum therapeutic levels (24–72h), with a narrow target range (INR 2–3), a renal clearance of 90%, unpredictable between- and within-patient responses, the existence of interactions with certain foods and other drugs, and a purported inherent increase in bleeding risk.11 Because of the above, routine monitoring of coagulation becomes mandatory in this group of patients.
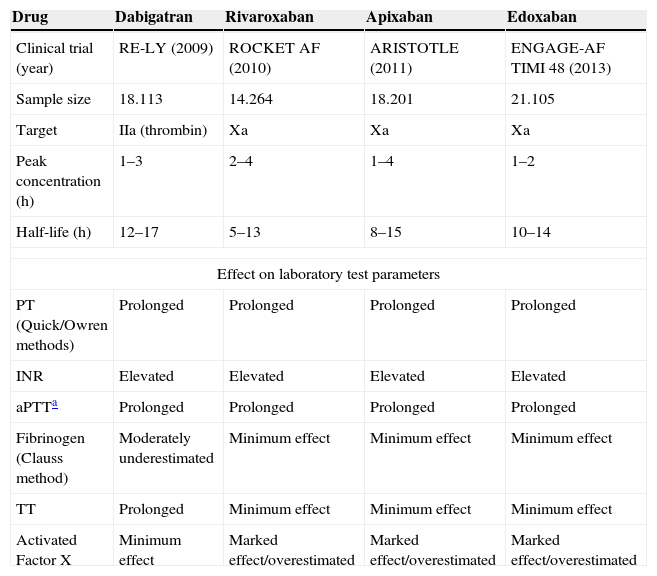
The above inconveniences, together with the high worldwide prevalence of AF associated to population aging (between 3 and 4%), have probably incentivized the study and development of new drugs that optimize and facilitate adequate prevention of cardioembolic phenomena.5 In this regard, since 2009 a total of four large clinical trials on the use of NOAs have been published.12–15Table 1 summarizes the main advantages and properties of these new drugs. Firstly, NOAs have been shown to offer better prevention of ischemic stroke and embolic events compared with warfarin.14–18 Secondly, a decrease in severe spontaneous bleeding is observed, especially as regards intracranial hemorrhage. However, it must be noted that dabigatran has been associated to an increased risk of gastrointestinal bleeding.14 Their effects do not require monitoring, thereby improving patient comfort – though this may be a problem in individuals with treatment adherence problems. It must be emphasized that in the case of important bleeding, excessive dosing, or administration before surgery, we can monitor the effects of these drugs upon coagulation.6 Nevertheless, no laboratory value has yet been found to reliably determine NOA activity, since some specific parameters may be under- or overestimated (Table 1).
Principal characteristics of the new oral anticoagulants (NOAs).
| Drug | Dabigatran | Rivaroxaban | Apixaban | Edoxaban |
|---|---|---|---|---|
| Clinical trial (year) | RE-LY (2009) | ROCKET AF (2010) | ARISTOTLE (2011) | ENGAGE-AF TIMI 48 (2013) |
| Sample size | 18.113 | 14.264 | 18.201 | 21.105 |
| Target | IIa (thrombin) | Xa | Xa | Xa |
| Peak concentration (h) | 1–3 | 2–4 | 1–4 | 1–2 |
| Half-life (h) | 12–17 | 5–13 | 8–15 | 10–14 |
| Effect on laboratory test parameters | ||||
| PT (Quick/Owren methods) | Prolonged | Prolonged | Prolonged | Prolonged |
| INR | Elevated | Elevated | Elevated | Elevated |
| aPTTa | Prolonged | Prolonged | Prolonged | Prolonged |
| Fibrinogen (Clauss method) | Moderately underestimated | Minimum effect | Minimum effect | Minimum effect |
| TT | Prolonged | Minimum effect | Minimum effect | Minimum effect |
| Activated Factor X | Minimum effect | Marked effect/overestimated | Marked effect/overestimated | Marked effect/overestimated |
In the same way as with the classical anticoagulants, NOAs can interact with other drugs associated to glycoprotein P including diltiazem, verapamil, atorvastatin, etc.7 Recently, Ruff et al. published the results of a study that pooled the available information from phase III studies on the use of NOAs.16 The conclusions of the authors underscored the benefits of these new drug substances. However, despite the large sample size of the metaanalysis (71,683 patients), we consider that it has a number of methodological limitations. On one hand, the populations in each study were not analogous in terms of cardiovascular risk and the probability of embolic events (as assessed using the CHADS2 classification).17 On the other hand, the different NOAs cannot be pooled as a “single drug” versus warfarin: in effect, as can be seen in Table 1, not all the NOAs have the same characteristics and therapeutic targets.
Independent of the above, the main problem with NOAs is the control of bleeding after severe trauma. The current recommendations on reversion of the effect of these drugs are set within the context of scheduled surgery and other procedures, though in the case of conditions as common as trauma, special care is required.7,18,19 In this respect, activated charcoal is advised if the NOA was administered in the previous 2h.6,7,20 In the case of dabigatran, the Summary of Product Characteristics contemplates the possibility of extrarenal filtration, since 68% of the drug is dialyzable. However, this is complicated to do in severe trauma patients, since a specific vascular access would be needed for the dialysis technique, with the patient under anticoagulation. Likewise, the available time window is not enough to allow complete elimination of the drug.20–23 Of note is the fact that fresh frozen plasma (FFP) does not revert thrombin inhibition. Therefore, since it does not improve patient coagulopathy, we consider the recommendation of the European Heart Rhythm Association regarding the use of FFP as a volume expander to be questionable.7
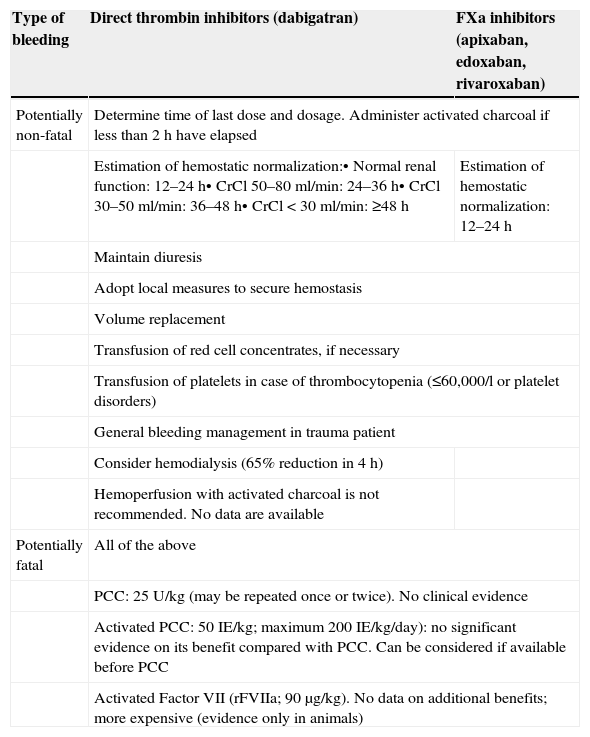
The latest European guides on the management of bleeding and coagulopathy related to trauma offer a series of recommendations referred to patients receiving anticoagulation therapy.3 In this regard, the early use of prothrombin complex concentrate (PCC) for the emergent reversion of patients anticoagulated with vitamin K-dependent drugs is advised (grade 1B recommendation). The guides indicate that if this strategy is followed, PCC should be administered with thromboelastometric evidence of a delay in the activation of coagulation (grade 2C recommendation).3 If the patient is being treated with NOAs, the PCC dose must be greater and should be established on an individualized basis, weighing thromboembolic risk against the need for rapid and effective correction of such induced coagulopathy.6,24–27 Recommendation number 32 of the European guides suggests the control of anti-Factor Xa activity in patients treated with rivaroxaban, apixaban or edoxaban (grade 2C recommendation).3 If bleeding is considered to be life-threatening, the reversion of rivaroxaban, apixaban and edoxaban with high-dose PCC (25–50U/kg) is advised (grade 2C recommendation). Treatment with Factor IIa antagonists (dabigatran) prolongs activated partial thromboplastin time (aPTT) and thrombin time–high PCC doses being inefficient in such situations (grade 2B recommendation). In these cases the participation of an experienced hematologist is advised.3 Accordingly, specific treatment should be provided to revert the effects upon Factor IIa. In the case of recombinant activated Factor VII (rFVIIa), the guides recommend (recommendation 33) consideration of its use after serious bleeding, and if the traumatic coagulopathy persists despite conventional attempts to control the bleeding, in the absence of secondary intracranial hemorrhage following isolated traumatic brain injury (grade 2C recommendation). However, the guides make no specific mention of the use of rFVIIa in the context of patients under treatment with NOAs.3 From the clinical perspective, it must be remembered that NOA interruption or reversion can imply an increased thrombotic risk, because of the shorter drug half-life and the use of pro-hemostatic agents, which are not true antidotes to such drugs (Table 2).6
Possible measures in the event of bleeding with the new oral anticoagulants (NOAs).
| Type of bleeding | Direct thrombin inhibitors (dabigatran) | FXa inhibitors (apixaban, edoxaban, rivaroxaban) |
|---|---|---|
| Potentially non-fatal | Determine time of last dose and dosage. Administer activated charcoal if less than 2h have elapsed | |
| Estimation of hemostatic normalization:• Normal renal function: 12–24h• CrCl 50–80ml/min: 24–36h• CrCl 30–50ml/min: 36–48h• CrCl<30ml/min: ≥48h | Estimation of hemostatic normalization: 12–24h | |
| Maintain diuresis | ||
| Adopt local measures to secure hemostasis | ||
| Volume replacement | ||
| Transfusion of red cell concentrates, if necessary | ||
| Transfusion of platelets in case of thrombocytopenia (≤60,000/l or platelet disorders) | ||
| General bleeding management in trauma patient | ||
| Consider hemodialysis (65% reduction in 4h) | ||
| Hemoperfusion with activated charcoal is not recommended. No data are available | ||
| Potentially fatal | All of the above | |
| PCC: 25U/kg (may be repeated once or twice). No clinical evidence | ||
| Activated PCC: 50IE/kg; maximum 200IE/kg/day): no significant evidence on its benefit compared with PCC. Can be considered if available before PCC | ||
| Activated Factor VII (rFVIIa; 90μg/kg). No data on additional benefits; more expensive (evidence only in animals) | ||
PCC: prothrombin complex concentrate; CrCL: creatinine clearance.
In conclusion, aging of the population is associated to an increased prevalence of age-related disorders such as atrial fibrillation, the management of which is in constant evolution. The use of NOAs has opened new perspectives in the prevention of ischemic stroke in patients with AF or other emboligenic processes, though on the other hand they indirectly increase the probability of having to deal in hospital with NOA-anticoagulated trauma patients. At present we are in the wait of development of drugs or monoclonal antibodies such as idarucizumab (ClinicalTrials.gov identifier: NCT02104947), to antagonize the effects of NOAS and thus allow good overall control of treatment with these new oral anticoagulants and optimization of their use in all possible scenarios.28–30 Once specific measures for NOA reversion after severe trauma become available, we probably will have overcome the major problem of bleeding management in patients of this kind upon admission to hospital.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Egea-Guerrero JJ, Quintana Díaz M. Nuevos anticoagulantes orales en el paciente traumatizado grave: ¿enemigo a las puertas? Med Intensiva. 2015;39:167–171.