To compare the therapeutic efficacy of intramuscular midazolam (MDZ-IM) with that of intravenous diazepam (DZP-IV) for seizures in children.
DesignRandomized clinical trial.
SettingPediatric emergency department.
Patients: Children aged 2 months to 14 years admitted to the study facility with seizures.
InterventionPatients were randomized to receive DZP-IV or MDZ-IM.
Main measurementsGroups were compared with respect to time to treatment start (min), time from drug administration to seizure cessation (min), time to seizure cessation (min), and rate of treatment failure. Treatment was considered successful when seizure cessation was achieved within 5min of drug administration.
ResultsOverall, 32 children (16 per group) completed the study. Intravenous access could not be obtained within 5min in four patients (25%) in the DZP-IV group. Time from admission to active treatment and time to seizure cessation was shorter in the MDZ-IM group (2.8 versus 7.4min; p<0.001 and 7.3 versus 10.6min; p=0.006, respectively). In two children per group (12.5%), seizures continued after 10min of treatment, and additional medications were required. There were no between-group differences in physiological parameters or adverse events (p=0.171); one child (6.3%) developed hypotension in the MDZ-IM group and five (31%) developed hyperactivity or vomiting in the DZP-IV group.
ConclusionGiven its efficacy and ease and speed of administration, intramuscular midazolam is an excellent option for treatment of childhood seizures, enabling earlier treatment and shortening overall seizure duration. There were no differences in complications when applying MDZ-IM or DZP-IV.
Comparar la eficacia de midazolam intramuscular (MDZ-IM) con la de diazepam intravenoso (DZP-IV) para convulsiones en niños.
DiseñoEnsayo clínico aleatorizado.
ÁmbitoServicio de Urgencias Pediátricas.
PacientesNiños de entre 2 meses y 14 años internados con convulsiones.
IntervenciónLos pacientes fueron aleatorizados para recibir DZP-IV o MDZ-IM.
Mediciones principalesTiempo hasta el inicio del tratamiento (minutos), tiempo entre la administración del medicamento y el cese de la convulsión (minutos), tiempo hasta el cese de la convulsión (minutos), y tasa de fallo del tratamiento. El tratamiento fue considerado exitoso cuando las convulsiones cesaron en los 5min tras la administración del medicamento.
ResultadosCompletaron el estudio 32 niños (16 por grupo). No fue posible obtener acceso intravenoso en 4 pacientes (25%) del grupo DZP-IV. El tiempo entre la internación y el tratamiento fue menor en el grupo MDZ-IM (2,8 vs. 7,4 min; p<0,001), así como el tiempo hasta el cese de la convulsión (7,3 vs. 10,6 min; p=0,006). En 2 niños de cada grupo (12,5%), las convulsiones continuaron después de 10min de tratamiento. No hubo diferencias entre los grupos en los parámetros fisiológicos o eventos adversos (p=0,171); un niño (6,3%) del grupo MDZ-IM presentó hipotensión, y 5 del grupo DZP-IV (31%) presentaron hiperactividad o vómitos.
ConclusiónDada su eficacia, facilidad y velocidad de administración, MDZ-IM es una excelente opción para el tratamiento de convulsiones infantiles, posibilitando un tratamiento precoz y reduciendo la duración de la convulsión. No hubo diferencias en las complicaciones al aplicar MDZ-IM o DZP-IV.
Epileptic seizures are a common cause of pediatric emergency department visits. The vast majority of seizures cease within 5min; however, some are prolonged and may progress to status epilepticus (SE).1 SE, defined as continuous or recurring seizure activity lasting longer than 30min, is associated with major morbidity rate and carries a mortality rate of up to 20%.2 Prolonged seizure activity leads to failure of cerebral autoregulation, which consequently reduces cerebral blood flow and eventually results in cerebral hypoxia.2,3
ImportanceBenzodiazepines (BZDs) have been used in the urgent care of seizures for over 40 years, and are considered first-line therapy for this purpose. The BZDs, which act on GABAA receptors, are effective in the treatment of various types of seizure, have a rapid onset of action after intravenous administration, excellent penetration into the central nervous system, and a good safety profile. Persistent generalized seizure activity increases benzodiazepine resistance; therefore, there is a consensus as to the need for immediate treatment of seizures.2,4–8 Lorazepam, diazepam, and midazolam are the three BZDs used in this setting. Lorazepam is the drug of choice due to its rapid onset of action and prolonged effect, but no parenteral formulations are available in Brazil. Consequently, intravenous diazepam, which provides seizure control in 85–90% of the cases,2,3 is the first-line drug of choice for acute treatment of seizures in Brazilian practice.
Goals of this investigationWhen venous access is challenging or cannot be obtained, the most common alternatives have been rectal diazepam and intranasal midazolam, with some studies suggesting intramuscular midazolam as an additional option.7,9 The difficulty of administering BZDs rectally or intranasally and the erratic absorption provided by these routes jeopardize anticonvulsant efficacy.5,6,8,10–13 Median time to achievement of intravenous access in children ranges from 5 to 7min.8,11 Difficulties in obtaining venous access delay treatment and, consequently, increase the risk of progression to status epilepticus. Thus far, no consensus has been established as to the best route for administration of BZDs in the event of failed intravenous access.1,5
The objective of this study was to compare the therapeutic efficacy of intramuscular midazolam (MDZ-IM) with that of intravenous diazepam (DZP-IV) in children admitted to the referral service of a pediatric emergency department with epileptic seizures.
Patients and methodsStudy design and settingThis randomized clinical trial was carried out between August 2010 and August 2011 in a sample of children admitted to the pediatric emergency department of Santa Maria University Hospital (PSPed-HUSM) with epileptic seizures. PSPed is a tertiary referral center in Southern Brazil, staffed by dedicated pediatricians and pediatric residents, which treats approximately 4000 pediatric patients per month. The study was approved by the local Research Ethics Committee (protocol no. 0184.0.243.000-10) in accordance with the Declaration of Helsinki 1964 revised in 2008. In view of the nature of the study condition and to provide the best possible benefit in this urgent setting, the committee authorized immediate patient allocation and treatment without prior consent. Subjects’ parents or legal guardians were then notified of the trial, and those who agreed to the use of patient data for research purposes were asked to provide written informed consent.
The study protocol was presented by the authors and approved by the medical officers and nursing staff of the department, who received specific training in its use.
Selection of participantsInclusion criteria: children between the ages of 2 months and 14 years who were admitted to the emergency department with seizures, regardless of type or potential trigger, and in whom anticonvulsant medication was indicated and ordered by the attending physician. Children could be enrolled in the study more than once.
Exclusion criteria: children in whom venous access had been obtained in the prehospital setting and those with a known history of coagulopathy or hepatic and/or renal impairment.
InterventionsPatients admitted to the pediatric emergency department with seizures were randomly allocated to one of the two treatment groups: (a) Intravenous diazepam (DZP-IV), 0.5mg/kg IV (maximum dose 10mg) at a concentration of 5mg/ml and with an application speed of 5mg/min, or (b) intramuscular midazolam (MDZ-IM), 0.5mg/kg IM (maximum dose 15mg) at a concentration of 5mg/ml.
Patients were randomized in blocks of 10. Five slips of paper marked “DZP-IV” and five slips marked “MDZ-IM” were placed into a brown paper envelope. At the time of admission, the nurse in charge of medication administration took a slip of paper from the envelope at random and administered the corresponding drug as standardized in the study protocol, after adjusting the dose for weight and age.
Methods and measurementsIdentifying information and physiological parameters were recorded. The following variables were used as outcome measures: time from admission to drug administration (including time required to obtain intravenous access), time from drug administration to cessation of seizures, and total time from admission to cessation of seizures.
Heart rate and pulse oximetry were monitored in all patients throughout treatment. Vital signs were recorded on admission and every 5min thereafter until discharge or transfer. Airway suctioning, supplemental oxygen, or tracheal intubation were provided as necessary or when ordered by the attending physician. Immediate adverse drug reactions were assessed in the first 10min after administration of MDZ-IM or DZP-IV. The emergency department where the study was performed is equipped with crash carts containing all the necessary equipment and medications for treatment of cardiorespiratory instability.
Outcomes and analysisTreatment was considered successful when cessation of seizures was achieved within 5min of administration of a single dose of study drug (DZP or MDZ). Requirement of a second dose of study drug or additional medications was defined as treatment failure.
Inability to obtain venous access within 4min was defined as “failed intravenous access.” This 4-min cutoff was chosen on the basis of mean time to establish intravenous access by Pediatric Emergency nursing staff during emergency care.
Statistical analysis: continuous variables were expressed as means and standard deviations. Comparisons were performed using Student's t-test (for normally distributed variables) or the Mann–Whitney U test (in case of wide variability). Categorical variables were expressed as percentages and comparisons were performed with the chi-square or Fisher's exact tests.
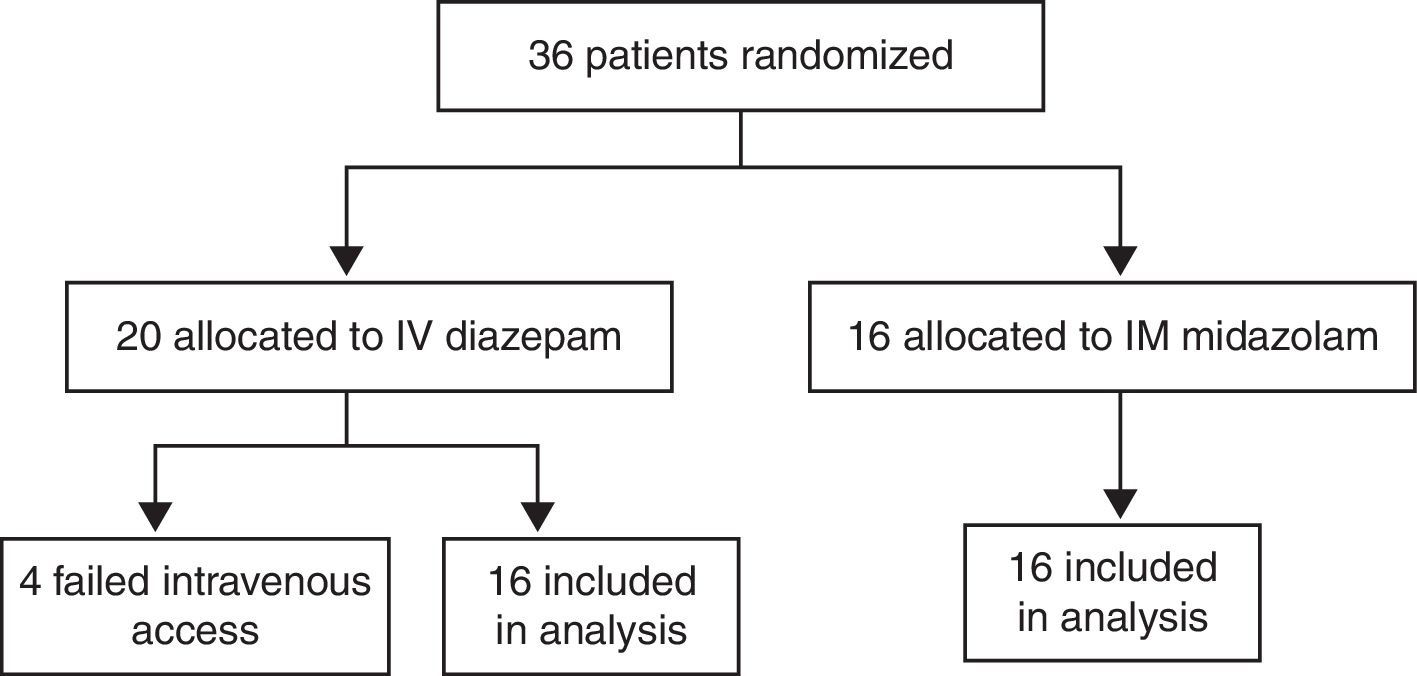
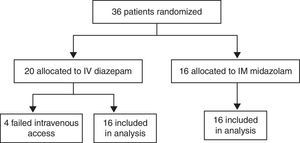
ResultsCharacteristics of study subjectsOf the 180 children admitted to the Pediatric Emergency Department at Santa Maria University Hospital (Brazil) with a chief complaint of convulsive seizures, 144 were postictal on arrival. The remaining 36 children were enrolled in the study. Of these, 16 were allocated to the MDZ-IM group and 20 to the DZP-IV group. All children allocated to the midazolam group completed the study. Of those allocated to the diazepam arm, four (20%) were excluded due to failed intravenous access, and the remaining 16 completed the study (Fig. 1). The parents or guardians of all 32 children who completed the study had provided informed consent for participation.
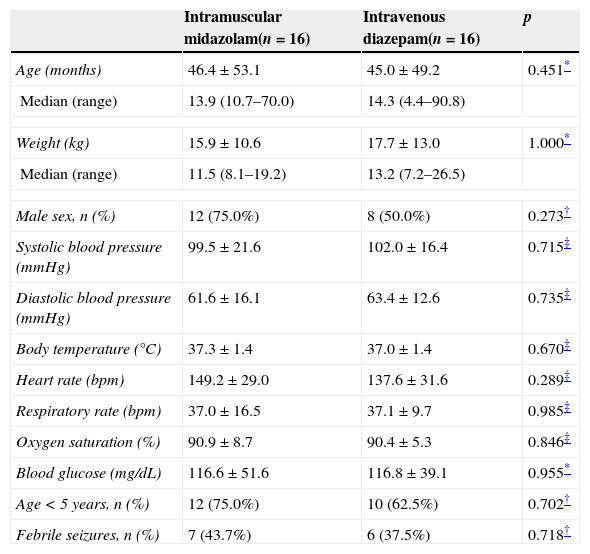
There were no significant between-group differences in age, weight, sex, seizure etiology, vital signs, or blood glucose levels on admission. In both groups, most children were under the age of 5 (Table 1).
Sample profile by group.
| Intramuscular midazolam(n=16) | Intravenous diazepam(n=16) | p | |
|---|---|---|---|
| Age (months) | 46.4±53.1 | 45.0±49.2 | 0.451* |
| Median (range) | 13.9 (10.7–70.0) | 14.3 (4.4–90.8) | |
| Weight (kg) | 15.9±10.6 | 17.7±13.0 | 1.000* |
| Median (range) | 11.5 (8.1–19.2) | 13.2 (7.2–26.5) | |
| Male sex, n (%) | 12 (75.0%) | 8 (50.0%) | 0.273† |
| Systolic blood pressure (mmHg) | 99.5±21.6 | 102.0±16.4 | 0.715‡ |
| Diastolic blood pressure (mmHg) | 61.6±16.1 | 63.4±12.6 | 0.735‡ |
| Body temperature (°C) | 37.3±1.4 | 37.0±1.4 | 0.670‡ |
| Heart rate (bpm) | 149.2±29.0 | 137.6±31.6 | 0.289‡ |
| Respiratory rate (bpm) | 37.0±16.5 | 37.1±9.7 | 0.985‡ |
| Oxygen saturation (%) | 90.9±8.7 | 90.4±5.3 | 0.846‡ |
| Blood glucose (mg/dL) | 116.6±51.6 | 116.8±39.1 | 0.955* |
| Age<5 years, n (%) | 12 (75.0%) | 10 (62.5%) | 0.702† |
| Febrile seizures, n (%) | 7 (43.7%) | 6 (37.5%) | 0.718† |
In 14 patients in each group (87.5%), seizure activity ceased after administration of a single BZD dose, which shows that both intramuscular midazolam and intravenous diazepam are effective anticonvulsants. Two patients in each group (12.5%) required a second dose of study drug or additional medications for seizure control (treatment failure).
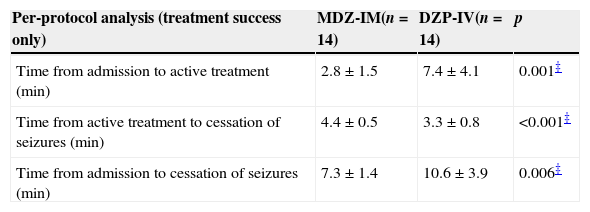
In the MDZ-IM group, active treatment was instituted significantly sooner than in the DZP-IV group (2.8 versus 7.4min; p=0.001). Total time to cessation of seizures was also significantly shorter in the MZD-IM group (time between admission and seizure cessation, 7.3 versus 10.6min; p=0.006) (Table 2). However, time from drug administration to cessation of seizures was significantly shorter in the DZP-IV group (3.3 versus 4.4min; p=0.001).
Main outcomes.
| Per-protocol analysis (treatment success only) | MDZ-IM(n=14) | DZP-IV(n=14) | p |
|---|---|---|---|
| Time from admission to active treatment (min) | 2.8±1.5 | 7.4±4.1 | 0.001‡ |
| Time from active treatment to cessation of seizures (min) | 4.4±0.5 | 3.3±0.8 | <0.001‡ |
| Time from admission to cessation of seizures (min) | 7.3±1.4 | 10.6±3.9 | 0.006‡ |
| Intention-to-treat analysis (including treatment failures) | MDZ-IM(n=16) | DZP-IV(n=16) | p |
|---|---|---|---|
| Time from admission to active treatment (min) | 3.8±2.8 | 7.4±4.0 | 0.001* |
| Time from active treatment to cessation of seizures (min) | 7.9±12.6 | 5.7±9.1 | 0.003* |
| Time from admission to cessation of seizures (min) | 11.7±14.6 | 13.1±10.5 | 0.007* |
| Failed intravenous access (n, %) | 4 (25%) | 0.0001† | |
| Treatment failure (n, %) | 2 (12.5%) | 2 (12.5%) | 1.000† |
| ICU transfer (n, %) | 1 (6.25%) | 2 (12.5%) | 1.000† |
| Adverse drug reactions (n, %) | 1 (6.3%) | 5 (31%) | 0.171† |
DZP-IV, intravenous diazepam; ICU, intensive care unit; MDZ-IM, intramuscular midazolam.
As noted above, two patients in each group (12.5%) were considered to have failed treatment due to persistence of seizures more than 5min after BZD administration. The between-group differences in time to active treatment and time to cessation of seizures held true regardless of inclusion or exclusion of the patients that failed treatment (Table 2).
All children in both groups exhibited cyanosis and psychomotor agitation on admission and required supplemental oxygen by nasal cannula or non-rebreather mask. One child in the DZP-IV group progressed to SE of 40-min duration, requiring phenytoin for seizure control, and one child had a seizure duration of 6min, requiring only an additional dose of IV diazepam. In the MDZ-IM group, one child had a seizure lasting 10min, requiring intravenous diazepam and phenytoin. Additionally, one child in the MDZ group had SE that lasted 55min and did not respond to intravenous diazepam. In this case, cessation was achieved after rectal diazepam and an additional dose of intramuscular midazolam.
One child (6.25%) in the MDZ group required intubation, artificial ventilation, and ICU admission due to respiratory failure on admission to the ED (SaO2=64%). Two children (12.5%) in the DZP group required intubation and ICU admission (one due to severe head trauma and one due to respiratory failure with SaO2=80%).
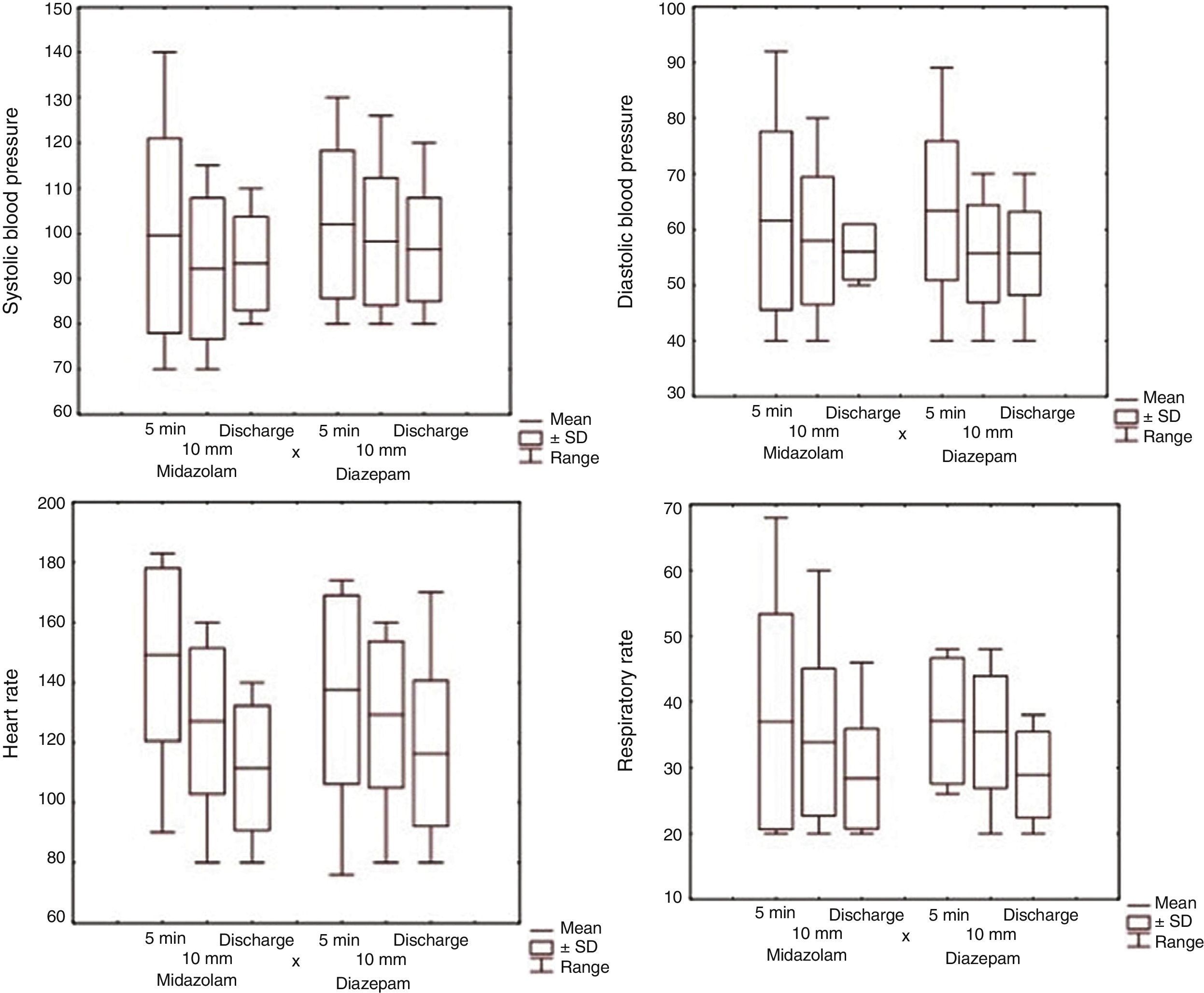
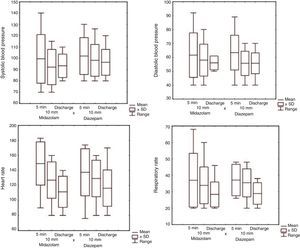
All children were monitored until cessation of seizures or discharge. There were no significant between-group differences in vital signs (Fig. 2).
Possible immediate adverse drug reactions (those occurring within 10min of study drug administration) included hypotension in one child (6.3%) in the MDZ-IM group, two children (12.6%) with hyperactivity and salivation, one (6.3%) with nausea, and two (12.6%) with vomiting in the DZP-IV group. Nevertheless, the between-group difference in adverse reactions did not reach statistical significance (Table 2).
DiscussionIn this randomized clinical trial where intramuscular midazolam was compared with intravenous diazepam for treatment of seizures in the pediatric emergency department, the following findings were observed: (a) in a reasonably high percentage of patients (20%), intravenous access cannot be achieved within 5min of admission; (b) time from admission to active treatment and time from admission to cessation of seizures are significantly shorter with IM midazolam; and, the previous finding notwithstanding, (c) time from drug administration to cessation of seizures is significantly shorter with IV diazepam instead of IM midazolam.
Current treatment protocols propose that all children with convulsive seizures of >5-min duration should be managed according to established treatment algorithms for status epilepticus, in view of the complications and risk of neurological damage associated with prolonged seizure activity.6 The first 10min of management should focus on protecting the airway and maintaining its patency, providing supplemental oxygen, measuring blood glucose, obtaining intravenous access, and treating the seizure with an intravenous benzodiazepine.3,6,8
One may presume that the minimum time required for a child to reach the emergency department after the onset of seizures, when transported from the home by parents, is 5min. Studies have estimated that at least 5–7min are required to obtain intravenous access in pediatric patients.8,11 The sum of these times means at least 10–12min will have elapsed between seizure onset and active treatment, which increases the risk of complications.
When intravenous access cannot be obtained, buccal or intranasal administration of midazolam has been advocated, with an effectiveness rate in the region of 60%.13 Despite good absorption through the mucous membranes of the mouth and nose, administration of midazolam through these routes can be extraordinarily challenging due to the involuntary movements of the convulsing child and due to the presence of airway secretion, which impedes proper absorption.2,13 Rectal administration of diazepam is associated with erratic absorption and poor therapeutic success rates (27%).4,12
Midazolam has been used in the treatment of seizures since the 1980s,14 with a good safety profile in terms of respiratory depression and good absorption via several routes (intranasal, buccal, rectal, and intramuscular).13,15–17 A study conducted in pediatric emergency departments in Australia and New Zealand showed that, when venous access could not be obtained, 49% of the physicians used rectal diazepam and 41% used intramuscular midazolam, which shows increasing preference for the intramuscular route.18
As in previous studies, IV diazepam has a faster onset of action than IM midazolam after administration (3.3min versus 4.4min), due to the shorter time to peak serum levels and, consequently, earlier achievement of therapeutic levels in the CNS.1,11,19 Nevertheless, actual time to cessation of seizures (i.e. time from admission to the emergency department to cessation of seizure activity) was significantly shorter in the IM midazolam group (7.3min versus 10.6min), which corroborates the observations of a similar trial.20
These results suggest that, when treating children with convulsive seizures in whom venous access is expected to be difficult or unlikely, IM administration of midazolam is safe, effective, and perhaps superior to intravenous diazepam. It bears stressing that seizure duration is directly associated with the speed of benzodiazepine administration.5 Similar findings have been reported in the treatment of behavioral disturbances in adult patients (where intravenous access is also challenging), where IM administration was superior to IV sedation in terms of time to cessation of psychomotor agitation (21min versus 30min).21 Therefore, even though BZDs act more rapidly when administered by the intravenous route, early intramuscular administration of midazolam provides superior therapeutic efficacy, due to faster administration as well as due to the excellent absorption of midazolam by this route.
Failed intravenous access is widely recognized as a hindrance to implementation of urgent care protocols in the pediatric emergency department. It is estimated that 2.5–16min are required to obtain peripheral venous access in adults, with a failure rate of 10–40%. In children, this rate ranges from 14% to 70%, with failure being most common in infants.11,22–24 Venous access could not be obtained in 20% of the 20 children randomized to the DZP-IV arm of this study, which provides further evidence of the importance of intramuscular administration of anticonvulsants in this setting.
Adverse effects were more frequent in the diazepam group, as expected in view of previous comparisons with midazolam and lorazepam,9,19 although the difference did not reach statistical significance. The adverse effects of BZDs are usually dose-dependent and associated with repeated administration, and manifest most commonly as reduced oxygen saturation and central hypoventilation.2,5 These findings are rapidly reversed with administration of supplemental oxygen, airway suctioning and positional maneuvers and, in a minority of cases, bag-valve-mask ventilation.
The etiology of seizures varies according to age and geographical region. However, most studies report that approximately one-third of children admitted to an emergency department with convulsions are having a febrile seizure,25–27 as was the case in the present study.
One limitation of this study is that the sample size precludes any conclusions about adverse effects and drug safety, which would require a larger population. Furthermore, due to the design and nature of the study, personnel in charge of administering drugs were not blinded to allocation, which may have introduced bias. Despite this limitation, the times to seizure cessation obtained in this study were highly favorable, especially in view of the study setting (the dedicated pediatric emergency department of a large, university-affiliated tertiary care center). One may presume that at smaller hospitals, where staff might have less skill or experience obtaining intravenous access in children, the difference would be even more significant.
Another limitation of the present study is the fact that, although the cause of seizure could be initially identified at the emergency room in most patients, this initial identification does reach a 100% level of certainty. Therefore, emergency room professionals may have failed to identify, at first, a child with an underlying neurological disease leading to unresponsiveness to seizure treatment.
The results of the study suggest that intramuscular midazolam significantly shortens seizure duration in children as compared with intravenous diazepam. Therefore, intramuscular midazolam is a good alternative to intravenous diazepam, in view of its anticonvulsant efficacy and speed and ease of administration in the pediatric emergency care setting.
Conflicts of interestThe authors have no conflicts of interest to declare.
Authors’ contributionAll authors took part in data collection and analysis, literature review, and drafting of the manuscript.
Work center: Pediatric Emergency Department, Hospital Universitário de Santa Maria Universidade Federal de Santa Maria (UFSM), Av. Roraima, Prédio 22, Campus, Bairro Camobi, Zip Code: 97105-900 – Santa Maria, RS, Brazil.