The integration of the ventricular function is essential when making decisions over a patient subjected to cardiac electrostimulation in order to understand the structure followed in the new cardiac stimulation and resynchronizing therapy guides. To support the importance of ventricular function in cardiac electrostimulation it is important to know: (a) the deleterious effect of stimulation on the right ventricle apex; (b) the effect over the left ventricular function produced by complete blockage of the left branch, and (c) left ventricular disfunction as arrythmogenic substrate. When it comes to decide what type of cardiac electrostimualtion to apply we will know: the percentage of ventricular stimulation needed and its ventricular function. A normal ventricular function will enable electrostimulation from the right ventricle apex or alternative site. On the contrary, if this value is lower than 50% the most recommended electrostimulation is cardiac resynchronization (CRT-P), which will be accompanied by defibrillation (CRT-D) if FEVI is lower than 35%.
La integración de la función ventricular en la toma de decisiones del paciente sometido a electroestimulación cardiaca resulta fundamental para comprender la estructuración de las nuevas guías sobre estimulación cardiaca y terapia de resincronización. Para argumentar la importancia de la función ventricular en la electroestimulación cardiaca es necesario conocer: a) el efecto deletéreo de la estimulación desde el ápex del ventrículo derecho; b) el efecto del bloqueo completo de rama izquierda sobre la función ventricular izquierda, y c) la disfunción ventricular izquierda como sustrato arritmogénico. Así, cuando decidimos el modo de electroestimulación cardiaca a aplicar debemos conocer el porcentaje de estimulación ventricular que precisará y su función ventricular. Si esta es normal, permitirá estimular desde el ápex del ventrículo derecho o desde sitios alternativos al ápex. Por el contrario, si es menor del 50% es recomendable la resincronización cardiaca (CRT-P) acompañada de desfibrilación (CRT-D) si la FEVI es menor del 35%.
The integration of ventricular function is essential when making decisions regarding patients subjected to cardiac electrostimulation in order to understand how the new cardiac stimulation and resynchronizing therapy guides are structured.1 In relation to the present review, it must be taken into account that when we speak of left ventricular dysfunction, we are actually referring to left ventricular systolic dysfunction, not to left ventricular diastolic dysfunction, which is a also a cause of cardiovascular symptoms. Furthermore, cardiac electrostimulation not only comprises conventional pacemakers but also implantable cardioverter-defibrillators (ICDs) and cardiac resynchronization therapy without defibrillation (CRT-P) or with defibrillation (CRT-D). On the other hand, cardiac electrostimulation has presently extended beyond the “stimulation of survival” principle, which aims to achieve a certain heart rate regarded as adequate, and has become a form of “physiological stimulation” designed to secure adequate cardiac function, and which can be analyzed through the concepts of “atrioventricular synchrony” and “intraventricular synchrony”.
On the other hand, consideration is required of three premises, described below, which underscore the “importance of ventricular function in cardiac electrostimulation”.
Deleterious effect of pacing from the right ventricular apexSince the 1980s, studies conducted in humans2 have shown pacing in sinus rhythm to be characterized by a left ventricle stimulation sequence with early depolarization zones that start at lower septal level and on the anterior aspect, together with other late depolarization zones at apical level and in the basal segments of the inferolateral region. However, when pacing is made from the right ventricular apex there is a significant delay in left ventricular activation, with the observation of early stimulation zones located in the middle septal region and other late activation zones at the inferolateral base. The total left ventricular activation time is prolonged, thereby resulting in a “loss of the normal left ventricular activation sequence” that simulates complete left branch block, with clinical repercussions.
A number of clinical studies have demonstrated the inconveniences of pacing from the right ventricular apex. Andersen et al.,3 in patients with sick sinus syndrome, paced one group of subjects exclusively from the atrium (AAI)–a pacing mode that preserves the “left ventricular activation sequence”–and another group exclusively from the right ventricular apex (VVI)–a pacing mode that loses the “left ventricular activation sequence”. This second group of patients showed an increase in mortality of cardiovascular origin and a greater incidence of atrial fibrillation episodes.
The MOST study,4 also carried out in patients with sick sinus syndrome, used two pacing modes: (a) dual chamber (DDD), with one electrode in the right atrium and another in the right ventricular apex, in which the left ventricular activation sequence was lost with a high percentage of ventricular pacing (% pacing); and (b) single chamber, with an electrode in the right ventricular apex (VVI) programmed with a low lower frequency limit, implying a low percentage of ventricular pacing. A greater number of hospital admissions due to heart failure and/or atrial fibrillation episodes was recorded in those patients with a higher percentage of ventricular pacing when the latter exceeded 80%.
Lastly, the DAVID study5 involved patients with left ventricular dysfunction subjected to ICD implantation as primary prevention measure. The subjects were divided into two groups: a DDD pacing group (receiving a high percentage of ventricular pacing) and a VVI pacing group (receiving a low percentage of ventricular pacing). The trial had to be suspended prematurely because of high mortality among the patients in the group with a greater percentage of stimulation.
In addition to these clinical findings, the following functional alterations related to pacing from the right ventricular apex have been described:
- -
Paradoxical septal motion.6
- -
Shortened relaxation and filling times.7
- -
Mitral valve regurgitation.8
- -
Increased left atrial size.9
- -
Reduced global and regional left ventricular ejection fraction.10
A number of structural11 and molecular alterations have also been described12 that reaffirm the deleterious effects of pacing from the right ventricular apex as a condition or entity in its own right–hence the descriptive term “pacing-induced myocardiopathy”.13
Effect of complete left branch block upon left ventricular functionComplete left branch block (CLBB) causes a delay in contraction of the lateral wall of the left ventricle with respect to the right ventricle and the interventricular septum–producing inefficient contraction that lowers cardiac output and the left ventricular ejection fraction (LVEF). This situation defines a clinical condition characterized by: CLBB (QRS >120ms), LVEF <35% and New York Heart Association (NYHA) functional class II–III. These patients can benefit from specific cardiac electrostimulation known as cardiac resynchronization therapy (CRT).14
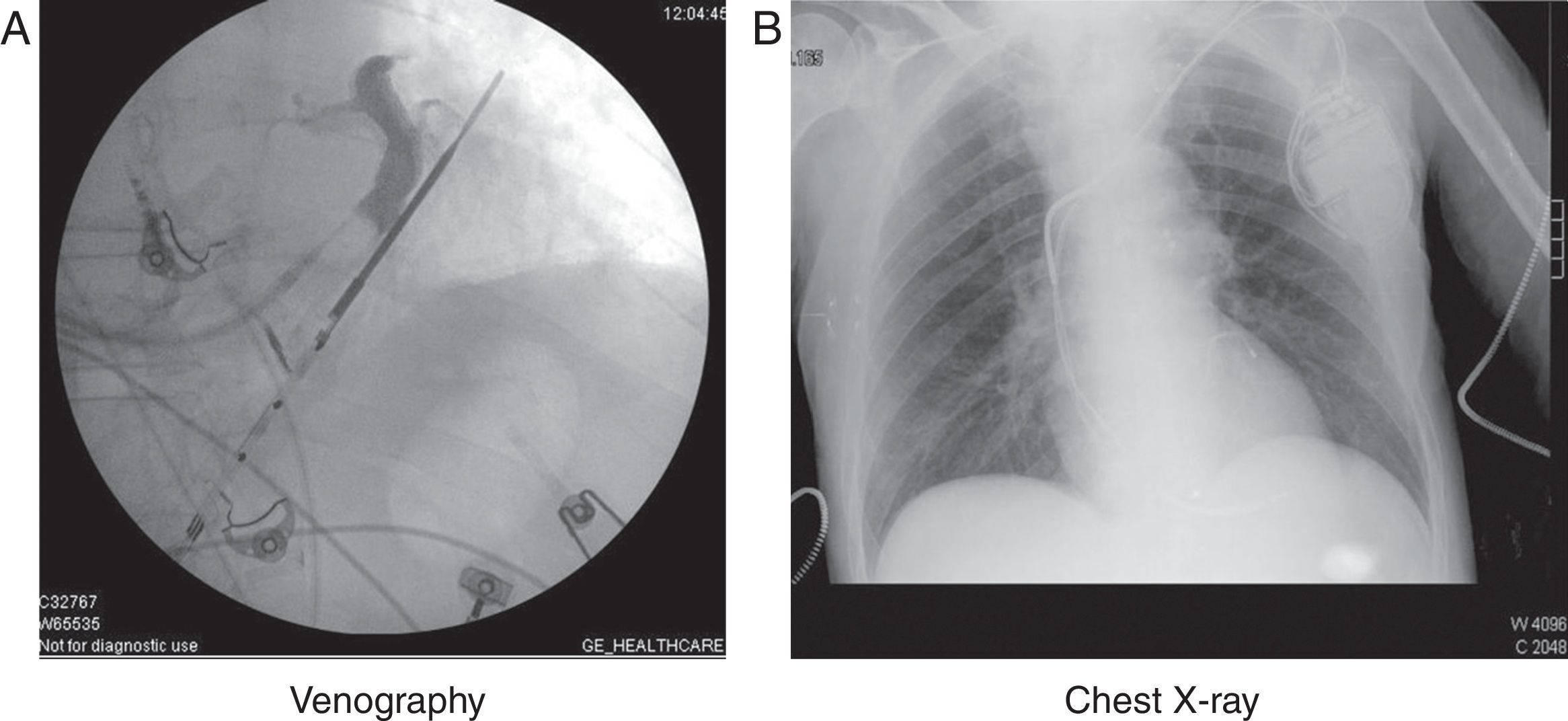
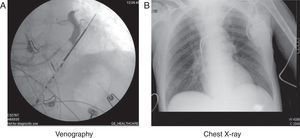
On the basis of a DDD pacemaker (if the patient is not in permanent atrial fibrillation), the usual technique involves placing an electrode from the endocardium through the coronary sinus to the epicardium of the left ventricle–preferentially in the basal inferolateral and lateral segments. Occlusive retrograde venography through the coronary sinus is performed during the procedure to visualize the coronary venous system and select the appropriate vein. Synchronized pacing from this position neutralizes the delay produced by the CLBB (Fig. 1).
The MIRACLE trial14 compared patients with the above described clinical profile assigned to either conventional medical treatment or resynchronization therapy. The CRT group was seen to present a significant percentage of asymptomatic patients.
We now know that patients who respond to CRT have a longer duration QRS, and those with a duration of over 150ms are the most responsive individuals. When echocardiography evidences a delay in the inferolateral segments with respect to the septum (ventricular dyssynchrony), in the absence of associated QRS prolongation, CRT is not indicated.15
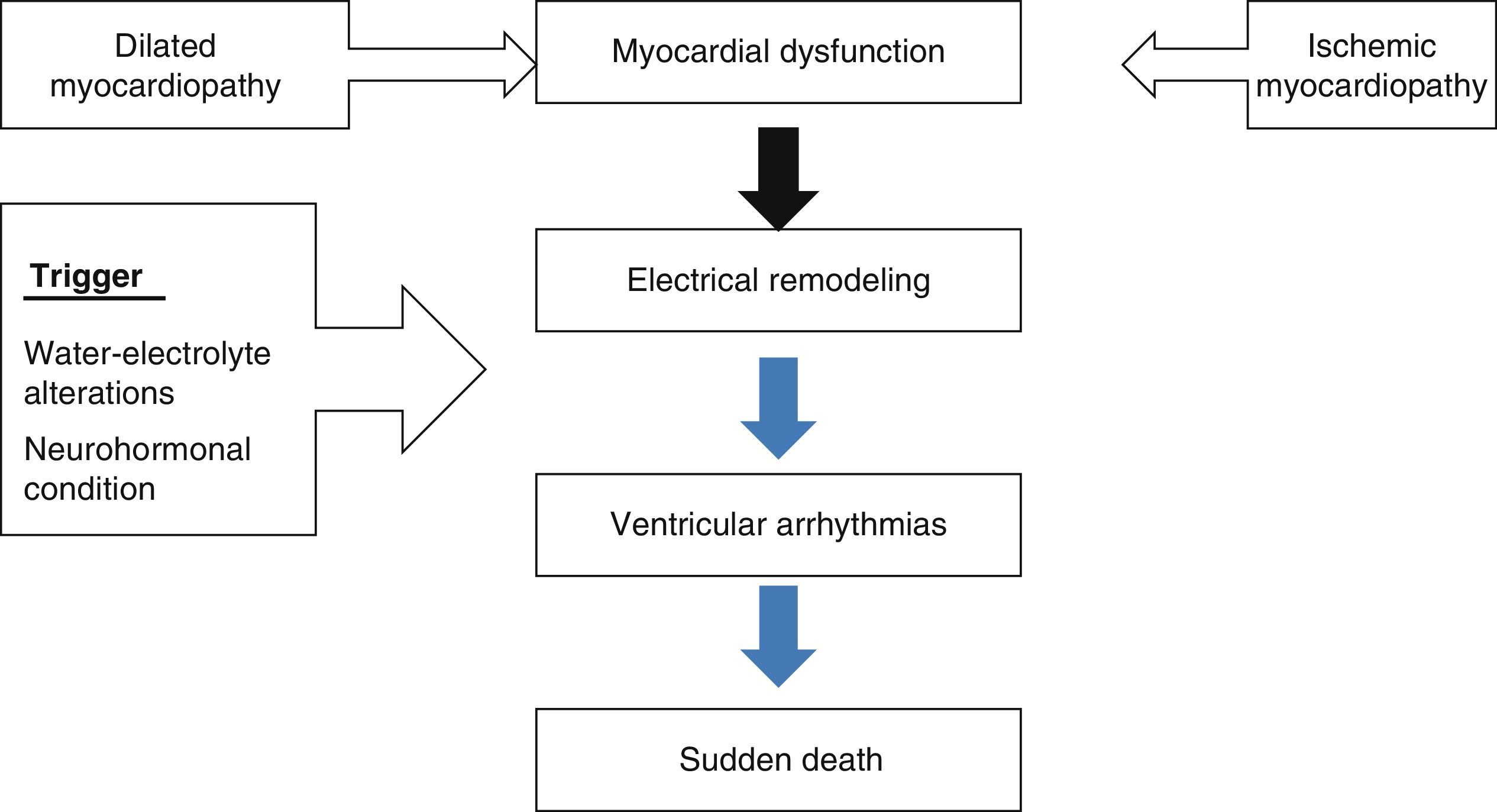
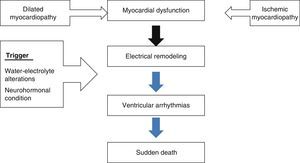
Left ventricular dysfunction as arrhythmogenic substrateMyocardial dysfunction gives rise to electrical remodeling independently of whether the origin of the disorder is ischemic or non-ischemic. When a trigger (electrolytic alterations, neurohormonal disorders, alcohol, etc.) acts upon this substrate, ventricular arrhythmias can be triggered that may result in sudden death (Fig. 2). These circumstances have been known since the 1980s, with the observation of an increase in mortality in patients with myocardial dysfunction16 secondary to the appearance of ventricular arrhythmias (ventricular tachycardia and ventricular fibrillation).17 The placement of a defibrillator in patients with this clinical profile therefore could contribute to reduce the mortality rate (Fig. 3).
The MADIT II study18 showed that in patients with left ventricular dysfunction of ischemic origin and LVEF <35%, the implantation of an ICD results in lesser mortality than in patients treated only with antiarrhythmic drugs.
On the other hand, according to the DEFINITIVE trial,19 carried out in patients with ventricular dysfunction of non-ischemic origin and LVEF <35%, defibrillatory implantation was likewise seen to result in lesser mortality, though in these cases the benefit was less pronounced due to an increase in fatalities more attributable to cardiac contractility failure than to arrhythmias.
In terms of mortality, patients with left ventricular dysfunction secondary to complete left branch block also benefit from resynchronization therapy to which defibrillation is moreover added.20
The three profiles described above correspond to indications of ICD for the primary prevention of sudden death. Furthermore, between 15–20% of these patients require anti-bradycardia treatment. It is currently debated whether LVEF should be used as the sole criterion for deciding defibrillator implantation.21 On one hand there are patients with moderate LVEF who suffer sudden death, and who could have benefited from such therapy had they been identified on time. On the other hand, of the patients with severely depressed ventricular function (LVEF <35%) that carry an ICD, approximately 60% receive no treatment during the first three years after implantation. Cardiac MRI with late gadolinium enhancement performed in the first days after acute myocardial infarction is viewed as a technique that will provide new criteria for defining those patients who stand to benefit most from ICD implantation as a primary prevention measure.22,23
CommentBased on the analysis of the three abovementioned profiles, alternatives have been sought to neutralize or avoid the deleterious effects of pacing from the right ventricular apex. On one hand, considering the hypothesis that “pacing from the right ventricular apex simulates complete left branch block” and that the latter can be corrected by cardiac resynchronization therapy, this technique would appear to be useful for neutralizing such deleterious effects. In this regard, the PACE trial,24 conducted in patients with normal LVEF and bradycardia, compared two pacing modes: resynchronization in one group and pacing from the right ventricular apex in the other. After one year of follow-up, a decrease in mean LVEF was observed in the patients paced from the right ventricular apex that persisted after a period of two years.25 However, on carrying out an individualized analysis in the group of patients paced from the right ventricular apex, only 9% were found to have depressed LVEF (<50%).
The DANPACE study,26 designed to assess the benefit of dual chamber stimulation in patients with sick sinus syndrome and preserved LVEF, recorded no clinical differences in terms of hospital admission due to atrial fibrillation or heart failure between patients paced from the apex and individuals with a low percentage of stimulation.
On examining the prevalence of ventricular dysfunction in patients paced from the right ventricular apex and starting with normal LVEF (>50%), a 9% ventricular dysfunction rate was observed after one year of pacing,24 versus 13% in patients paced for 15 years26 and 15% in patients paced for 24 years.13 It therefore can be affirmed that the deleterious effects of pacing from the right ventricular apex in patients with normal LVEF (>50%) are seen in only a small number of patients (in general terms 1/10), and there are no criteria allowing us to identify such individuals beforehand.
The situation is different when the patients start with LVEF <50%. In this regard, the BLOCK trial27 evaluated subjects with LVEF <50% and complete atrioventricular block (requiring high percentages of ventricular pacing) subjected to two cardiac electrostimulation treatments (pacing from the right ventricular apex/cardiac resynchronization). The patients subjected to pacing from the right ventricular apex showed increased mortality, more admissions due to heart failure, and an increase in left ventricle end-systolic volume (LVESV).
Another proposal for minimizing the deleterious effect of pacing from the right ventricular apex is to perform pacing from a point of the conduction system that does not result in loss of the “normal left ventricular activation sequence”–a situation known as “pacing from sites alternative to the right ventricular apex”. Two pacing points have been proposed: (a) the ventricular septum, which includes the right ventricle outflow tract (RVOT) at different levels (high and middle); and (b) the bundle of His. A number of studies involving small patient samples have been published on this subject.28–32 In general terms, when the patients have a normal LVEF (>50%), no benefits with respect to pacing from the apex are observed. In contrast, when LVEF is depressed (<50%), the ejection fraction experiences less worsening than when pacing is performed from the apex–though no clinical improvements in terms of exercise capacity, quality of life or survival are observed.
On the other hand, implantation in the bundle of His or in para-Hisian zones is scantly effective, since only about 50% of the patients can be controlled with this procedure, and high thresholds are moreover required.33 In turn, when pacing from the RVOT, the electrode is effectively placed in the septum in only one-third of the cases. In the rest of the cases it is positioned in the anterior wall or right ventricle free wall. In this regard, positioning of the electrode in the anterior wall or lateral wall of the right ventricle affords no functional benefits.34
Lastly, in patients paced from the right ventricular apex and who require “replacement”, it is considered that those individuals with depressed LVEF, high percentages of right ventricle stimulation (>80%) and no symptoms or only moderate symptoms of heart failure could benefit from an upgrade to resynchronization therapy–improvements being observed in both functional (increased LVEF) and clinical terms (improved functional capacity and quality of life).35
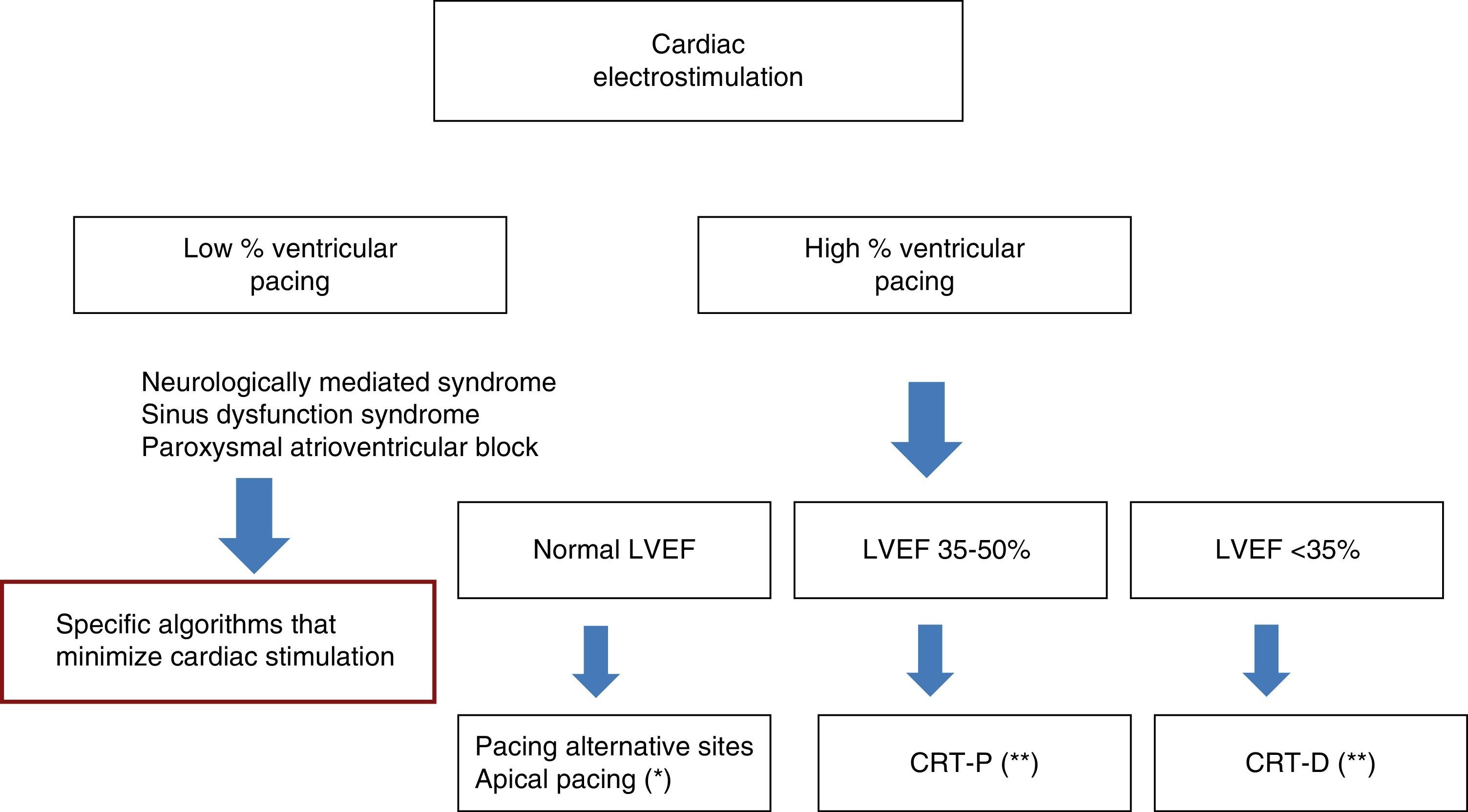
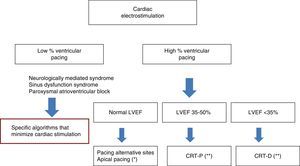
In sum, when deciding the cardiac electrostimulation mode to be used in a given patient, we first must analyze the required percentage of ventricular pacing. If this percentage is low, as in the case of neurologically mediated syndromes, sinus dysfunction syndrome or paroxysmal atrioventricular block, we can pace from the right ventricular apex using algorithms that minimize ventricular pacing (not been addressed in this review).
In contrast, when a high percentage of pacing is contemplated (>60–80%), we must assess ventricular function. If the latter is found to be normal (LVEF >50%), we can pace from the right ventricular apex, taking into account that about 10% of the patients will develop left ventricular dysfunction, and these individuals will have to be identified by echocardiography after one year, with the assessment of possible upgrading to resynchronization. Alternatively, we can pace from other sites such as the ventricular septum (provided we are sure that pacing really takes place from the septum), or resort to Hisian or para-Hisian pacing.
However, in patients with LVEF less than 50% and over 35%, we can opt for cardiac resynchronization therapy. In patients with ischemic heart disease it is advisable to use cardiac MRI to make sure that there is no scarring in the left ventricle segments used for pacing, thereby minimizing the number of non-responders. Lastly, if LVEF <35%, we should add ICD to resynchronization therapy in order to protect the patient against the risk of sudden death. It must be remembered that these indications are to be established on an individualized basis, taking into account the life expectancy of the patient as conditioned by the existing non-cardiac morbidities. In this respect, a life expectancy of over one year is recommended (Fig. 4).
Selection of cardiac electrostimulation mode. *Follow-up during the first year, detecting the group of patients who develop ventricular dysfunction. **Under revision, pending introduction of new criteria fundamentally based on cardiac imaging (late gadolinium enhancement). CRT-D: cardiac resynchronization with defibrillation; CRT-P: cardiac resynchronization without defibrillation; LVEF: left ventricular ejection fraction.
The authors declare that they have no conflicts of interest.
Please cite this article as: Nicolás-Franco S, Rodríguez-González FJ, Nicolás-Boluda A, Sánchez-Martos A. Importancia de la función ventricular en la elección del modo de electroestimulación cardiaca. Med Intensiva. 2015;39:172–178.