Pre-emptive isolation refers to the application of contact precaution measures in patients with strongly suspected colonization by multiresistant bacteria.
ObjectiveTo assess the impact of an intervention program involving the implementation of a consensus-based protocol of pre-emptive isolation (CPPI) on admission to a polyvalent ICU of a general hospital.
MethodsA comparative analysis of 2 patient cohorts was made: a historical cohort including patients in which pre-emptive isolation was established according to physician criterion prior to starting CPPI (from January 2010 to February 2011), and a prospective cohort including patients in which CPPI was implemented (from March to November 2011). CPPI included the identification and diffusion of pre-emptive isolation criteria, the definition of sampling methodology, the evaluation of results, and the development of criteria for discontinuation of pre-emptive isolation. Pre-emptive isolation was indicated by the medical staff, and follow-up was conducted by the nursing staff. Pre-emptive isolation was defined as “adequate” when at least one multiresistant bacteria was identified in any of the samples. Comparison of data between the 2 periods was made with the chi-square test for categorical variables and the Student t-test for quantitative variables. Statistical significance was set at P<.05.
ResultsAmong the 1740 patients admitted to the ICU (1055 during the first period and 685 during the second period), pre-emptive isolation was indicated in 199 (11.4%); 111 (10.5%) of these subjects corresponded to the historical cohort (control group) and 88 (12.8%) to the posterior phase after the implementation of CPPI (intervention group). No differences were found in age, APACHE II score or patient characteristics between the 2 periods. The implementation of CPPI was related to decreases in non-indicated pre-emptive isolations (29.7 vs. 6.8%, P<.001), time of requesting surveillance cultures (1.56 vs. 0.37 days, P<.001), and days of duration of treatment (4.77 vs. 3.58 days, P<.001). In 44 patients (22.1%) in which pre-emptive isolation was indicated, more than one multiresistant bacteria was identified, with an “adequate pre-emptive isolation rate” of 19.8% in the first period and 25.0% in the second period (P<.382).
ConclusionsThe implementation of CPPI resulted in a significant decrease in pre-emptive isolations which were not indicated correctly, a decrease in the time elapsed between isolation and collection of samples, and a decrease in the duration of isolation measures in cases in which isolation was unnecessary, without increasing the rate of “adequate pre-emptive isolation”.
El aislamiento preventivo consiste en la aplicación de medidas de aislamiento de contacto en pacientes con alta sospecha de estar colonizados por bacterias multirresistentes.
ObjetivoEvaluar el impacto de un programa de intervención basado en la implantación de un Protocolo Consensuado de Aislamiento Preventivo (PCAP) al ingreso en una UCI polivalente de un hospital general.
MétodoAnálisis comparativo de 2 cohortes de pacientes, una histórica, que incluye pacientes a los que se indicó el aislamiento preventivo a juicio del médico responsable (enero de 2010 a febrero de 2011), y otra prospectiva, que incluye los pacientes a los que se aplicó el PCAP (marzo a noviembre de 2011). El PCAP incluyó la identificación y divulgación de los criterios de aislamiento preventivo, la metodología a seguir en cuanto a toma de muestras, la valoración de los resultados y los criterios de retirada del aislamiento. La indicación del aislamiento fue realizada por el personal médico, y un equipo de enfermería realizó el seguimiento. Se definió el aislamiento preventivo como «adecuado» cuando en alguna de las muestras iniciales se identificó una bacteria multirresistente. Para la comparación de resultados entre los 2 periodos se utiliza la chi cuadrado para variables cualitativas y la t de Student para variables cuantitativas. Se aceptan como significativas diferencias con p<0,05.
ResultadosDe los 1.740 pacientes ingresados en UCI (1.055 en el primer periodo y 685 en el segundo) se indicó el aislamiento preventivo en 199 (11,4%), de los que 111 (10,5%) correspondieron a la fase histórica (grupo control) y 88 (12,8%) a la fase posterior a la implantación del PCAP (grupo de intervención). No se han detectado diferencias en la edad, el APACHE II y las características de los pacientes entre los 2 periodos. La aplicación del PCAP se ha relacionado con una disminución de los aislamientos preventivos no indicados (29,7 vs. 6,8%, p<0,001), una disminución del tiempo en la solicitud de las muestras de vigilancia (1,56 vs. 0,37 días, p<0,001), y una disminución de la duración en días del aislamiento (4,77 vs. 3,58 días, p<0,001). En 44 pacientes (22,1%) en los que se indicó el aislamiento preventivo se identificaron más de una bacteria multirresistente, siendo la tasa de «aislamiento preventivo adecuado» del 19,8% en el primer periodo y del 25,0% en el segundo (p<0,382).
ConclusionesTras la instauración de PCAP se han reducido significativamente los aislamientos preventivos no indicados correctamente, se ha disminuido el tiempo entre el aislamiento y la toma de muestras, además de reducirse la duración del aislamiento en los casos en que no es necesario, sin que haya aumentado la tasa de «aislamiento preventivo adecuado».
Pre-emptive isolation involves measures designed to avoid contact with patients strongly suspected to be colonized or infected by multiresistant bacteria (MRB).1 The first references to pre-emptive isolation correspond to epidemic or endemic episodes due to methycillin-resistant Staphylococcus aureus (MRSA), where the admission to hospital of patients colonized or infected by this pathogen was shown to be the origin of a subsequent epidemic.2,3 Some authors justify the adoption of pre-emptive isolation measures in high risk patients, and these measures combined with other interventions designed to prevent cross-transmission have been useful in reducing the incidence of new colonization or infection by this pathogen.4–7
Departments of Intensive Care Medicine or Intensive Care Units (ICUs) have a high risk of cross-transmission of MRB, due to the characteristics of the admitted patients, the many control or treatment interventions made, and the use of complex apparatuses that can act as transmission vectors or reservoirs. As a result, some ICUs have proposed the adoption of pre-emptive contact isolation measures upon the admission of patients at a high risk of being colonized by MRB.8,9
The criteria for indicating such isolation have not been well established, and consensus is likewise lacking regarding the samples that must be collected to study the presence of MRB, and the criteria for suspending isolation.10 In our hospital, pre-emptive isolation of critical patients has been indicated for years, in the absence of any pre-established protocol. This has resulted in different care problems (absence of initial vigilance samples, inadequate prolongation of isolation, unjustified indications of isolation, etc.). With the aim of optimizing our pre-emptive isolation strategy, a consensus-based protocol for pre-emptive isolation (CPPI) was developed for application at the time of patient admission to the ICU. This protocol included the criteria or reasons for isolation, the mandatory samples to be collected for study, the criteria for suspending isolation, and the measures to be adopted in those patients in which isolation is indicated. The present study reports the clinical impact of the application of this protocol in the multidisciplinary ICU of a general hospital.
Materials and methodsStudy designAn interventional, comparative study was made of two patient cohorts following a protocolized intervention. The retrospective historical cohort included those patients in which pre-emptive isolation was indicated prior to introduction of the CPPI (January 2010 to February 2011), while the prospective interventional cohort included those patients in which the CPPI was used (March to November 2011).
Description of the Intensive Care UnitOur ICU is a multidisciplinary unit with 18 beds in semi-circularly distributed individual rooms that can be isolated by transparent glass doors. The rooms are equipped with individual washing basins and alcoholic solution dispensing units. Six of the rooms are under negative pressure and are equipped with independent air extractors. The nursing shifts comprise one nurse for every two beds, and a nursing assistant for every 6 beds. All the staff members have received basic training in invasive procedures, and there are written protocols for each of the techniques used. The ICU participates annually in a nosocomial infection vigilance program (ENVIN-HELICS registry). In the year 2011 the invasive device-related infection rate was 12.4 episodes per 1000 days of stay in the ICU (national study: 11.3 episodes per 1000 days of stay). The unit also employs an MRB vigilance program that includes the obtainment of biological samples (rectal and oropharyngeal) once a week (Tuesdays) from all admitted patients.
Management of the projectA working group was created for the study, composed of nurses, nursing assistants and physicians of the ICU, and the hospital nosocomial infection control program supervisor. The working group was in charge of developing and implementing the pre-emptive isolation recommendations in the ICU, and of informing all the team members about the CPPI. The indication of isolation was established by the medical staff, and the nurses and nursing assistants were in charge of applying the decision, and received authorization to demand compliance with isolation on the part of all the healthcare staff intervening in the care of the patient and the visiting relatives. The nursing team completed case report forms (CRFs) of both the historical cases (by reviewing the case histories) and the prospective cases.
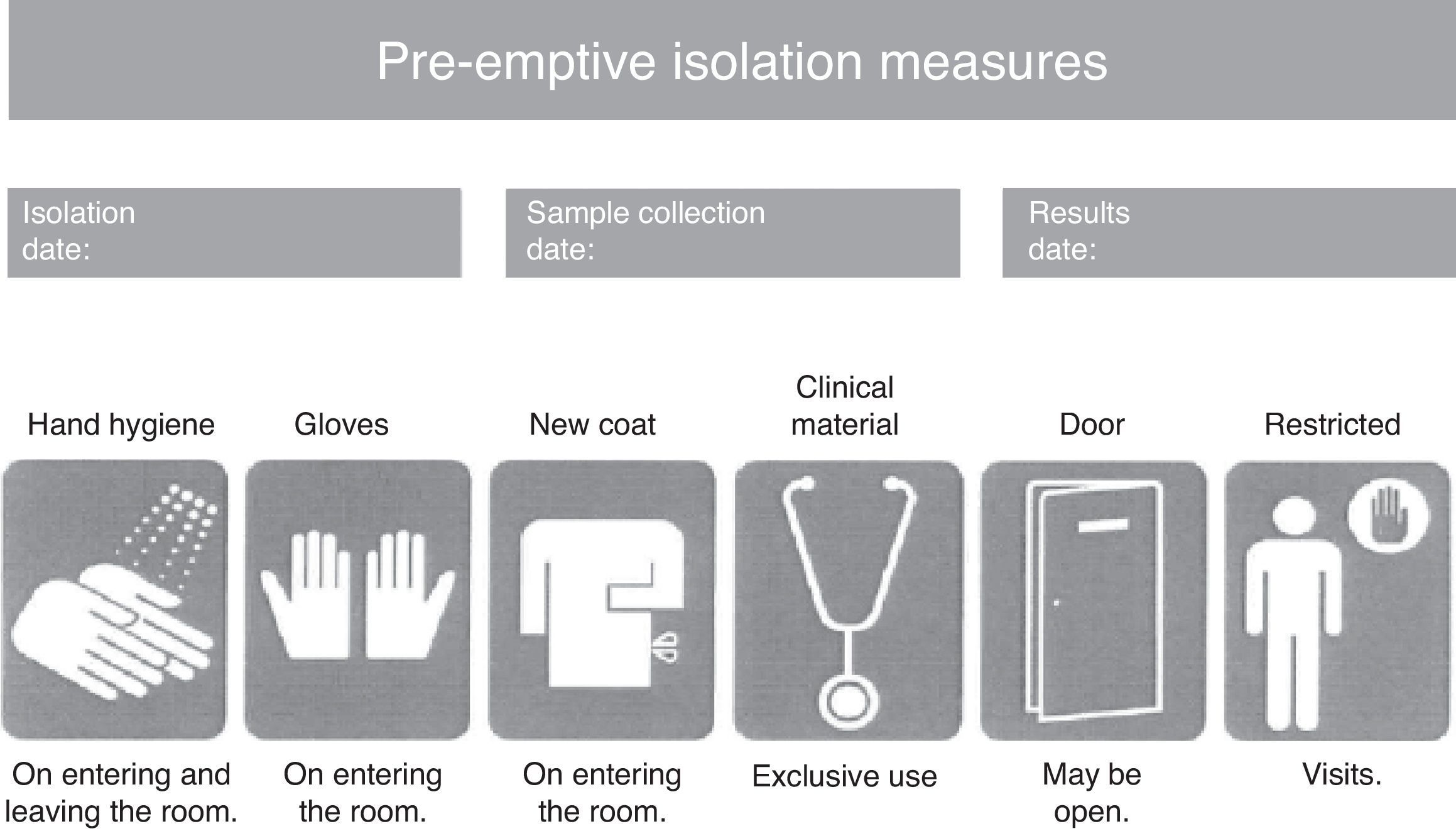
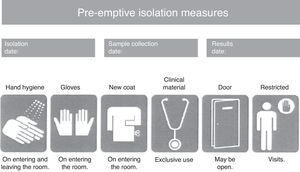
Consensus-based protocol for pre-emptive isolation (CPPI)The CPPI included the clinical criteria indicating pre-emptive isolation, the biological samples to be collected for determining the presence of MRB, the pre-emptive isolation measures to be adopted, and the criteria for suspending isolation. Pre-emptive isolation criteria. The predominant MRB in the hospital where the intervention is applied (multiresistant Pseudomonas aeruginosa) were selected based on a review of the literature and the experience of the working group that designed the CPPI. The following clinical situations were to be assessed upon patient admission to the ICU, using a checklist: (a) previous admission (>7 days) to a hospital center over the last 6 months; (b) frequent contact with healthcare services (hemodialysis, chemotherapy, day hospital, etc.); (c) antibiotic treatment during more than 7 days in the 15 days before admission; (d) history of colonization or infection by an MRB; and (e) chronic ulcers or wounds (pressure, vascular, surgical, etc.). Study samples. In coordination with the Department of Microbiology, rectal and pharyngeal smears were obtained in the first 24h of admission to the ICU. Other samples (blood, respiratory secretion, urine, wound exudates, etc.) were also obtained where considered necessary by the physician attending the patient. Isolation measures. A pre-emptive isolation identification card was placed on the door of the room, indicating the starting date of pre-emptive isolation, the vigilance sample collection date, and the date on which the microbiological results became known. Likewise, the card specified the measures to be adopted during pre-emptive isolation (Fig. 1), including hygiene of the hands on entering and leaving the room, the use of single-use gloves and coat for entering the room, clinical material exclusively used with the patient (sphygmomanometer, stethoscope, etc.), the restriction of visits to the necessary minimum, and the advisability of closing the door to the room (though closing was not considered mandatory). Criteria for suspending isolation. The suspension of pre-emptive isolation was indicated when the control sample cultures failed to identify any MRB in the 5 days after sample collection. Multiresistant pathogens requiring obligate isolation. The following MRB were considered to require obligate contact isolation: methycillin- and/or vancomycin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus spp., extended spectrum betalactamase- or carbapenemase-producing enterobacteria, Pseudomonas aeruginosa resistant to three or more antipseudomonal antibiotic families, and imipenem-resistant Acinetobacter spp. The presence of any of these MRB modified pre-emptive isolation to contact isolation,1,11,12 and required obligate reporting in the hospital isolation registry systems.
Case report form (CRF)A case report form was used to record the patient demographic data, background disease, risk factors (comorbidities and extrinsic factors at the time of admission), criteria indicating isolation, sample collection date and results of the sample studies, and isolation starting and ending dates. The identification of MRB in patients admitted to the ICU was carried out by the Department of Microbiology of the Reference Laboratory of Catalonia, following the usual procedures applicable to vigilance samples.
Assessment criteriaThe criteria used for assessing application of the CPPI were the “correctly indicated isolation” rate, the “adequate isolation” rate, the days of stay in the ICU until the request for vigilance samples, and the duration (in days) of pre-emptive isolation. The “correctly indicated isolation” rate was defined as the number of isolations made following the approved indications included in the checklist, divided by the total number of pre-emptive isolations multiplied by 100, while the “adequate isolation” rate was defined as the number of pre-emptive isolations in which the presence of an MRB requiring obligate isolation in the ICU of our hospital was confirmed, divided by the number of indicated pre-emptive isolations multiplied by 100. The “adequate isolation” rate was likewise calculated for each of the reasons for patient isolation. The clinical impact of the CPPI was assessed on the basis of the duration of pre-emptive isolation and the time elapsed between the indication of pre-emptive isolation and the collection of vigilance samples.
Statistical analysisContinuous variables were expressed as the mean and standard deviation (SD) or as the median and percentiles 25 and 75, depending on whether the variable exhibited a normal distribution or not. Categorical variables in turn were expressed as frequencies and percentages. The comparison of groups (control group versus intervention group) was based on the chi-squared test or Fisher exact test for qualitative variables, and the Student t-test or Mann–Whitney U-test in the case of quantitative variables. Statistical significance was considered for p<0.05.
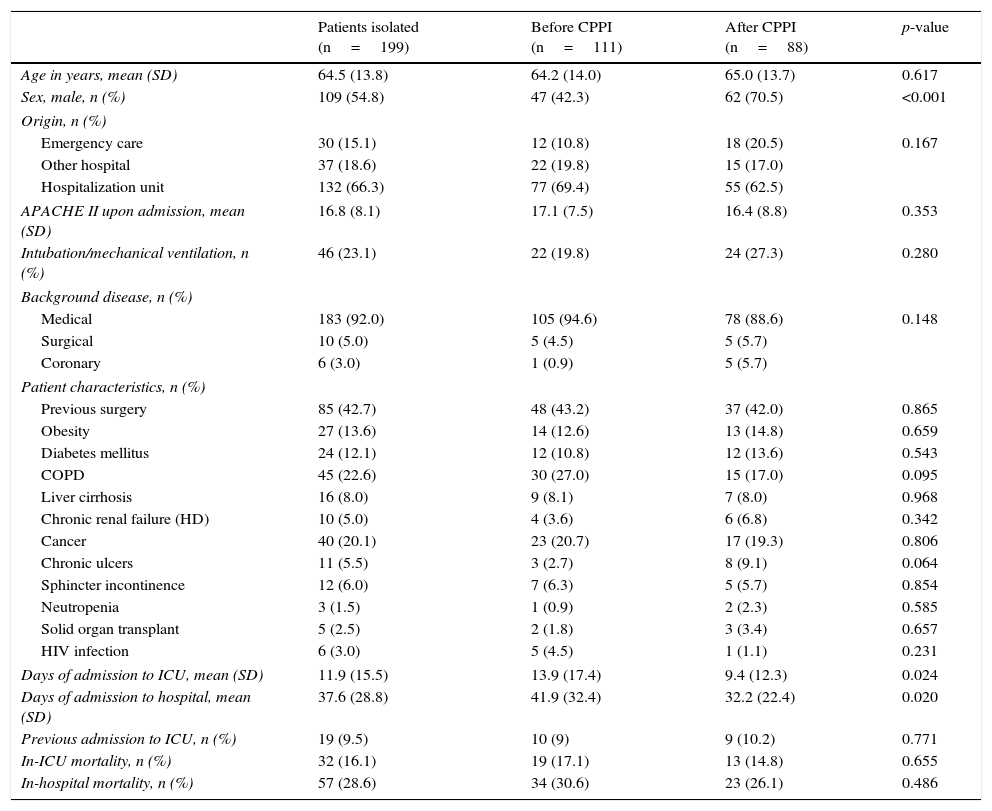
ResultsOf the 1740 patients admitted to the ICU (1055 in the first study period and 685 in the second), pre-emptive isolation was indicated in 199 cases (11.4%). A total of 111 of these patients (10.5%) corresponded to the historical phase (control group) and 88 (12.8%) to the phase following introduction of the CPPI (intervention group). The mean age (±SD) of the patients in which isolation was indicated was 64.5±13.8 years; 109 were males (54.8%); and severity as rated by the APACHE II score upon admission to the ICU was 16.8±8.1. There was a predominance of medical cases (n=183; 92.0%). Nineteen of the patients (9.5%) had been previously admitted to intensive care. The mean duration of stay in the ICU was 11.9±15.5 days, versus a mean hospital stay of 37.6±28.8 days. Thirty-two patients (16.1%) died in the ICU, and another 25 patients (12.6%) died in hospital after discharge from the ICU. The cumulative in-hospital mortality rate was 28.6%. Table 1 shows the characteristics of the study groups. There were no differences between groups in terms of age, background disease, severity score or comorbidities. However, the male gender was seen to predominate in the intervention group (70.5% versus 42.3% in the control group; p<0.001).
Characteristics of the study population in each of the periods analyzed.
| Patients isolated (n=199) | Before CPPI (n=111) | After CPPI (n=88) | p-value | |
|---|---|---|---|---|
| Age in years, mean (SD) | 64.5 (13.8) | 64.2 (14.0) | 65.0 (13.7) | 0.617 |
| Sex, male, n (%) | 109 (54.8) | 47 (42.3) | 62 (70.5) | <0.001 |
| Origin, n (%) | ||||
| Emergency care | 30 (15.1) | 12 (10.8) | 18 (20.5) | 0.167 |
| Other hospital | 37 (18.6) | 22 (19.8) | 15 (17.0) | |
| Hospitalization unit | 132 (66.3) | 77 (69.4) | 55 (62.5) | |
| APACHE II upon admission, mean (SD) | 16.8 (8.1) | 17.1 (7.5) | 16.4 (8.8) | 0.353 |
| Intubation/mechanical ventilation, n (%) | 46 (23.1) | 22 (19.8) | 24 (27.3) | 0.280 |
| Background disease, n (%) | ||||
| Medical | 183 (92.0) | 105 (94.6) | 78 (88.6) | 0.148 |
| Surgical | 10 (5.0) | 5 (4.5) | 5 (5.7) | |
| Coronary | 6 (3.0) | 1 (0.9) | 5 (5.7) | |
| Patient characteristics, n (%) | ||||
| Previous surgery | 85 (42.7) | 48 (43.2) | 37 (42.0) | 0.865 |
| Obesity | 27 (13.6) | 14 (12.6) | 13 (14.8) | 0.659 |
| Diabetes mellitus | 24 (12.1) | 12 (10.8) | 12 (13.6) | 0.543 |
| COPD | 45 (22.6) | 30 (27.0) | 15 (17.0) | 0.095 |
| Liver cirrhosis | 16 (8.0) | 9 (8.1) | 7 (8.0) | 0.968 |
| Chronic renal failure (HD) | 10 (5.0) | 4 (3.6) | 6 (6.8) | 0.342 |
| Cancer | 40 (20.1) | 23 (20.7) | 17 (19.3) | 0.806 |
| Chronic ulcers | 11 (5.5) | 3 (2.7) | 8 (9.1) | 0.064 |
| Sphincter incontinence | 12 (6.0) | 7 (6.3) | 5 (5.7) | 0.854 |
| Neutropenia | 3 (1.5) | 1 (0.9) | 2 (2.3) | 0.585 |
| Solid organ transplant | 5 (2.5) | 2 (1.8) | 3 (3.4) | 0.657 |
| HIV infection | 6 (3.0) | 5 (4.5) | 1 (1.1) | 0.231 |
| Days of admission to ICU, mean (SD) | 11.9 (15.5) | 13.9 (17.4) | 9.4 (12.3) | 0.024 |
| Days of admission to hospital, mean (SD) | 37.6 (28.8) | 41.9 (32.4) | 32.2 (22.4) | 0.020 |
| Previous admission to ICU, n (%) | 19 (9.5) | 10 (9) | 9 (10.2) | 0.771 |
| In-ICU mortality, n (%) | 32 (16.1) | 19 (17.1) | 13 (14.8) | 0.655 |
| In-hospital mortality, n (%) | 57 (28.6) | 34 (30.6) | 23 (26.1) | 0.486 |
APACHE: Acute Physiology and Chronic Health Evaluation; SD: standard deviation; COPD: chronic obstructive pulmonary disease; HD: hemodialysis; CPPI: consensus-based protocol for pre-emptive isolation; ICU: Intensive Care Unit; HIV: human immunodeficiency virus.
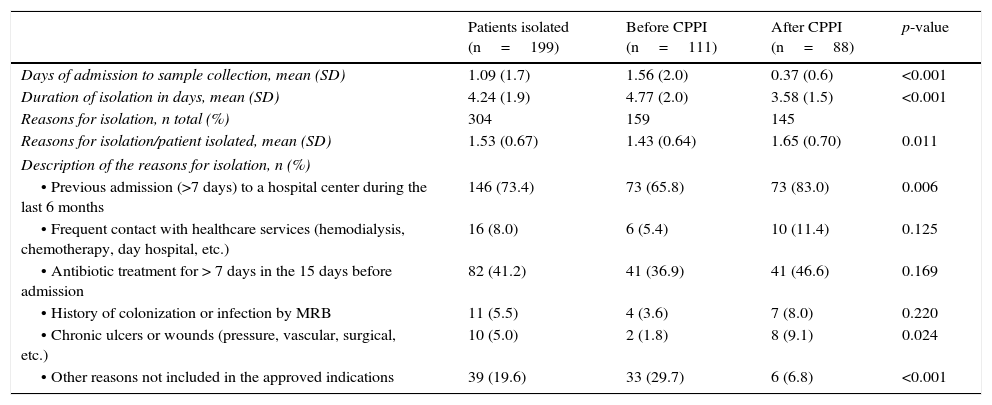
The reasons for indicating pre-emptive isolation are reported in Table 2, differentiating between the period before and after introduction of the CPPI. In both periods the predominant indication was admission to a hospital center during more than 7 days in the last 6 months (including current admission) and the use of antibiotics during 7 or more days in the 15 days prior to admission to the ICU. The rest of the indications were much less frequent. The second period was characterized by a significant increase in the indication of isolation due to the presence of chronic ulcers or wounds (1.8% versus 6.8%; p=0.024) and a significant decrease in indications without clinical criterion for isolation (29.7% versus 6.8%; p<0.001). The “correctly indicated isolation” rate was seen to increase following introduction of the CPPI, from 70.3% to 93.2% (p<0.001).
Characteristics of pre-emptive isolation in each of the periods analyzed.
| Patients isolated (n=199) | Before CPPI (n=111) | After CPPI (n=88) | p-value | |
|---|---|---|---|---|
| Days of admission to sample collection, mean (SD) | 1.09 (1.7) | 1.56 (2.0) | 0.37 (0.6) | <0.001 |
| Duration of isolation in days, mean (SD) | 4.24 (1.9) | 4.77 (2.0) | 3.58 (1.5) | <0.001 |
| Reasons for isolation, n total (%) | 304 | 159 | 145 | |
| Reasons for isolation/patient isolated, mean (SD) | 1.53 (0.67) | 1.43 (0.64) | 1.65 (0.70) | 0.011 |
| Description of the reasons for isolation, n (%) | ||||
| • Previous admission (>7 days) to a hospital center during the last 6 months | 146 (73.4) | 73 (65.8) | 73 (83.0) | 0.006 |
| • Frequent contact with healthcare services (hemodialysis, chemotherapy, day hospital, etc.) | 16 (8.0) | 6 (5.4) | 10 (11.4) | 0.125 |
| • Antibiotic treatment for > 7 days in the 15 days before admission | 82 (41.2) | 41 (36.9) | 41 (46.6) | 0.169 |
| • History of colonization or infection by MRB | 11 (5.5) | 4 (3.6) | 7 (8.0) | 0.220 |
| • Chronic ulcers or wounds (pressure, vascular, surgical, etc.) | 10 (5.0) | 2 (1.8) | 8 (9.1) | 0.024 |
| • Other reasons not included in the approved indications | 39 (19.6) | 33 (29.7) | 6 (6.8) | <0.001 |
MRB: multiresistant bacteria; SD: standard deviation; CPPI: consensus-based protocol for pre-emptive isolation
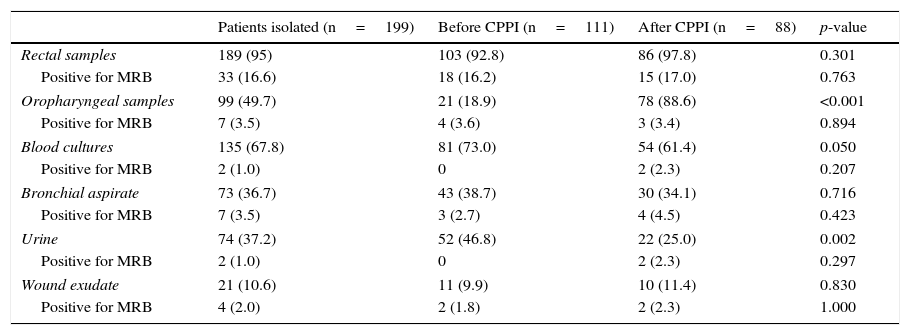
One or more samples for vigilance study were collected in 189 patients (95% of the total). Rectal samples predominated (95% of the patients), along with pharyngeal exudates (49.7%) and blood samples (67.8%). Following introduction of the CPPI in the second study period, a significant increase was recorded in the number of oropharyngeal samples (18.9% versus 88.6%; p<0.001), with a decrease in urine (46.8% versus 25.0%; p=0.002) and blood samples (73.0% versus 61.4%; p=0.050). In turn, the time elapsed between admission to the ICU and sample collection decreased significantly between the two study periods, from 1.56±2.0 days in the control group to 0.37±0.6 days in the intervention group (p<0.001). Likewise, the duration of pre-emptive isolation decreased from 4.77±2.0 days to 3.58±1.4 days (p<0.001). Table 3 shows the samples obtained in each period, as well as their yield in identifying MRB. The rectal samples were seen to be more effective in identifying MRB in both study periods (16.2 versus 17.0%; p=0.763), while the effectiveness of the rest of the samples was very low (<5%).
Yield (effectiveness) of the vigilance samples.
| Patients isolated (n=199) | Before CPPI (n=111) | After CPPI (n=88) | p-value | |
|---|---|---|---|---|
| Rectal samples | 189 (95) | 103 (92.8) | 86 (97.8) | 0.301 |
| Positive for MRB | 33 (16.6) | 18 (16.2) | 15 (17.0) | 0.763 |
| Oropharyngeal samples | 99 (49.7) | 21 (18.9) | 78 (88.6) | <0.001 |
| Positive for MRB | 7 (3.5) | 4 (3.6) | 3 (3.4) | 0.894 |
| Blood cultures | 135 (67.8) | 81 (73.0) | 54 (61.4) | 0.050 |
| Positive for MRB | 2 (1.0) | 0 | 2 (2.3) | 0.207 |
| Bronchial aspirate | 73 (36.7) | 43 (38.7) | 30 (34.1) | 0.716 |
| Positive for MRB | 7 (3.5) | 3 (2.7) | 4 (4.5) | 0.423 |
| Urine | 74 (37.2) | 52 (46.8) | 22 (25.0) | 0.002 |
| Positive for MRB | 2 (1.0) | 0 | 2 (2.3) | 0.297 |
| Wound exudate | 21 (10.6) | 11 (9.9) | 10 (11.4) | 0.830 |
| Positive for MRB | 4 (2.0) | 2 (1.8) | 2 (2.3) | 1.000 |
MRB: multiresistant bacteria; CPPI: consensus-based protocol for pre-emptive isolation. Data expressed as n (%).
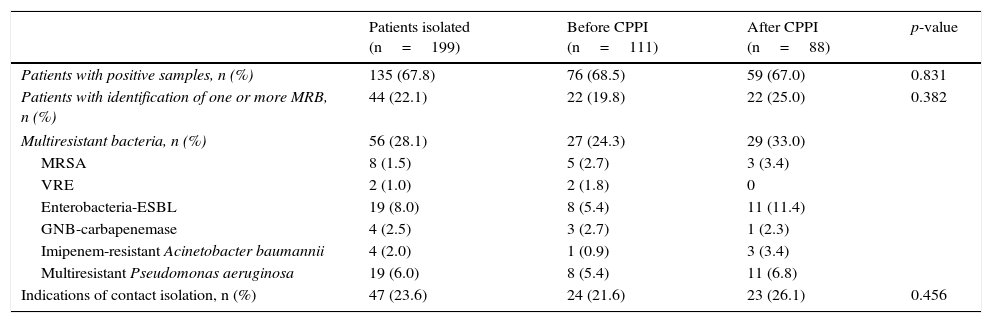
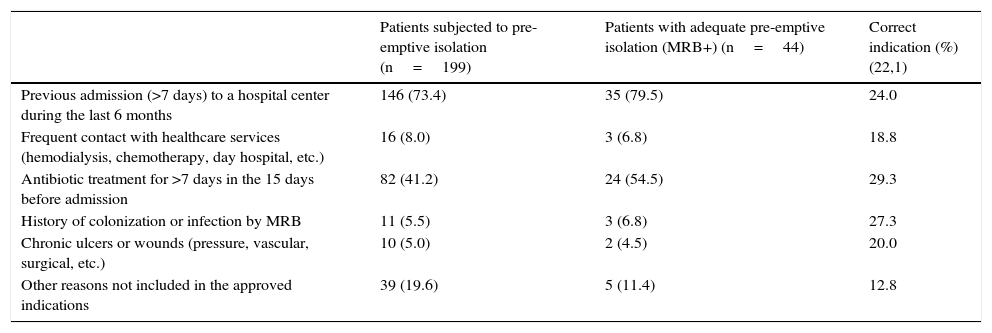
One or more pathogens were identified in the vigilance samples or in the samples obtained upon admission in 135 of the patients subjected to pre-emptive isolation (67.8%). In turn, 56 MRB were identified in 44 of these patients (22.1%)–their distribution in the two study periods being shown in Table 4. The “adequate isolation” rate was 19.8% in the period before application of the CPPI (control group) and 25% in the period after application of the CPPI (intervention group) (p=0.382). The “adequate isolation” rates corresponding to the different criteria used to indicate pre-emptive isolation are shown in Table 5. Of note is the use of antibiotics during more than 7 days in the 15 days before admission to the ICU (29.3%), a history of colonization or infection by some MRB (27.3%), and admission to a hospital center in the last 6 months (24%).
Microorganisms identified in the vigilance and clinical samples upon admission to the Intensive Care Unit in the patients subjected to pre-emptive isolation in each of the periods analyzed.
| Patients isolated (n=199) | Before CPPI (n=111) | After CPPI (n=88) | p-value | |
|---|---|---|---|---|
| Patients with positive samples, n (%) | 135 (67.8) | 76 (68.5) | 59 (67.0) | 0.831 |
| Patients with identification of one or more MRB, n (%) | 44 (22.1) | 22 (19.8) | 22 (25.0) | 0.382 |
| Multiresistant bacteria, n (%) | 56 (28.1) | 27 (24.3) | 29 (33.0) | |
| MRSA | 8 (1.5) | 5 (2.7) | 3 (3.4) | |
| VRE | 2 (1.0) | 2 (1.8) | 0 | |
| Enterobacteria-ESBL | 19 (8.0) | 8 (5.4) | 11 (11.4) | |
| GNB-carbapenemase | 4 (2.5) | 3 (2.7) | 1 (2.3) | |
| Imipenem-resistant Acinetobacter baumannii | 4 (2.0) | 1 (0.9) | 3 (3.4) | |
| Multiresistant Pseudomonas aeruginosa | 19 (6.0) | 8 (5.4) | 11 (6.8) | |
| Indications of contact isolation, n (%) | 47 (23.6) | 24 (21.6) | 23 (26.1) | 0.456 |
GNB: gram negative bacilli; BLEE: extended-spectrum betalactamase; MRB: multiresistant bacteria; VRE: vancomycin-resistant Enterococcus spp.; CPPI: consensus-based protocol for pre-emptive isolation; MRSA: methicillin-resistant Staphylococcus aureus.
Reasons for indicating pre-emptive isolation and percentage correct indication.
| Patients subjected to pre-emptive isolation (n=199) | Patients with adequate pre-emptive isolation (MRB+) (n=44) | Correct indication (%) (22,1) | |
|---|---|---|---|
| Previous admission (>7 days) to a hospital center during the last 6 months | 146 (73.4) | 35 (79.5) | 24.0 |
| Frequent contact with healthcare services (hemodialysis, chemotherapy, day hospital, etc.) | 16 (8.0) | 3 (6.8) | 18.8 |
| Antibiotic treatment for >7 days in the 15 days before admission | 82 (41.2) | 24 (54.5) | 29.3 |
| History of colonization or infection by MRB | 11 (5.5) | 3 (6.8) | 27.3 |
| Chronic ulcers or wounds (pressure, vascular, surgical, etc.) | 10 (5.0) | 2 (4.5) | 20.0 |
| Other reasons not included in the approved indications | 39 (19.6) | 5 (11.4) | 12.8 |
MRB: multiresistant bacteria.
The application of a consensus-based protocol for pre-emptive isolation (CPPI) in the multidisciplinary ICU of a general hospital has been found to be associated to a significant increase in correct patient isolations and to a decrease in delay in obtaining samples at patient admission to intensive care, as well as a shortening of pre-emptive isolations in those patients who did not need isolation measures. In contrast, there was no significant increase in the adequate isolation rate, which remained in the range of 25% after introduction of the CPPI, thereby meaning that four pre-emptive isolations would have to be indicated in order to avoid one risk of cross-transmission in a patient with MRB.
Although different clinical and microbiological criteria have been proposed for indicating pre-emptive isolation of patients admitted to hospital,1,11,12 no specific criteria have been developed in the case of patients requiring admission to the ICU. In our study, the most frequent reason for indicating pre-emptive isolation was admission to a hospital center during more than 7 days in the 6 months before admission to the ICU. This definition includes patients admitted to hospital during more than 7 days before admission to the ICU. On the other hand, the use of antimicrobials during more than 7 days in the 15 days before admission was also used as criterion for pre-emptive patient isolation. In many cases both criteria coincide in one same patient. The yield or effectiveness of these two criteria in identifying patients with MRB is in the range of 25%. Both criteria have been previously identified in multivariate analyses designed to explore risk factors for methicillin-resistant Staphylococcus aureus carrier status among patients upon admission to hospital13–17 or the ICU.18 These criteria, with some modifications, were proposed by the American Thoracic Society and the Infectious Disease Society of America in screening for patients at risk of presenting MRB as a cause of healthcare-related pneumonia.19 Posteriorly, Nseir et al. analyzed the capacity of these criteria to predict MRB colonization or infection upon admission to the ICU,20 and identified the use of antibiotic treatment in the three days before admission to the ICU, previous hospitalization during two days or more in the three months before admission to the ICU, and patient referral from sociosanitary care centers as independent factors associated to MRB upon admission to the ICU.
The application of contact precaution measures in hospitalized patients is not without risks, and the medical literature has associated such measures to a decrease in patient care by the healthcare staff, an increase in patient depression and anxiety, lesser satisfaction with the care received, and an increase in recorded adverse effects.21–25 A study of adverse effects in the ICU has shown isolated patients to suffer more such effects, and particularly a larger number of errors in prescribing anticoagulants, as well as a greater frequency of detection of hypo- and hyperglycemia.26 The results of our study indicate the need to establish more specific criteria in order to optimize the indication of this type of isolation in critical patients, and to avoid needless isolation.
In the present study, la identification of MRB was made fundamentally in rectal samples, and less frequently in oropharyngeal samples. The identification of MRB in clinical samples has been very low, which justifies the active search for MRB upon admission to the ICU through the collection of vigilance samples. At present, it is debated whether such sampling should be limited to patients at risk of colonization or should be performed on a universal basis.26,27
The creation of mixed working groups headed by nursing staff is an important development for improving the application of pre-emptive isolation strategies.28 Our study shows the application of a CPPI headed by nursing staff to be associated to a greater number of correctly indicated isolations, a greater number of recommended samplings and a decrease in unnecessary samplings, and a shortening of the time elapsed between patient admission to the ICU and the collection of samples for vigilance studies. All this has been possible thanks to the active participation of the ICU nursing staff, which has carried the responsibility of managing the identification of isolation criteria and the collection of vigilance samples. Capacitation of the nursing staff in turn has been completed with the responsibility of complying (and ensuring compliance) with the pre-emptive isolation norms on the part of all the healthcare staff members that assist the patients and their families.
Our study has shown that application of the CPPI results in a significant decrease in pre-emptive isolation time. This is attributable to shortening of the interval between patient admission to the ICU and vigilance sample collection and processing, as well as to the decision to suspend isolation in the case of patients for which the sample results had not been received from the Department of Microbiology by day 5 of admission. This decision was made on examining the results of the vigilance samples corresponding to the control group (i.e., the pre-CPPI period), where it was seen that the MRB-positive cases were reported within the first 5 days after sending the samples to the laboratory. The risk of MRB in those samples without reported findings after 5 days was zero.
The main limitation of this study is represented by the fact that it is a single-center investigation, and the conclusions drawn are only applicable to that setting. Likewise, part of the information was obtained on a retrospective basis, and some of the variables entered in the CRFs were not found in the case histories.
In conclusion, our study for the first time proposes pre-emptive isolation criteria referred to a polyvalent ICU, with the creation of multidisciplinary working groups in order to optimize their application with the purpose of minimizing the spread of MRB in the ICU. The clinical impact of the CPPI has been evidenced by an increase in the “correctly indicated isolation” rate and a decrease in delay in sample collection, unnecessary isolation days, and the duration of indicated patient isolation – thereby reducing the total days of pre-emptive isolation. However, in reference to critical patients, we need new studies or the introduction of new rapid MRB identification techniques allowing the early identification of MRB carries and the avoidance of unnecessary pre-emptive isolations.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The authors thank Marta Gas-Amat, Secretary of the Department of Intensive Care Medicine of Hospital del Mar, for her collaboration in preparing the CRFs and database, and Sergio Mojal, of the Statistics Department of the Fundación Instituto Mar de Investigaciones Médicas (IMIM) of Barcelona.
Please cite this article as: Álvarez Lerma F, Granado Solano J, García Sanz A, López Martínez C, Herrera Sebastián R, Salvat Cobeta C, et al. Optimización de los aislamientos preventivos en una UCI polivalente mediante la aplicación de un plan de intervención. 2015;39:543–551.