Parthanatos is a form of programmed cell death mediated by apoptosis-inducing factor (AIF). However, there are not data on parthanatos in septic patients. The objective of the current study was to explore whether parthanatos is associated with mortality of septic patients.
DesignObservational and prospective study.
SettingThree Spanish Intensive Care Units during 2017.
PatientsPatients with sepsis according to Sepsis-3 Consensus criteria.
InterventionsSerum AIF concentrations were determined at moment of sepsis diagnosis.
Main variable of interestMortality at 30 days.
ResultsThere were included 195 septic patients, and non-surviving (n=72) had serum AIF levels (p<0.001), lactic acid (p<0.001) and APACHE-II (p<0.001) that surviving (n=123). Multiple logistic regression analysis showed that patients with serum AIF levels>55.6ng/mL had higher mortality risk (OR=3.290; 95% CI=1.551−6.979; p=0.002) controlling for age, SOFA and lactic acid.
ConclusionsParthanatos is associated with mortality of septic patients.
Parthanatos es un tipo de muerte celular programada mediada por el factor inductor de apoptosis (AIF). Sin embargo, no hay datos sobre Parthanatos en pacientes sépticos. Por ello, el objetivo de este estudio fue explorar si Parthanatos está asociado con la morlaidad de los pacientes sépticos.
DiseñoEstudio observacional y prospective.
ÁmbitoTres Unidades de Cuidados Intensivos españolas durante 2017.
PacientesPacientes con sepsis en base a los criterios del Consenso Sepsis-3.
IntervencionesSe determinaron las concentraciones séricas de AIF en el momento del diagnóstico de la sepsis.
Variable de interés principalMortalidad a los 30 días.
ResultadosSe incluyeron 195 pacientes sépticos, y los que fallecieron (n=72) presentaron mayores concentraciones séricas de AIF (p<0.001) y de ácido láctico (p<0.001), y mayor puntuación APACHE-II (p<0.001) que los pacientes supervivientes (n=123). El análisis de regresión logística múltiple mostró que los pacientes con concentraciones séricas de AIF>55.6ng/mL tuvieron mayor riesgo de fallecer (OR=3.290; 95% CI=1.551−6.979; p=0.002) controlando por edad, SOFA y ácido láctico.
ConclusionesParthanatos está asociado con la mortalidad de pacientes sépticos.
Many deaths and a great consumption of healthcare resources are produced yearly due to sepsis.1,2 Parthanatos is a form of programmed cell death mediated by poly ADP-ribose polymerase 1 (PARP1) and apoptosis-inducing factor (AIF).3–8 Toxic stimuli such as reactive oxygen species (ROS), ischemia, alkylating agents (e.g., methyl-nitro-nitroso-guanidine; MNNG), ultraviolet radiation (UVR), ionizing radiation (IR) activate PARP-1 in the nucleus. PARP-1 overactivation produces free Poly (ADP-Ribose) (PAR) by Poly (ADP-Ribose) Glycohydrolase (PARG)-mediated hydrolyzation. PAR serves as a death signal from the nucleus to mitochondria, inducing the release of AIF from mitochondrial intermembrane space to cytosol. Afterwards AIF translocate to the nucleus and induces chromatinolysis.3–8
Parthanatos exists in different diseases (neurological diseases, retinal diseases, diabetes, renal diseases, tumors, cardiovascular diseases and ischemia-reperfusion injury).3–8 Parthanatos (with PARP-1 activation, AIF translocation from mitochondria to the nucleus and DNA fragmentation) has been found in patients with infection by Mycobacterium tuberculosis, hepatitis B virus, cytomegalovirus, John Cunningham (JC) virus, herpes simplex virus and avian influenza A (H5N1) virus.9–13 In one study, mice received injection intraperitoneal of lipopolysaccharide (LPS) and the authors found PARP-1 activation, PAR formation, AIF translocation from mitochondria to the nucleus and cell death in macrophages; and that the administration of agents that block PARP-1 (3-AB and erlotinib) reduced all these phenomena in macrophages and increased survival of mice.14 In other study, mice were underwent to cecal ligation and puncture (CLP), and human alveolar epithelial cells were treated with LPS and the authors found increased AIF release from mitochondria to the nucleus and cell death of human alveolar epithelial cells; and that the administration of heat shock protein 70 (HSP70) reduced AIF release from mitochondria and cell death of human alveolar epithelial cells, and reduced septic lung injury and sepsis mortality of mice.15 However, there are not data on parthanatos in septic patients. The primary objective of the current study was to explore whether parthanatos is associated with mortality of septic patients. The secondary objective was to explore whether parthanatos is also associated with mortality of septic shock patients.
Material and methodsDesign and subjectsIn this observational study were recruited septic patients from three Intensive Care Units during 2017 with approval of Ethics Committee of each hospital (H. Universitario de Canarias, H. Universitario Nuestra Señora de Candelaria and H. Universitario de la Palma). Informed and signed consent was obtained from patients or their relatives.
Sepsis and septic shock were defined according to Sepsis-3 Consensus.16 Exclusion criteria were hematological tumor, solid tumor, human immunodeficiency virus (HIV), white blood cell count <1000/μL, radiation therapy, chemoterapy, immunosuppressive therapy (such as methotrexate, micofenolato, infliximab, tocilizumab, rituximab, etc.), oral steroid agents and age <18 years.
In each patient was registered age and sex. We also registered the history of chronic renal failure, diabetes mellitus, ischemic heart disease or chronic obstructive pulmonary disease. Besides, we registered leukocytes, platelets, activated partial thromboplastin time (aPTT), international normalized ratio (INR), pressure arterial of oxygen (PaO2), fraction inspired of oxygen (FIO2), creatinine, bilirubin, lactic acid. In addition, we registered Sepsis-related Organ Failure Assessment [SOFA] score,17 Acute Physiology and Chronic Health Evaluation (APACHE)-II score,18 site of infection, and the presence of septic shock or of bloodstream infection. The main outcome variable was 30-day mortality.
Some of those patients were included in a previous publication by our team (determining serum survivin concentrations)19 and in the current research serum AIF concentrations were determined.
Determination of serum AIF concentrationsSera from patients at the time of sepsis diagnosis were obtained and frozen at -80°C. All determinations of serum AIF levels were performed at the same time at the end of patient recruitment with the Human Apoptosis Inducing Factor ELISA Kit (Elabscience, Houston, Texas, United States). The assay detection limit was 0.16ng/mL, and the intra-assay and inter-assay coefficient of variation (CV) were <10%.
Statistical methodsMedians (percentile 25−75) and Mann–Whitney U test were used to describe and compare continuous variables. Frequencies (percentages) and chi-square test were used to describe and compare categorical variables. A receiver operating characteristic (ROC) analysis was carried out to determine the utility of serum AIF levels, SOFA score, APACHE-II score and acid lactic levels for mortality prediction. We compared area under curve (AUC) for mortality prediction of serum AIF levels with SOFA score, APACHE-II score, lactic acid levels, the combination of serum AIF levels and SOFA score, the combination of serum AIF levels and APACHE-II score, and the combination of serum AIF levels and lactic acid levels using the method of DeLong et al.20 We constructed Kaplan-Meier survival curves using cut-point of serum AIF of 55.6ng/mL (level selected by Youden J index). A multiple logistic regression analysis was performed to determine whether patients with serum AIF levels>55.6ng/mL had higher mortality risk controlling for age, SOFA and lactic acid. We did not include septic shock in the regression analysis because it is one variable of SOFA score; and the same variables were included in another regression analysis performed specifically with septic shock patients. Statistical analysis was performed with SPSS 25.0 (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.).
ResultsThere were included 195 septic patients, and non-surviving patients (n=72) in respect to surviving patients (n=123) were older (p=0.001), had lower platelet count (p=0.001), and had higher rate of septic shock (p=0.001), aPTT (p=0.01), creatinine (p=0.02), lactic acid (p<0.001), APACHE-II (p<0.001), SOFA (p<0.001) and serum AIF levels (p<0.001) (Table 1).
Comparisons between non-surviving and surviving septic patients on demographic and clinical characteristics at moment of sepsis diagnosis.
| Survivors (n=123) | Non-survivors (n=72) | p-Value | |
|---|---|---|---|
| Sex female — n (%) | 43 (35.0) | 25 (34.7) | 0.97 |
| Age — median years (p 25−75) | 55 (44−66) | 64 (54−74) | 0.001 |
| COPD — n (%) | 13 (10.6) | 12 (16.7) | 0.22 |
| Ischemic heart disease — n (%) | 11 (8.9) | 5 (6.9) | 0.62 |
| Diabetes mellitus — n (%) | 36 (29.3) | 28 (38.9) | 0.17 |
| Chronic renal failure — n (%) | 7 (5.7) | 6 (8.3) | 0.48 |
| PaO2/FIO2 ratio — median (p 25−75) | 168 (116−261) | 170 (99−237) | 0.36 |
| Leukocytes (cells/mm3) — median*103 (p 25−75) | 14.1 (9.3−18.4) | 13.8 (7.6−19.8) | 0.61 |
| Platelets (cells/mm3) — median*103 (p 25−75) | 197 (133−274) | 133 (71−219) | 0.001 |
| INR — median (p 25−75) | 1.27 (1.10−1.53) | 1.42 (1.13−1.86) | 0.052 |
| aPTT (seconds) — median (p 25−75) | 32 (28−40) | 36 (30−45) | 0.01 |
| Bilirubin (mg/dl) — median (p 25−75) | 0.90 (0.41−1.50) | 0.80 (0.50−2.00) | 0.97 |
| Lactic acid (mmol/L) — median (p 25−75) | 1.70 (1.10−3.30) | 3.30 (1.43−5.38) | <0.001 |
| Creatinine (mg/dl) — median (p 25−75) | 1.15 (0.80−1.90) | 1.50 (0.95−2.75) | 0.02 |
| Septic shock — n (%) | 51 (41.5) | 48 (66.7) | 0.001 |
| Bloodstream infection — n (%) | 20 (16.3) | 9 (12.5) | 0.48 |
| Sepsis focus | 0.62 | ||
| Respiratory — n (%) | 72 (58.5) | 44 (61.1) | |
| Abdominal — n (%) | 32 (26.0) | 16 (22.2) | |
| Neurological | 3 (2.4) | 0 | |
| Urinary — n (%) | 6 (4.9) | 4 (5.6) | |
| Skin — n (%) | 6 (4.9) | 3 (4.2) | |
| Endocarditis — n (%) | 4 (3.3) | 4 (5.6) | |
| Osteomyelitis — n (%) | 0 | 1 (1.4) | |
| Empiric antimicrobial treatment adequate | 0.90 | ||
| Unknown due to negative cultures — n (%) | 67 (54.5) | 39 (54.2) | |
| Adequate — n (%) | 49 (39.8) | 27 (37.5) | |
| Inadequate — n (%) | 2 (1.6) | 2 (2.8) | |
| Unknown due to antigenuria diagnosis — n (%) | 5 (4.1) | 4 (5.6) | |
| Microorganism responsibles | |||
| Unknwon — n (% | 67 (54.5) | 39 (54.2) | 0.97 |
| Gram-positive — n (%) | 26 (21.1) | 18 (25.0) | 0.53 |
| Gram-negative — n (%) | 30 (24.4) | 14 (19.4) | 0.43 |
| Fungii— n (%) | 3 (2.4) | 3 (4.2) | 0.50 |
| Anaerobe — n (%) | 1 (0.8) | 1 (1.4) | 0.70 |
| APACHE-II score — median (p 25−75) | 18 (14−23) | 23 (18−28) | <0.001 |
| SOFA score — median (p 25−75) | 9 (7−11) | 11 (9−14) | <0.001 |
| AIF (ng/mL) — median (p 25−75) | 43 (37−51) | 51 (43−60) | <0.001 |
COPD=chronic obstructive pulmonary disease; PaO2/FIO2=pressure of arterial oxygen/fraction inspired oxygen; aPTT=activated partial thromboplastin time; INR=international normalized ratio; APACHE=Acute Physiology and Chronic Health Evaluation; SOFA=Sepsis-related Organ Failure Assessment; AIF=apoptosis-inducing factor.
Multiple logistic regression analysis showed that patients with serum AIF levels>55.6ng/mL had higher mortality risk (OR=3.290; 95% CI=1.551−6.979; p=0.002) controlling for age, SOFA and lactic acid (Table 2). And this higher mortality risk was also present specifically in septic shock patients (OR=3.027; 95% CI=1.078−8.502; p=0.04) controlling for the same variables (Table 2).
Multiple logistic regression analyses to predict mortality at 30 days.
| Odds ratio | 95% confidence interval | p-Value | |
|---|---|---|---|
| All patients | |||
| Serum AIF levels >55.6ng/mL (yes vs no) | 3.290 | 1.551−6.979 | 0.002 |
| Age (years) | 1.024 | 1.000−1.048 | 0.054 |
| SOFA score (points) | 1.108 | 1.004−1.221 | 0.04 |
| Lactic acid (mmol/L) | 1.134 | 1.001−1.285 | 0.048 |
| Patients with septic shock | |||
| Serum AIF levels >55.6ng/mL (yes vs no) | 3.027 | 1.078−8.502 | 0.04 |
| Age (years) | 1.040 | 1.003−1.077 | 0.03 |
| SOFA score (points) | 1.142 | 0.993−1.313 | 0.06 |
| Lactic acid (mmol/L) | 1.136 | 0.974−1.326 | 0.11 |
SOFA=Sepsis-related Organ Failure Assessment; AIF=apoptosis-inducing factor.
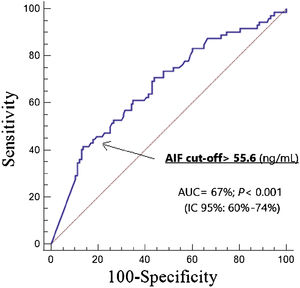
ROC analysis showed an AUC for mortality prediction by serum AIF levels of 0.67 (95% CI=0.60−0.74; p<0.001) (Fig. 1), of 0.67 (95% CI=0.60−0.74; p<0.001) by SOFA score, of 0.71 (95% CI=0.64−0.77; p<0.001) by APACHE-II score, and of 0.66 (95% CI=0.59−0.73; p<0.001) by lactic acid levels.
We no found significant differences when we compared AUC for mortality prediction of serum AIF levels with SOFA score (p=0.98), APACHE-II score (p=0.41), lactic acid levels (p=0.84), the combination of serum AIF levels and SOFA score (p=0.92), the combination of serum AIF levels and APACHE-II score (p=0.71), and the combination of serum AIF levels and lactic acid levels (p=0.64).
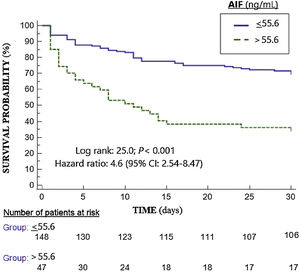
The mortality prediction by 55.6ng/mL cutt-point of serum AIF levels had 42% (30%–54%) sensitivity, 86% (79%–92%) specificity, 3.0 (1.8-5.1) positive likelihood ratio, 0.7 (0.6–0.8) negative likelihood ratio, 64% (51%–75%) positive predictive value and 72% (67%–76%) negative predictive value. A higher mortality rate in those patients with serum AIF levels>55.6ng/mL (Hazard ratio=4.6; 95% CI=2.54–8.47; p<0.001) was found in Kaplan–Meier analysis (Fig. 2).
DiscussionThe new and main findings of our study were that septic patients and shock septic patients with high serum AIF levels had higher mortality risk controlling for age, SOFA and lactic acid levels.
Previously, there has been found parthanatos (with PARP-1 activation, AIF translocation from mitochondria to the nucleus and DNA fragmentation) in patients with infection by different microorganisms.9–13 In studies of mice receiving LPS14 and human alveolar epithelial cells receiving LPS had been found AIF release from mitochondria to the nucleus and cell death.15 However, there are not data on parthanatos in septic patients. Thus, that we know, our study is the first one reporting that serum AIF levels are associated with mortality of septic patients.
Parthanatos is a form of programmed cell death initiated by PARP-1 activation in the nucleus, which produces PAR that serves as a death signal from the nucleus to mitochondria, inducing the release of AIF from mitochondrial to cytosol and afterwards to the nucleus promoting chromatinolysis.3–8 We think that our findings in respect to the association between high serum AIF levels and higher mortality rate in septic patients could mean that a higher PARP-1 activation and higher parthanatos could contribute on sepsis mortality. However, a limitation of our study was that we did not taken samples of tissues to assess PARP-1 activation, PAR formation, AIF translocation from mitochondria to the nucleus and chromatinolysis and to explore its association with serum AIF levels.
The interesting findings of studies with septic animals that the use of different agents (3-AB, erlotinib or HSP70) have reduced AIF release from mitochondria to the nucleus, cell death and sepsis mortality of mice are promising facts.14,15
We must recognize some limitations in our study such as that the number of patients lost and their reason was not recorded, the lack of serum AIF levels in healthy subjects or in the follow-up of patients, that we have not registered serum levels of C-reactive protein, procalcitonin and other inflammatory biomarkers21 to compare its prognostic reliability, and that the time from starting antibiotics and from ICU admission to patient inclusion time were not registered.
We no found differences on mortality prediction between serum AIF levels and SOFA score, APACHE-II score and lactic acid levels; and neither between serum AIF levels and the combination of serum AIF levels with those sepsis severity scores or lactic acid levels. However, the key point of serum AIF levels is that could be modulate according the results of animals models. We think that the findings of our study and those of animal studies could motivate research to establish the rol of serum AIF levels for septic patient prognosis prediction and research to establish the potential rol of agents blocking AIF release from mitochondria to the nucleus in the prognosis of those patients.
ConclusionsParthanatos is associated with mortality of septic patients.
Key messages- -
Serum AIF levels during were higher in non-surviving patients
- -
Serum AIF levels could predict sepsis mortality
- -
Serum AIF levels are associated with sepsis mortality
- -
LLo conceived, designed and coordinated the study, participated in acquisition and interpretation of data, and drafted the manuscript
- -
MMM and RO participated in acquisition of data
- -
AFGC, FGB and APC carried out the determinations of serum caspase-8 concentrations
- -
AJ participated in the interpretation of data
All authors revised the manuscript critically for important intellectual content and made the final approval of the version to be published.
FundingNone.
Conflicts of interestThe authors declare that they have no competing interests.