To evaluate the impact of the recommendations of the SEMICYUC (2012) on severe influenza A.
DesignA prospective multicenter observational study was carried out.
SettingICU.
PatientsPatients infected with severe influenza A (H1N1) from the GETGAG/SEMICYUC registry.
InterventionsAnalysis of 2 groups according to the epidemic period of the diagnosis (2009–2011; 2013–2015).
VariablesDemographic, temporal, comorbidities, severity, treatments, mortality, late diagnosis and place of acquisition.
ResultsA total of 2205 patients were included, 1337 (60.6%) in the first period and 868 (39.4%) in the second one. Age and severity on admission were significantly greater in the second period, as well as co-infection. With regard to the impact of the recommendations, in the second period the diagnosis was established earlier (70.8 vs. 61.1%, p<0.001), without changes in the start of treatment. Patients received less corticosteroid treatment (39.7 vs. 44.9%, p<0.05), more NIMV was used (47.4 vs. 33.2%, p<0.001) and more vaccination was made (11.1 vs. 1.7%, p<0.001), without changes in mortality (24.2 vs. 20.7%). A decrease in nosocomial infection was also noted (9.8 vs. 16%, p<0.001). Patients needed less MV with more days of ventilation, more vasopressor drug use and more ventral decubitus.
ConclusionsThe management of patients with severe influenza A (H1N1) has changed over the years, though without changes in mortality. The recommendations of the SEMICYUC (2012) have allowed earlier diagnosis and improved corticosteroid use. Pending challenges are the delay in treatment, the vaccination rate and the use of NIMV.
Evaluar el impacto de las recomendaciones SEMICYUC 2012 en la gripe A grave.
DiseñoProspectivo multicéntrico observacional.
ÁmbitoUCI.
PacientesPacientes con virus influenza A (H1N1) grave (registro GETGAG/SEMICYUC).
IntervencionesAnálisis de 2 grupos según el periodo epidémico del diagnóstico (2009-2011; 2013-2015).
VariablesDemográficas, temporales, comorbilidades, gravedad, tratamientos, mortalidad, diagnóstico tardío y lugar de adquisición.
ResultadosSe incluyeron 2.205 pacientes, 1.337 (60,6%) en el primer periodo y 868 (39,4%) en el segundo. La edad, la gravedad al ingreso y la coinfección bacteriana fueron significativamente mayores en el segundo periodo. Respecto al impacto de las recomendaciones, en el segundo periodo el diagnóstico fue más precoz (70,8 vs. 61,1%, p<0,001), sin cambios en el inicio del tratamiento. Se administraron menos corticoides (39,7 vs. 44,9%, p<0,05), se utilizó más VMNI (47,4 vs. 33,2%, p<0,001) y se objetivó una mayor tasa de vacunación (11,1 vs. 1,7%, p<0,001), sin cambios en la mortalidad (24,2 vs. 20,7%). También se evidenció una disminución de la infección adquirida en el hospital (9,8 vs. 16%, p<0,001). Asimismo, los pacientes requirieron menos VM con más días de ventilación, más vasopresores y más decúbito prono.
ConclusionesEl manejo de los pacientes con gripe A (H1N1) grave se ha modificado con los años, sin cambios en la mortalidad. Las recomendaciones de la SEMICYUC del año 2012 han mejorado el diagnóstico precoz y el uso de corticoides. Queda por mejorar el retraso en el tratamiento, la tasa de vacunación y la utilización de la VMNI.
Influenza is a health problem in view of the deaths it can cause,1,2 its potential complications, and the associated economic and social costs. The severe influenza A (H1N1)pdm09 pandemic recorded in Spain in 2009 constituted a serious challenge for Intensive Care Units (ICUs), which were able to respond quickly thanks to coordinated efforts from the Spanish Society of Critical and Intensive Care Medicine and Coronary Units (Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias, SEMICYUC) in collaboration with its infectious diseases and sepsis, acute respiratory failure and organization and management working groups. The infectious diseases and sepsis working group then created a network of hospitals and investigators (the Spanish Severe Influenza A Working Group [Grupo Español de Trabajo Gripe A Grave, GETGAG]) with the purpose of contributing their cases to a national registry that remains active today, and which over these years has been able to offer real time data on the evolution of patients with severe forms of influenza A (H1N1)pdm09 infection. This registry helps characterize the clinical course of the disease and its risk factors, as well as modify approaches to prevention, diagnosis and treatment, and has had a favorable impact upon patient prognosis.3–8 Thanks to this large database, a number of studies have investigated the impact of the pandemic of 20093,9–11 and have compared the characteristics of the patients with severe influenza A during the pandemic versus the patients affected in a post-pandemic period (2010–2011). In the year 2012, the SEMICYUC from the GETGAG published a consensus document with a series of recommendations for the diagnosis and treatment of influenza A (H1N1)pdm09 in critical adult patients admitted to the ICU10, with the provision of clear guidelines for the early diagnosis and management of the disease. The main recommendations of the consensus document included: (1) diagnostic testing as soon as possible of all patients with fever and flu symptoms requiring hospital admission; (2) the administration of oseltamivir on an early basis (<48h from symptoms onset); (3) empirical antibiotic coverage, due to the possibility of bacterial coinfection; (4) the avoidance of corticosteroids, due to the lack of scientific evidence supporting their use; (5) the avoidance of noninvasive mechanical ventilation (NIMV) as technique of choice; and (6) influenza vaccination of the entire population at risk.
In addition to these recommendations, two recent studies have evidenced the important impact of diagnostic delays and in-hospital acquisition of the infection upon the prognosis of these patients.12,13 Both of these parameters have been independently related to mortality – thus underscoring the need for early diagnosis and treatment of the infection in epidemic periods targeted to all patients admitted with respiratory manifestations, and the importance of prevention and control of the infection in order to lessen the risk of transmission of the influenza virus in hospitalized patients.
The present study analyzes and compares the characteristics of the patients admitted to the ICU with a diagnosis of influenza A (H1N1)pdm09 infection in two time periods (2009–2011 and 2013–2015), with the purpose of evaluating the impact of applying the recommendations of the SEMICYUC (2012) and the evolution of diagnostic delays and in-hospital acquisition of the infection over the years.
MethodsStudy design and populationA prospective, multicenter, observational cohort study was carried out. Between 1 January 2009 and 28 March 2015, the GETGAG/SEMICYUC registry compiled data on the patients diagnosed with microbiologically confirmed influenza A (H1N1)pdm09 infection and admitted to 148 ICUs throughout Spain. All the patients with flu symptoms admitted to the participating ICUs were subjected to influenza A or B detection testing, and the investigators voluntarily entered all the patients with positive influenza A (H1N1)pdm09 infection in the national registry. Patient identification was established on an anonymized basis, and informed consent was not considered necessary due to the non-interventional nature of the study. The GETGAG/SEMICYUC registry was approved by the Clinical Research Ethics Committee of Joan XXIII University Hospital (Tarragona, Spain), and analysis of the study data was approved by the Clinical Research Ethics Committee of Parc de Salut Mar (Barcelona, Spain).
The patients included in the study were admitted to the ICU with clinical manifestations of respiratory infection, and the presence of influenza virus A or B was investigated in all of them based on real time polymerase chain reaction (RT-PCR) testing according to the recommendations of the Centers for Disease Control and Prevention (CDC).14 The clinical manifestations comprised two or more of the following signs and symptoms: fever (>38°C), cough, bronchial expectoration and muscle pain. These manifestations were required to be associated to clinical evidence of organ or systemic failure (respiratory failure, hemodynamic instability, renal failure or altered consciousness). The exclusion criteria were patients under 15 years of age and a diagnosis of influenza A (H3N2) or influenza B infection.
The patients were classified into two groups according to the epidemic period of the diagnosis: first period (2009–2011) and second period (2013–2015). The cut-off point was established with the purpose of comparing two homogeneous groups coinciding with the introduction of the mentioned recommendations on the diagnosis and treatment of the infection. These recommendations were published in a consensus document10 in the journal of the SEMICYUC (Medicina Intensiva) in January 2012. The recommendations were likewise presented at different national and international congresses and were disclosed electronically on the website of the Society and via e-mail.
Data collectionA clinical registry form was developed for data collection, including demographic information (age, gender), variables related to hospital stay (time from hospital admission to the diagnosis of influenza A infection, time from symptoms onset to the start of antiviral treatment, days of stay in the ICU), comorbidities, previous influenza vaccinations, season of the epidemic, severity of the disease, treatment measures (antiviral agents, inotropic drugs, corticosteroids, mechanical ventilation (MV), renal replacement therapy) and mortality in the ICU. The severity of the infection was scored using the Acute Physiology and Chronic Health Evaluation II (APACHE II) and the Sequential Organ Failure Assessment (SOFA) at the time of admission to the ICU. The information was supplied by the physicians of the participating ICUs, drawn from the patient case histories, laboratory data and radiological findings.
DefinitionsThe definitions of the different comorbidities have been given in previous publications related to this registry.9,15 Respiratory coinfection was defined as any bacterial respiratory infection diagnosed in a patient with influenza A (H1N1) infection upon admission to the ICU, while failed NIMV was defined as the need for MV in those patients subjected to NIMV immediately upon admission to the ICU as first respiratory support measure for the management of respiratory failure. Early diagnosis of the infection in turn was defined as a diagnosis established in the first 48h following admission to the hospital,13 and in-hospital acquired infection was defined as infection diagnosed from the seventh day of hospital stay in patients that had not received previous antiviral treatment.12
Statistical analysisCategorical variables were reported as frequencies and percentages, while continuous variables were reported as the mean and standard deviation in the presence of a normal data distribution, and as the median and interquartile range (IQR)(25–75%) in the absence of a normal distribution. Differences between groups were analyzed using the chi-squared test or Fisher exact test for categorical variables, and the Student t-test or Mann–Whitney U-test for continuous variables. Those variables found to be significant in the bivariate analysis were entered in a multivariate logistic regression model in order to evaluate the independent factors associated to mortality in each epidemic period. The odds ratios (ORs) and 95% confidence intervals (95%CIs) were calculated. Statistical significance was considered for p<0.05. The data were analyzed using the Statistical Package for the Social Sciences® version 15.0 (SPSS, Chicago, IL, USA) for MS Windows®.
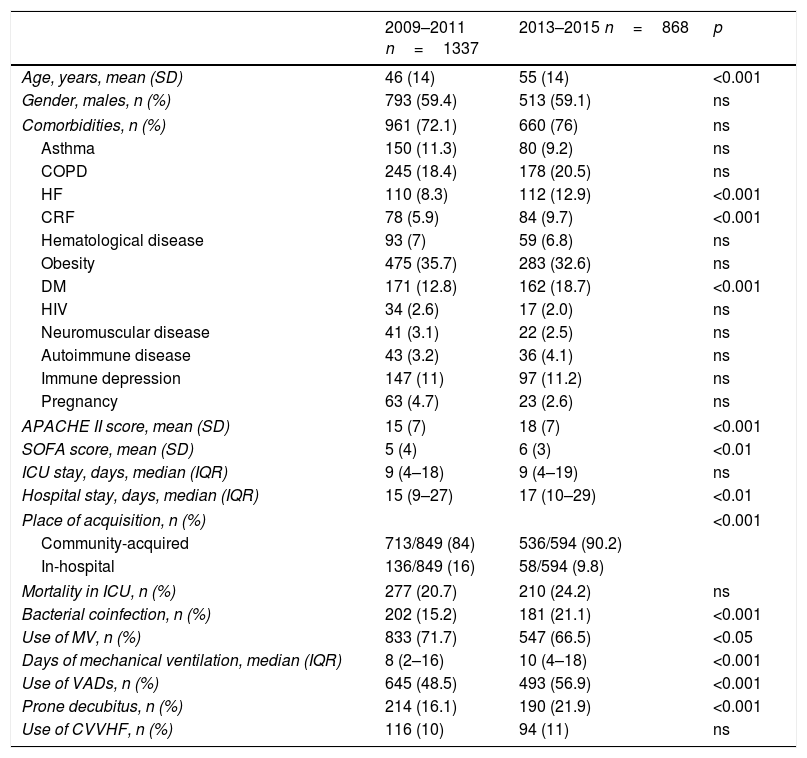
ResultsDuring the period between January 2009 and March 2015, the national GETGAG/SEMICYUC registry documented a total of 2421 patients admitted to the ICU with a diagnosis of influenza A (H1N1) infection. Of these patients, 216 were excluded due to a lack of data. Of the 2205 patients finally analyzed, 1337 (60.6%) corresponded to the first period and 868 (39.4%) to the second period. With respect to the first period, the patients in the second period were significantly older (55 [14] vs. 46 [14] years; p<0.001), with more comorbidities (heart failure: 12.9 vs. 8.3%; p<0.001; chronic renal failure: 9.7% vs. 5.9%; p<0.001; diabetes mellitus: 18.7% vs. 12.8%; p<0.001), greater severity upon admission (APACHE II score 18 [7] vs. 15 [7]; p<0.001), more organ failure (SOFA score 6 [3] vs. 5 [4]; p<0.01), and more bacterial coinfection (21.1% vs. 15.2%; p<0.001). No differences in terms of mortality were observed between the two groups (Table 1).
Descriptive characteristics of the patients admitted to the ICU with influenza A (H1N1)pdm09 infection in the two epidemic periods.
| 2009–2011 n=1337 | 2013–2015 n=868 | p | |
|---|---|---|---|
| Age, years, mean (SD) | 46 (14) | 55 (14) | <0.001 |
| Gender, males, n (%) | 793 (59.4) | 513 (59.1) | ns |
| Comorbidities, n (%) | 961 (72.1) | 660 (76) | ns |
| Asthma | 150 (11.3) | 80 (9.2) | ns |
| COPD | 245 (18.4) | 178 (20.5) | ns |
| HF | 110 (8.3) | 112 (12.9) | <0.001 |
| CRF | 78 (5.9) | 84 (9.7) | <0.001 |
| Hematological disease | 93 (7) | 59 (6.8) | ns |
| Obesity | 475 (35.7) | 283 (32.6) | ns |
| DM | 171 (12.8) | 162 (18.7) | <0.001 |
| HIV | 34 (2.6) | 17 (2.0) | ns |
| Neuromuscular disease | 41 (3.1) | 22 (2.5) | ns |
| Autoimmune disease | 43 (3.2) | 36 (4.1) | ns |
| Immune depression | 147 (11) | 97 (11.2) | ns |
| Pregnancy | 63 (4.7) | 23 (2.6) | ns |
| APACHE II score, mean (SD) | 15 (7) | 18 (7) | <0.001 |
| SOFA score, mean (SD) | 5 (4) | 6 (3) | <0.01 |
| ICU stay, days, median (IQR) | 9 (4–18) | 9 (4–19) | ns |
| Hospital stay, days, median (IQR) | 15 (9–27) | 17 (10–29) | <0.01 |
| Place of acquisition, n (%) | <0.001 | ||
| Community-acquired | 713/849 (84) | 536/594 (90.2) | |
| In-hospital | 136/849 (16) | 58/594 (9.8) | |
| Mortality in ICU, n (%) | 277 (20.7) | 210 (24.2) | ns |
| Bacterial coinfection, n (%) | 202 (15.2) | 181 (21.1) | <0.001 |
| Use of MV, n (%) | 833 (71.7) | 547 (66.5) | <0.05 |
| Days of mechanical ventilation, median (IQR) | 8 (2–16) | 10 (4–18) | <0.001 |
| Use of VADs, n (%) | 645 (48.5) | 493 (56.9) | <0.001 |
| Prone decubitus, n (%) | 214 (16.1) | 190 (21.9) | <0.001 |
| Use of CVVHF, n (%) | 116 (10) | 94 (11) | ns |
APACHE II: Acute Physiology and Chronic Health Evaluation II; SD: standard deviation; DM: diabetes mellitus; COPD: chronic obstructive pulmonary disease; VADs: vasoactive drugs; CVVHF: continuous venovenous hemofiltration; HF: heart failure; CRF: chronic renal failure; ns: nonsignificant; IQR: interquartile range; SOFA: Sequential Organ Failure Assessment; ICU: Intensive Care Unit; HIV: human immunodeficiency virus; MV: invasive mechanical ventilation.
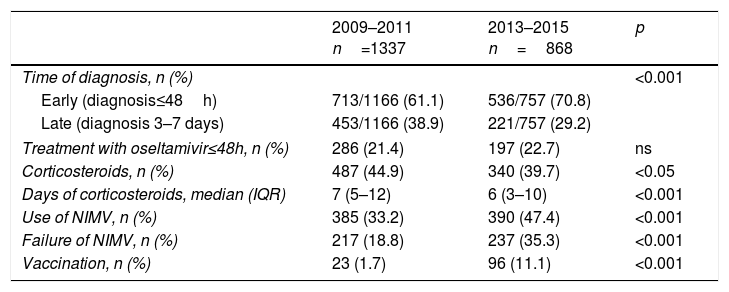
Table 2 shows the changes between the two periods corresponding to the variables defined in the recommendations of the SEMICYUC 2012. In the second period there was a greater percentage of early diagnoses (70.8 vs. 61.1%; p<0.001), with no changes in the percentage of patients that received oseltamivir in the first 48h (22.7% vs. 21.4%; p>0.05). Likewise, in the second period fewer corticosteroids were prescribed (39.7% vs. 44.9%; p<0.05) with fewer days of treatment (7 [5–12] vs. 6 [3–10]; p<0.001). In turn, the percentage use of NIMV was greater (47.4% vs. 33.2%; p<0.001), with a greater incidence of NIMV failure (35.3% vs. 18.8%; p<0.001), and a higher vaccination rate (11.1% vs. 1.7%; p<0.001) (Table 2).
Characteristics related to the recommendations of the SEMICYUC 2012 in the patients admitted to the ICU with influenza A (H1N1)pdm09 infection in the two epidemic periods.
| 2009–2011 n=1337 | 2013–2015 n=868 | p | |
|---|---|---|---|
| Time of diagnosis, n (%) | <0.001 | ||
| Early (diagnosis≤48h) | 713/1166 (61.1) | 536/757 (70.8) | |
| Late (diagnosis 3–7 days) | 453/1166 (38.9) | 221/757 (29.2) | |
| Treatment with oseltamivir≤48h, n (%) | 286 (21.4) | 197 (22.7) | ns |
| Corticosteroids, n (%) | 487 (44.9) | 340 (39.7) | <0.05 |
| Days of corticosteroids, median (IQR) | 7 (5–12) | 6 (3–10) | <0.001 |
| Use of NIMV, n (%) | 385 (33.2) | 390 (47.4) | <0.001 |
| Failure of NIMV, n (%) | 217 (18.8) | 237 (35.3) | <0.001 |
| Vaccination, n (%) | 23 (1.7) | 96 (11.1) | <0.001 |
ns: nonsignificant; IQR: interquartile range; NIMV: noninvasive mechanical ventilation.
In the second period the patients showed a decrease in hospital acquired infection (9.8% vs. 16%; p<0.001), were less subjected to invasive MV (66.5% vs. 71.7%; p<0.05), received more days of ventilation (10 [4–18] vs. 8 [2–16]; p<0.001) and required more vasoactive drug support (56.9% vs. 48.5%; p<0.001) and prone decubitus (21.9% vs. 16.1%; p<0.001) (Table 1).
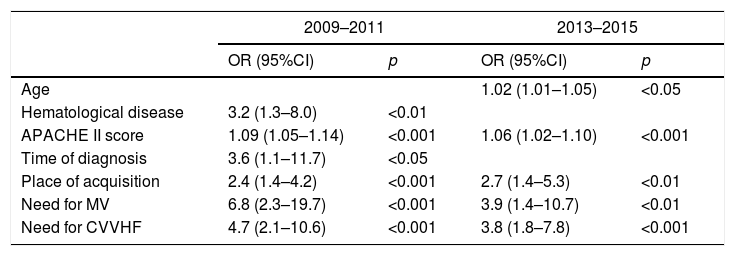
In both periods the APACHE II score, in-hospital acquisition of the infection, and the need for MV and renal replacement therapy were independently associated to mortality. In the first period the same applied to late diagnosis and hematological disease, while in the second period age was also associated to mortality (Table 3). In both periods the patients that died had greater delays in treatment than the survivors, though this variable was not independently correlated to mortality in either period.
Factors independently associated to patient mortality in the two epidemic periods of the study.
| 2009–2011 | 2013–2015 | |||
|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | |
| Age | 1.02 (1.01–1.05) | <0.05 | ||
| Hematological disease | 3.2 (1.3–8.0) | <0.01 | ||
| APACHE II score | 1.09 (1.05–1.14) | <0.001 | 1.06 (1.02–1.10) | <0.001 |
| Time of diagnosis | 3.6 (1.1–11.7) | <0.05 | ||
| Place of acquisition | 2.4 (1.4–4.2) | <0.001 | 2.7 (1.4–5.3) | <0.01 |
| Need for MV | 6.8 (2.3–19.7) | <0.001 | 3.9 (1.4–10.7) | <0.01 |
| Need for CVVHF | 4.7 (2.1–10.6) | <0.001 | 3.8 (1.8–7.8) | <0.001 |
APACHE II: Acute Physiology and Chronic Health Evaluation II; CVVHF: continuous venovenous hemofiltration; 95%CI: 95% confidence interval; OR: odds ratio; MV: invasive mechanical ventilation.
The present study shows that the profile and management of patients with severe influenza A (H1N1)pdm09 infection admitted to the ICU have experienced changes since the year 2009. On one hand, the intrinsic and non-modifiable characteristics of the patients have evolved over time. In effect, in recent years the patients admitted to the ICU are comparably older and in more serious condition, and present a greater incidence of bacterial coinfection. On the other hand, the recommendations of the SEMICYUC 2012 may have influenced the improvements seen in terms of an earlier diagnosis, a lesser use of corticosteroids, and improved vaccination percentages. Nevertheless, other aspects contemplated in the mentioned recommendations have not experienced changes over the years, such as delays in treatment or the use of NIMV despite the fact that this technique is not indicated as the treatment of choice in these patients. Likewise, other local initiatives referred to the prevention of infectious disease transmission may have influenced the improvements observed in terms of increased vaccination practice, early diagnosis and lessened in-hospital acquisition of the infection. The combined impact of all these factors probably explains why the mortality statistics in the two study periods remained constant.
In the second study period the patients were older and suffered more heart failure, chronic renal failure and diabetes mellitus. In addition, they were in more serious condition (APACHE II score), had a higher incidence of organ failure (SOFA score) upon admission to the ICU, and required more days of hospital stay. This change in the characteristics of the patients in the second period could reflect improved identification of the cases, with consequent earlier treatment – thereby avoiding admissions to the ICU. In the second period we also observed an increase in the incidence of bacterial coinfection, in concordance with the findings of other series.16–20 This circumstance was probably related to the older age and increased severity of the patients in that period. Both of these variables were identified in a recent study as risk factors for coinfection and were independently associated to mortality20.
The recommendations of the SEMICYUC firstly include early sampling and diagnostic testing in all patients with fever and respiratory symptoms requiring admission to hospital during the influenza epidemic periods. The observed increase in early diagnoses of the infection during the second period probably indicates that the recommendation was gradually implemented as the healthcare professionals incorporated influenza A to the differential diagnosis of patients with respiratory symptoms, particularly in the winter months.
On the other hand, and based on data from the 2009 pandemic, the early administration of oseltamivir was seen to be associated to improved patient outcomes.21,22 Different health organizations5,23 agree that antiviral therapy should be started as soon as possible (within the first 48h) in all patients with possible or confirmed influenza A H1N1 infection requiring admission to hospital, with serious progressive disease, or presenting complications – independently of the previous health condition of the patient or the vaccination history. However, a recent report by the expert committee of the World Health Organization (WHO) has considered the magnitude of the effect of oseltamivir in the treatment of influenza to be overestimated, and does not regard this drug as being essential in this scenario.24 Nevertheless, a subsequent report by the WHO continued to advise the use of oseltamivir as a key medication.25 In this respect, our data confirm that the time from symptoms onset to the administration of oseltamivir remained stable over time (about 5 days in both periods), and that the recommendation in this respect was only followed in about 21% of the cases in both study periods. Although the patients that died had a greater delay in treatment than the survivors in both periods, this variable was not seen to be independently correlated to mortality. Other factors associated to delays in treatment therefore likely exist and condition a poorer prognosis in these patients. Future studies should examine the usefulness of oseltamivir in the critical patient.
The recommendations of 2012 did not include corticosteroids in the severe influenza A treatment algorithm, due to the lack of evidence of any beneficial effect of such drugs. Many observational studies26–32 have evaluated the effect of treatment with corticosteroids in patients with influenza A (H1N1) infection, but none have been able to demonstrate any benefits of such therapy. Indeed, a meta-analysis of 23 studies observed an increase in mortality among patients with influenza A (H1N1) infection that had received corticosteroid treatment.33 The heterogeneity of the existing literature in terms of study design, study population, disease severity, dosage, and the time and duration of treatment constitutes an important limitation that precludes the drawing of firm conclusions. Nevertheless, it is reasonable to assume that if no advantages have been observed with corticosteroids in such diverse situations it is probably because there are no advantages. In this regard, during the second period we recorded a decrease in both the percentage of patients treated with corticosteroids and in the days of treatment.
Despite the fact that the recommendations were to avoid NIMV in cases of severe viral pneumonia, there was an increase both in the use of this technique and in its failure rate during the second period. The use of NIMV in respiratory failure due to influenza A infection has also been the subject of controversy in recent years. On one hand, the consensus document was based on the final report of the cases analyzed during the 2009 pandemic, in which a high NIMV failure rate was observed, associated to a poorer patient prognosis.34 A recent study has again demonstrated the importance of NIMV failure and its association to mortality, establishing a decision algorithm which in future could help define the profiles of patients with different NIMV failure risks.15 On the other hand, in 2013 another publication described a lesser NIMV failure rate in a concrete subgroup of patients in which the mortality rate was no greater than that seen among patients intubated from the start.35 Future studies are needed to confirm which patients could benefit from this technique. Although in our analysis the patients that died in both study periods showed a higher NIMV failure rate, this circumstance was not independently correlated to mortality in either period.
Finally, the recommendations included vaccination of the population at risk (elderly and institutionalized individuals, patients with chronic diseases, pregnant women and healthcare professionals). The prevalence of patients with more risk factors in the second period could account for the increase in vaccination rate in the population assisted during that period. However, it must be taken into account that in the year 2009 the vaccine was not yet available in Spain, and that in any case the vaccination rate during the second study period (11%) was still lower than expected. This therefore constitutes an opportunity for improvement in the general population.
The global mortality rate among the patients requiring admission to the ICU was approximately 22% and consistent with the data of a previous Spanish cohort and with the findings of studies conducted in other countries (14–46%).19,36–38 In addition to coinfection, a series of other factors such as age, hematological disease, the need for continuous renal replacement therapy, MV, the human immunodeficiency virus, diagnostic delays or in-hospital acquisition of the infection have been described as variables independently associated to mortality.9,12,13,36,37,39–41 Of all these factors, the last two (diagnostic delays and nosocomial acquisition) are the only variables amenable to modification and in which healthcare professionals are able to intervene with possibilities of improvement.
In our series, mortality was seen to increase with patient age, and during the second period age was seen to be independently correlated to mortality. In accordance with previous studies, hematological disease was independently associated to mortality, though not so immunosuppression or solid neoplasms. In-hospital acquisition of the infection was also independently associated to mortality in both study periods – this reflecting the responsibility of the healthcare professions in controlling transmission of the infection (washing of hands, vaccination of healthcare professionals, and control of healthcare professionals or relatives with influenza).
The present study underscores the need for campaigns targeted to society and to healthcare professionals in order to implement the recommendations derived from the objective analysis of national and international data. It likewise underscores the need for periodic re-evaluations of the effects of the actions taken in relation to diseases as prevalent as influenza, with a view to redirecting new intervention strategies as required. In the coming seasons in-hospital transmission of the infection can be expected to continue to decrease; an early diagnosis or suspicion can be expected to result in the early start of antiviral treatment if the latter shows clear benefits in application to severe influenza A infection; the vaccination rate can be expected to increase in the population at risk; the use of NIMV can be expected to become limited to selected cases only; and corticosteroid use can be expected to continue to decrease in these patients.
The present study has some limitations. The time periods were established based on the recommendations of 2012, and in this regard the first period included the year of the pandemic when a greater number of individuals were affected. Another limitation is the fact that we were not able to analyze the recommendation referred to empirical antibiotic treatment in cases of bacterial coinfection, due to a lack of data, or the types and doses of corticosteroid drugs used in both periods. Furthermore, the study only included adults admitted to Spanish ICUs; as a result, the findings cannot be generalized to children or to other countries. Lastly, patient management was not standardized, and the practices in each center were those dictated by the local protocols. However, the study has the strength of its prospective multicenter design and large sample size. It therefore may prove useful in re-evaluating procedures and defining new objectives.
ConclusionThe profile, diagnosis and management of patients with severe influenza A (H1N1)pdm09 have experienced changes since the 2009 epidemic, with no observed variations in mortality. In the second period the admitted patients were comparatively older, in more serious condition, and with a higher incidence of bacterial coinfections. The recommendations published by the SEMICYUC from the GETGAG in the year 2012 have partially influenced the observed improvements in terms of an early diagnosis, lessened corticosteroid use and an increase in vaccination practice. A number of aspects require improvement, however. Shortening the time from symptoms onset to the start of treatment; limiting NIMV to selected cases among this patient population; and boosting the annual vaccination rate in the population at risk (which remains low) should be some of the targets in the coming seasonal periods.
Financial supportThe registry of patients with influenza A has been developed by the GETGAG and is the property of the SEMICYUC.
AuthorshipJ. Marin-Corral: study design, data collection, statistical analysis and interpretation of results, writing of the manuscript.
C. Climent: data collection, writing of the manuscript.
R. Muñoz, M. Samper, I. Dot and C. Vilà: data collection.
J.R. Masclans: critical review of the manuscript referred to intellectual content.
A. Rodriguez: data collection, critical review of the manuscript referred to intellectual content.
I. Martin-Loeches: data collection, critical review of the manuscript referred to intellectual content.
F. Alvarez-Lerma: study design, data collection, interpretation of results and critical review of the manuscript.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Thanks are due to all the healthcare staff involved in the care of patients with severe influenza A (H1N1)pdm09 during admission to the ICU for their tireless effort. Thanks are also due to those who have strived to follow the recommendations with a view to improving patient management, as well as to all those physicians who enter data in the GETGAG/SEMICYUC, for their invaluable collaboration.
The members of the H1N1 GETGAG/SEMICYUC working group are cited in the Supplementary Material.
The members of the H1N1 GETGAG/SEMICYUC working group are cited in the Supplementary Material.
Please cite this article as: Marin-Corral J, Climent C, Muñoz R, Samper M, Dot I, Vilà C, et al. Pacientes con gripe por el virus influenza A (H1N1)pdm09 ingresados en la UCI. Impacto de las recomendaciones de la SEMICYUC. Med Intensiva. 2018;42:473–481.
This study has been presented to the LII National Congress of the Spanish Society of Critical and Intensive Care Medicine and Coronary Units (Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias, SEMICYUC), Madrid (Spain), June 2017 and to the 38th Meeting of the Catalan Society of Intensive and Critical Care Medicine (Sociedad Catalana de Medicina Intensiva y Crítica, SOCMIC), Barcelona (Spain), March 2017.