Cardio-surgical patient care requires a comprehensive and multidisciplinary approach to develop strategies to improve patient safety and outcomes. In the preoperative period, prophylaxis for frequent postoperative complications, such as de novo atrial fibrillation or bleeding, and prehabilitation based on exercise training, respiratory physiotherapy and nutritional and cognitive therapy, especially in fragile patients, stand out. There have been great advances, during the intraoperative phase, such as minimally invasive surgery, improved myocardial preservation, enhanced systemic perfusion and brain protection during extracorporeal circulation, or implementation of Safe Surgery protocols. Postoperative care should include goal-directed hemodynamic theraphy, a correct approach to coagulation disorders, and a multimodal analgesic protocol to facilitate early extubation and mobilization. Finally, optimal management of postoperative complications is key, including arrhythmias, vasoplegia, bleeding, and myocardial stunning that can lead to low cardiac output syndrome or, in extreme cases, cardiogenic shock. This global approach and the high degree of complexity require highly specialised units where intensive care specialists add value and are key to obtain more effective and efficient clinical results.
La atención al paciente cardioquirúrgico requiere una aproximación integral y multidisciplinar para desarrollar estrategias que mejoren la seguridad y el pronóstico de los pacientes. En la fase preoperatoria destacan la implantación de medidas de prevención de las complicaciones postoperatorias más frecuentes, como la fibrilación auricular de novo o el sangrado, y la prehabilitación basada en ejercicio físico, fisioterapia respiratoria y terapia nutricional y cognitiva, especialmente en aquellos pacientes más frágiles. En la fase quirúrgica se han producido grandes avances como el desarrollo de procedimientos mínimamente invasivos, las mejoras en la preservación miocárdica, la perfusión sistémica durante la circulación extracorpórea y la protección cerebral, o la implantación de los protocolos de Cirugía Segura. En el postoperatorio se debe establecer un manejo hemodinámico guiado por objetivos, un correcto abordaje de los trastornos de la coagulación y el uso de analgesia multimodal que posibilite la extubación y movilización precoz de los pacientes. Por último, es clave un óptimo manejo de las complicaciones postoperatorias, entre las que destacan las arritmias, la vasoplejia, el sangrado postquirúrgico y el aturdimiento miocárdico que puede derivar en bajo gasto o, en su caso más extremo, en shock cardiogénico. Este enfoque global y el elevado nivel de complejidad hacen necesario el ingreso del paciente en unidades de alto nivel de asistencia siendo clave el valor aportado a este proceso por los Servicios de Medicina Intensiva para obtener unos resultados clínicos más eficaces y eficientes.
Cardiovascular surgery is a highly complex procedure with good results for selected patients that increases both their quality of life and survival. In Spain, back in 2018,1 34 318 procedures were performed in 62 different cardiac surgery intensive care units.
The optimal management of these patients should be multidisciplinary, comprehensive, and patient-focused using the right protocols.2 Intensive medicine plays a key role in this clinical setting thanks to its contributions, values, and overall approach of the entire process.3,4 Intensivists play a key role for their knowledge of the procedure and capacity to handle the quality of the process through healthcare registries, outcome assessments, and actions for improvement. It is of paramount importance to admit patients to the intensive care unit (ICU) who, due to their instability or complexity, require preoperative optimization. Similarly, postoperative management is essential here too, both at the ICU setting and at the hospitalization floor for the early detection of any possible complications.
The Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC) strategic plan for 2018–20225 advocates for the quality of the surgical process by establishing specific actions aimed at improving the management of surgical patients and their families and working collaboratively with other medical specialties to achieve an effective, efficient, and safe process.
Advances in cardiac surgeryOver the last few years, cardiovascular surgery has evolved significantly thanks to the advances made in myocardial protection, systemic perfusion during extracorporeal circulation, and cerebral protection in situations of circulatory arrest, the implementation of safe surgical protocols during the intervention, the transesophageal echocardiography monitorization of technical complications, new minimally invasive surgical techniques, sutureless percutaneous heart valve implantation, and the development of multimodal recovery (ERAS) in cardiac surgery.
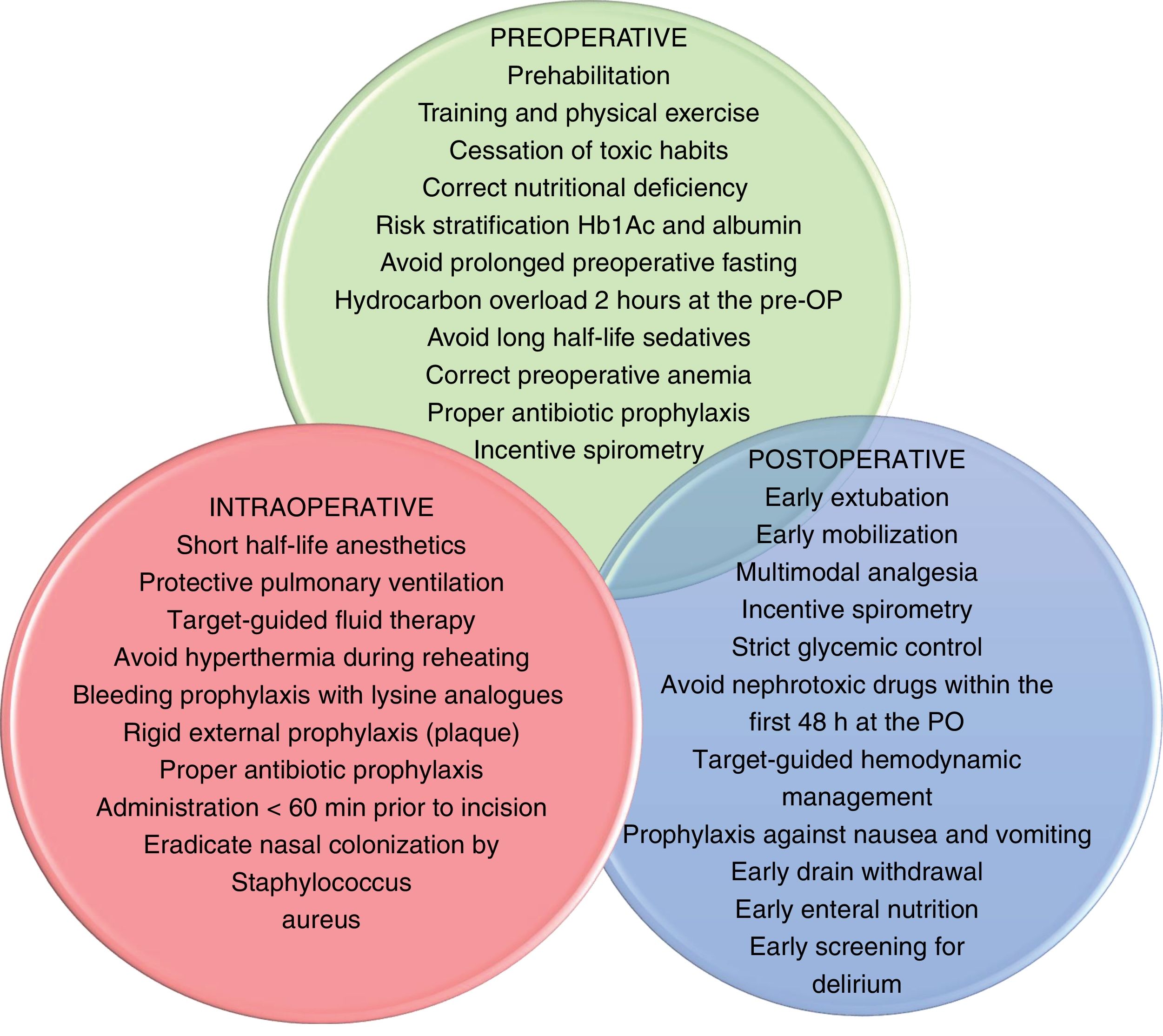
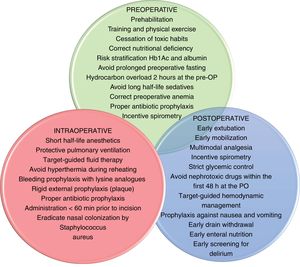
Enhanced recovery after surgery and fast-track surgeryERAS protocols reduce the impact of perioperative stress and speed up the patients’ recovery time. They include pre-, intra-, and postoperative indications; they obtain as much information as possible and preparation prior to the surgery, empower the patient, standardize certain processes, and optimize resources to reduce complications, hospital stays, and down times. These recommendations6 are shown on Fig. 1.
Preoperative optimizationPrehabilitation in frail patients7 by correcting nutritional deficits8 and preoperative anemia,9 and withdrawing certain drugs (antiplatelet drugs, anticoagulants, renin-angiotensin system inhibitors) improves postoperative results and reduces hemorrhagic, hemodynamic, and renal complications.10,11
Prophylaxis of de novo atrial fibrillation with beta-blockers or amiodarone is capable of reducing its incidence rate.12 Preconditioning with levosimendan in coronary patients with moderate-to-severe systolic dysfunction is a useful and cost-effective strategy to reduce the incidence rate of postoperative low cardiac output syndrome.13,14
Standard management of the uncomplicated postoperative courseThe early management of patients during postoperative cardiac surgery care (PCSC) requires a thorough physical examination and, at least, basic hemodynamic monitorization.15–17 (Table 1) Afterwards, the homeostasis of the internal medium needs to be reestablished, normothermia needs to be reached, the correct functioning of the epicardial pacemaker guaranteed, and antibiotic prophylaxis continued. It is essential to establish a multimodal analgesic strategy by combining different types of analgesics to reduce the doses of opioids, thus facilitating the patient’s extubation, rehabilitation, and early mobilization. Weaning from mechanical ventilation should start when the proper conditions of clinical stability are met. As it occurs in most critically ill patients, an adequate glycemic control should be kept followed by the detection and prevention of acute kidney injury, early administration of thromboprophylaxis (mechanical and, when possible, pharmacological), and prevention of delirium.
Recommended basic hemodynamic monitorization.
| Invasive arterial pressure |
| Continuous electrocardiogram |
| Temperature |
| Hydric balance |
| Mixed oxygen venous saturation (SvO2) or central venous oxygen saturation (SvcO2) (if not monitored through pulmonary artery catheterization): marker of the balance between oxygen supply and demand. |
| Gasometry: upon admission every 4 h–8 h and only as long as there is a change in the patient’s clinical situation. |
| Central venous pressure: it should be monitored because it is useful for tendency assessment or in the presence of extreme values. |
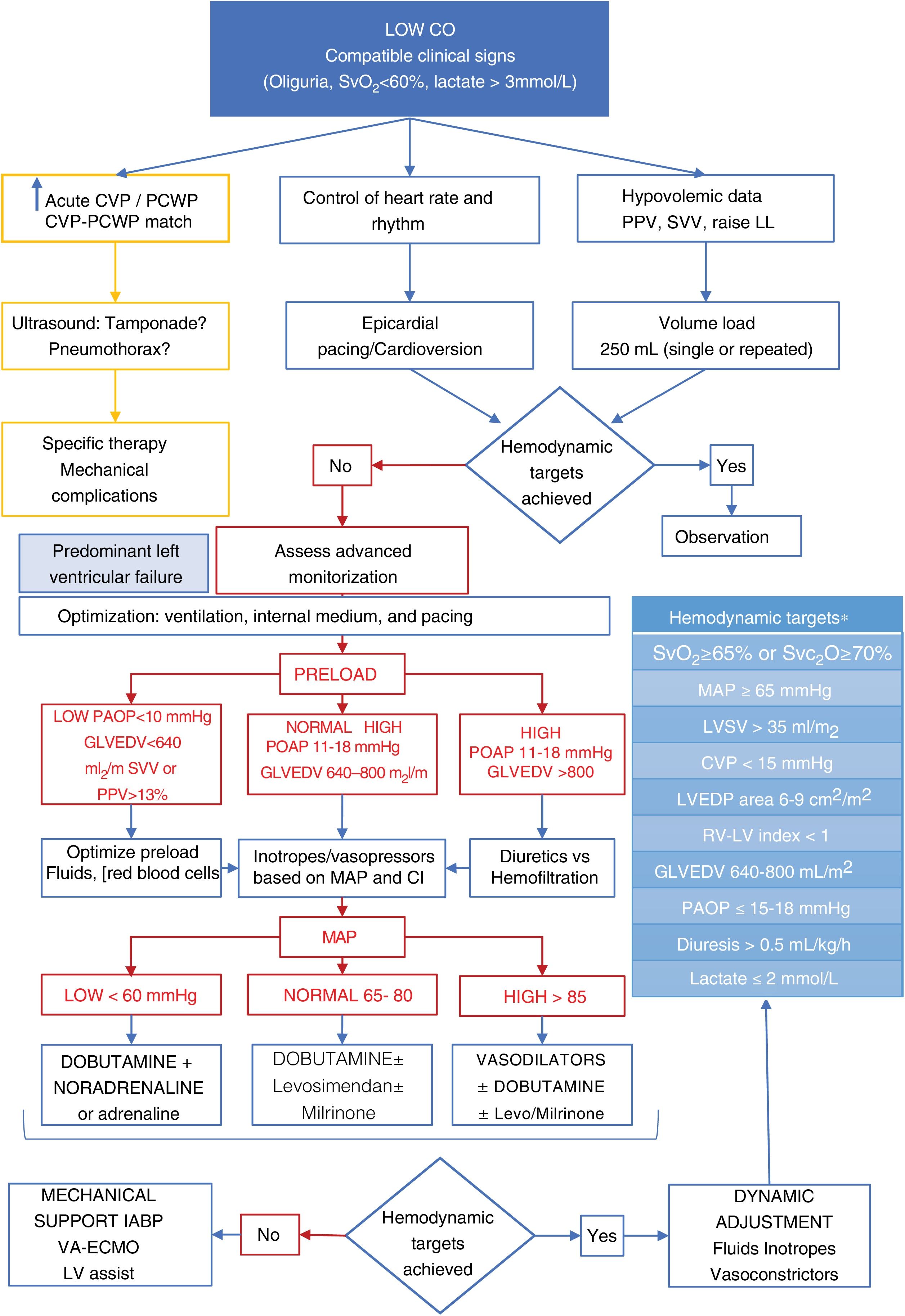
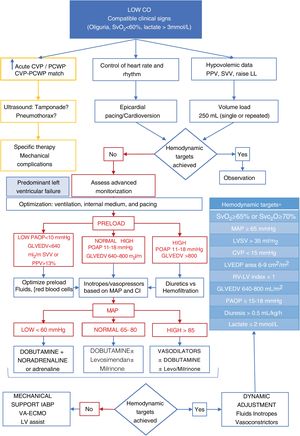
Resuscitation and hemodynamic management should be guided by objective targets18 (Fig. 2). Advanced monitorization is indicated in unstable patients, in those in whom the pathophysiology of the process needs to be deciphered or in patients who, due to their baseline characteristics, intraoperative evolution or type of intervention are considered of high surgical risk. Advanced hemodynamic monitoring systems allow us to obtain ongoing information on cardiac output (CO) and its determinants: preload or preload-dependency, contractility, and afterload.
Algorithm proposal for the management of postoperative low cardiac output syndrome. CVP, central venous pressure; GLVEDV, global left ventricular end-diastolic volume; LV, left ventricle; LVEDP, left ventricular end-diastolic pressure; LVSV, left ventricular systolic volume; MAP, mean arterial pressure; PAOP, pulmonary artery occlusion pressure; RV, right ventricle; SvO2, mixed venous oxygen saturation; SvcO2, central venous oxygen saturation.
*Adapted from Habicher M et al.18
Contractility is reduced during the immediate PCSC compared to the preoperative course. The magnitude and duration of this stage will depend on the previous cardiac dysfunction, existence of ischemic events, preoperative complications, and intraoperative evolution. The components that impact cardiac dysfunction the most are an increased left ventricular (LV) mass, hypothermia, the volume and route of cardioplegia administration, and prolonged ECC time.19
During the postoperative course we should take into account the presence of 2 hemodynamic patterns: pressure overload (left hypertrophy and impaired lusitropy) that affects the measurement of filling pressures, thus underestimating the volume needs and associated with possible tachycardia intolerance. The other one being volume overload (typical of valvular regurgitation) that also alters the measurement of preload-dependency and where the dynamic variables of response to volume have not been validated. In this context a continuous assessment of the response to the volume administered is required as well as its repercussion on the target parameters to avoid inadequate resuscitations.
Added to CO and its determinants, the pulmonary artery catheterization is the only system that provides us with information on SvO2 and pulmonary pressures. Systems based on transpulmonary thermodilution have been developed as an alternative to provide CO continuous measurements (and other parameters of great utility). These systems have recently been validated in patients with cardiogenic shock.20 However, they have limitations like the need for frequent recalibration in the presence of significant hemodynamic variations or loss of accuracy in the presence of arrhythmias or work of breathing.15–17 Echocardiography has been playing a key role during PCSC and, to this date, it is considered an essential imaging modality. Its utility lies in how fast it provides us with functional and anatomical information. It allows us to assess the ejection fraction, the CO through systolic volume assessment, the response to fluids, inotropes or vasoactive drugs, speed up the early diagnosis of complications (tamponade, valve failure…), and facilitate the appropriate therapeutic decision-making process.21
ComplicationsThe risk of perioperative complications depends on the patient’s baseline coronary artery disease, his functional reserve, the prevalence of comorbidities, type and character of the surgery, and duration of the entire surgical procedure. These are some of the most common postoperative complications:
De novo atrial fibrillation and other arrhythmiasAtrial fibrillation (AF) is the most common arrhythmia during PCSC.22 Its peak incidence rate occurs within 48 h–72 h and it usually subsides spontaneously within the first 24 h or with drug therapy. Beta-blockers and amiodarone are the cornerstone of prophylaxis and treatment of de novo postoperative AF. Here the strategy of monitoring heart rate and that of monitoring heart rhythm are equally valid. Antithrombotic therapy should be based on the individual assessment of risk of thromboembolism using scales like the CHA2DS2-VASc and the HAS-BLED.23 In presence of AF with hemodynamic instability we should consider the use of biphasic waveform synchronized shocks, initially at 100 joules, (200 joules in obese patients). For the management of sustained monomorphic ventricular tachycardias synchronized shocks at 50−100 joules should be used. Regarding disorganized or pulseless ventricular tachycardias non-synchronized shocks at maximum energy should be used.
The rate of complete postoperative atrioventricular block is between 4%24 and 11%.25 It is often transient, and the indications for definitive pacemaker implantation are the same ones as in non-operated patients.
Postoperative low cardiac output syndromeManagement of right-sided heart diseaseIt is defined as that hemodynamic situation where CO cannot fully satisfy the tissue metabolic demand. However, the triggers, clinical presentation, disease progression, and treatment are different to heart failure of medical origin. Its incidence rate is somewhere between 3% and 45%, and it is associated with a higher morbidity and mortality rate, ICU stays, and use of resources.26–29 Its clinical signs are varied and go from mild myocardial stunning that may require the transient use of inotropes with full recovery within 24 h–48 h to serious cases with cardiogenic shock, multiorgan failure, and death. The early identification of clinical signs and the proper management are essential to achieve good results.
Added to the aforementioned measures, we should also:
- 1
Rule out mechanical and/or reversible causes: pneumothorax, hemothorax, excessive bleeding, cardiac tamponade, and coronary graft spasm or occlusion, prosthetic valve dysfunction, and arrhythmias (Fig. 2).
- 2
Use a stepped approach following gradual sequencies of action. First, preload should be optimized, and rhythm should be kept under control by reversing tachyarrhythmias through cardioversion and/or over-pacing in the presence of hemodynamic repercussions. In the presence of bradyarrhythmias, relative bradycardias or atrioventricular block, epicardial cardiac pacing wires should be used. Afterwards, inotropes, vasoconstrictors and/or vasodilators should be used. The last step is circulatory assist devices like the intra-aortic balloon pump, venoarterial extracorporeal oxygenation or any other types of mechanical circulatory support ventricular assist devices (Fig. 2).
In the context of cardiac surgery, right ventricular (RV) failure is more prevalent in30 high-risk coronary artery disease with right coronary artery lesion, valvular heart disease—especially in the mitral valve—, the postoperative course of heart transplant, congenital coronary artery disease, pulmonary thromboendarterectomy due to chronic thromboembolic pulmonary hypertension (TPH), and patients with LV assist devices.
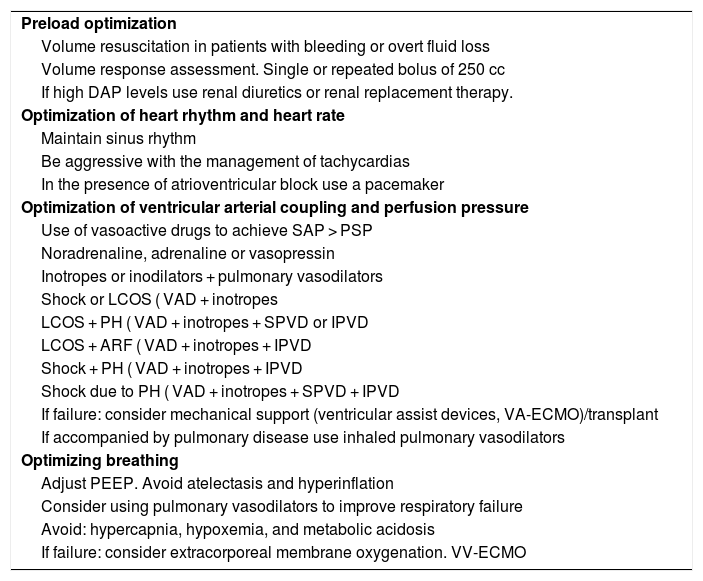
Its management requires optimizing preload, afterload, contractility, rhythm, and rate16 (Table 2). The RV cardiac output is influenced by the contraction of interventricular septum, based on the LV function, which is why in the presence of associated LV dysfunction, inotropes should be used. It is essential to avoid arterial hypotension to secure the correct perfusion of both ventricles.
Management of right ventricular failure while taking into consideration the patient’s hemodynamic situation, presence of pulmonary hypertension, and appearance of acute respiratory failure.
| Preload optimization |
| Volume resuscitation in patients with bleeding or overt fluid loss |
| Volume response assessment. Single or repeated bolus of 250 cc |
| If high DAP levels use renal diuretics or renal replacement therapy. |
| Optimization of heart rhythm and heart rate |
| Maintain sinus rhythm |
| Be aggressive with the management of tachycardias |
| In the presence of atrioventricular block use a pacemaker |
| Optimization of ventricular arterial coupling and perfusion pressure |
| Use of vasoactive drugs to achieve SAP > PSP |
| Noradrenaline, adrenaline or vasopressin |
| Inotropes or inodilators + pulmonary vasodilators |
| Shock or LCOS ( VAD + inotropes |
| LCOS + PH ( VAD + inotropes + SPVD or IPVD |
| LCOS + ARF ( VAD + inotropes + IPVD |
| Shock + PH ( VAD + inotropes + IPVD |
| Shock due to PH ( VAD + inotropes + SPVD + IPVD |
| If failure: consider mechanical support (ventricular assist devices, VA-ECMO)/transplant |
| If accompanied by pulmonary disease use inhaled pulmonary vasodilators |
| Optimizing breathing |
| Adjust PEEP. Avoid atelectasis and hyperinflation |
| Consider using pulmonary vasodilators to improve respiratory failure |
| Avoid: hypercapnia, hypoxemia, and metabolic acidosis |
| If failure: consider extracorporeal membrane oxygenation. VV-ECMO |
DAP, diastolic arterial pressure; IPVD, inhaled pulmonary vasodilators; LCOS: low cardiac output syndrome; PEEP, positive end-expiratory pressure; PH, pulmonary hypertension; PSP, pulmonary systolic pressure; SAP, systolic arterial pressure; SPVD, systemic pulmonary vasodilators; VA/VV ECMO, venoarterial/venovenous extracorporeal membrane oxygenation; VAD, vasoactive drugs.
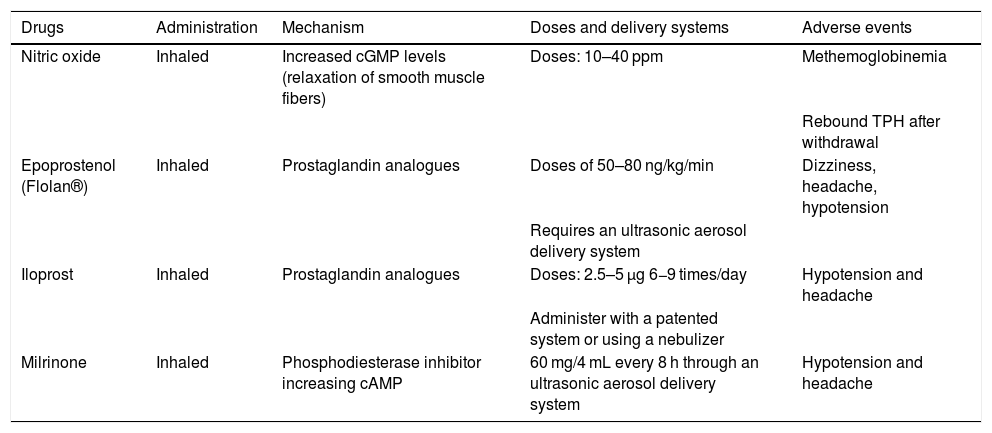
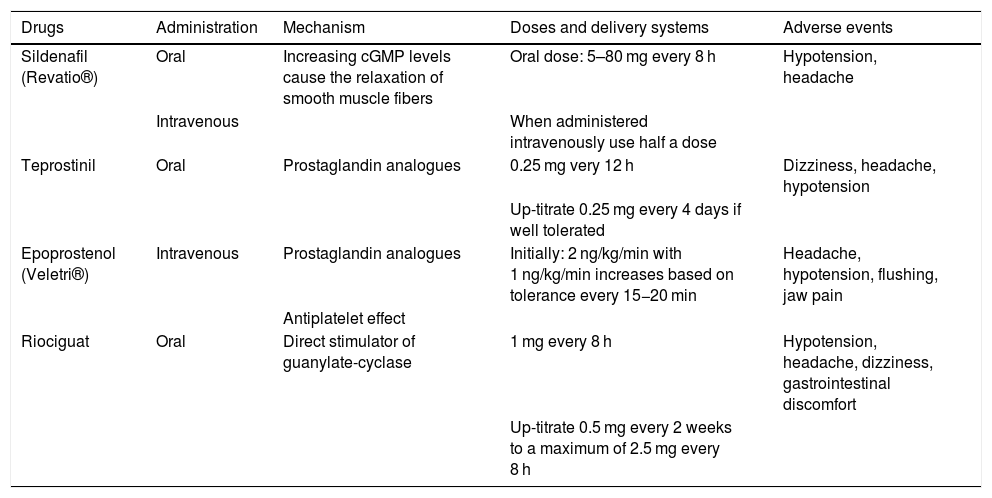
In order to reduce the RV afterload, pulmonary vasodilators (PVD) can be administered locally (inhaled or nebulized) or systemically (oral and intravenous) (Tables 3 and 4).31 Systemic vasodilation can cause arterial hypotension and create respiratory failure due to ventilation/perfusion (V/Q ratio) mismatch. Inhaled PVD cause less systemic hypotension and improve the V/Q ratio. Therefore, they are useful to treat chronic TPH, improve the V/Q ratio, and reduce the afterload of a dysfunctional RV.32
Inhaled pulmonary vasodilators.
| Drugs | Administration | Mechanism | Doses and delivery systems | Adverse events |
|---|---|---|---|---|
| Nitric oxide | Inhaled | Increased cGMP levels (relaxation of smooth muscle fibers) | Doses: 10–40 ppm | Methemoglobinemia |
| Rebound TPH after withdrawal | ||||
| Epoprostenol (Flolan®) | Inhaled | Prostaglandin analogues | Doses of 50–80 ng/kg/min | Dizziness, headache, hypotension |
| Requires an ultrasonic aerosol delivery system | ||||
| Iloprost | Inhaled | Prostaglandin analogues | Doses: 2.5–5 μg 6−9 times/day | Hypotension and headache |
| Administer with a patented system or using a nebulizer | ||||
| Milrinone | Inhaled | Phosphodiesterase inhibitor increasing cAMP | 60 mg/4 mL every 8 h through an ultrasonic aerosol delivery system | Hypotension and headache |
cGMP, cyclic guanosine monophosphate.
Systemic pulmonary vasodilators.
| Drugs | Administration | Mechanism | Doses and delivery systems | Adverse events |
|---|---|---|---|---|
| Sildenafil (Revatio®) | Oral | Increasing cGMP levels cause the relaxation of smooth muscle fibers | Oral dose: 5–80 mg every 8 h | Hypotension, headache |
| Intravenous | When administered intravenously use half a dose | |||
| Teprostinil | Oral | Prostaglandin analogues | 0.25 mg very 12 h | Dizziness, headache, hypotension |
| Up-titrate 0.25 mg every 4 days if well tolerated | ||||
| Epoprostenol (Veletri®) | Intravenous | Prostaglandin analogues | Initially: 2 ng/kg/min with 1 ng/kg/min increases based on tolerance every 15−20 min | Headache, hypotension, flushing, jaw pain |
| Antiplatelet effect | ||||
| Riociguat | Oral | Direct stimulator of guanylate-cyclase | 1 mg every 8 h | Hypotension, headache, dizziness, gastrointestinal discomfort |
| Up-titrate 0.5 mg every 2 weeks to a maximum of 2.5 mg every 8 h |
cGMP, cyclic guanosine monophosphate.
The type of drug that should be used depends on the clinical context.32–38 In patients with predominant respiratory failure and residual TPH, locally-acting drugs (inhaled nitric oxide [iNO] and/or inhaled prostacyclin) should be used to treat both entities simultaneously. In presence of hemodynamic instability, we should start with inodilators (dobutamine or milrinone) together with inhaled PVD (iNO and prostacyclin) to reduce the afterload thanks to the lower hemodynamic impairment associated. Once the situation has stabilized (significant reduction or withdrawal of vasoactive support) phosphodiesterase-5 inhibitors or prostaglandins can be started IV and at low doses. Based on the patient’s tolerance, the withdrawal of iNO and then prostacyclin can be considered (since the latter can still be used in extubated patients).
In hemodynamically stable patients without respiratory failure but significant residual TPH, home medication can be suggested and introduced gradually.
There are times that despite the optimal medical therapy, the patient’s cardiac output remains low. In these cases, it is required to initiate mechanical support measures.
Vasoplegic syndrome after cardiac surgeryIt has been reported that during PCSC up to half of the patients can develop vasoplegia (low systemic vascular resistances with CI > 2.2 L/kg/min) accompanied by distributive shock in up to 5% of them.39 Pathophysiology is similar to that of the vasoplegic shock reported in sepsis. It is important to recognize the situation early and start vasopressor therapy to achieve the proper organ perfusion. The most commonly used drug is noradrenaline. In cases that require high doses, vasopressin can be used as well.40 The role of angiotensin ii seems promising in these patients.41
Excessive postoperative bleedingExcessive postoperative bleeding is one the most common complications of cardiac surgery. It can be defined as bleeding over 1 L of blood within the first 24 h. Its incidence rate is around 33% and requires surgical reassessment in 3% to 7% of the cases.42 Proper management is a priority in this context since in up to 50% of the patients with reinterventions there is no surgical cause for the bleeding, and reassessment becomes an independent risk factor for the appearance of postoperative adverse events.43 Bleeding during PCSC is multifactorial and somehow triggered by the characteristics of the patient,44,45 the perioperative drugs used, and surgery per se.46 ECC causes a procoagulant state that triggers a pro-hemorrhagic situation (since it consumes coagulation and platelet factors) aggravated by hemodilution and the activation of the fibrinolytic system. The transfusion of hemoderivatives is directly associated with an increased morbidity and mortality rate in patients treated with cardiac surgery. It should be individualized based on clinical and functional parameters like tissue oxygenation rather than hemoglobin values. Nevertheless, without perioperative ischemic data, hemoglobin values between 7 g/dL and 8 g/dL are well tolerated during PCSC.46 Bleeding prophylaxis with tranexamic acid during the entire cardiac surgery is recommended. The management of postoperative bleeding should be conducted using target-guided algorithms. The first step is to eliminate the factors that exacerbate coagulopathy (correcting hypothermia and acidosis), reverse residual heparin induced anemia through a protamine booster shot and optimize primary hemostasis (platelet levels > 100 000 cells/cc). The first thing target-guided therapy based on viscoelastic testing recommends is to correct hyperfibrinolysis (tranexamic acid), and then the firmness of the blood clot (fibrinogen). Finally, if the synthesis of thrombin is still compromised, a prothrombin complex concentrate should be administered.
ConclusionsThe optimal perioperative management of patients treated with cardiovascular surgery requires a comprehensive and multidisciplinary approach including the proper medical-surgical training. Preoperative optimization, minimally invasive techniques, the prophylaxis of arrhythmias and postoperative bleeding, target-guided hemodynamic management, and multimodal analgesia to facilitate extubation and early mobilization are key elements in the intensified recovery of these patients. The high level of complexity reported turns the intensive medicine unit into the optimal environment to achieve the best results possible.
AuthorsJJJR, CLJ, MJLG, and JLPV participated in the writing and final approval of this manuscript.
Conflicts of interestNone reported.
Please cite this article as: Jiménez Rivera JJ, Llanos Jorge C, López Gude MJ, Pérez Vela JL. Manejo perioperatorio en cirugía cardiovascular. Med Intensiva. 2021;45:175–183.