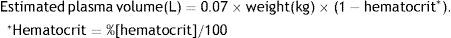Plasmapheresis is an extracorporeal technique that eliminates macromolecules involved in pathological processes from plasma. A review is made of the technical aspects, main indications in critical care and potential complications of plasmapheresis, as well as of other extracorporeal filtration techniques such as endotoxin-removal columns and other devices designed to eliminate cytokines or modulate the inflammatory immune response in critical patients.
La plasmaféresis es una técnica extracorpórea mediante la cual se procede a la eliminación de macromoléculas del plasma que se consideran mediadores de procesos patológicos. En este artículo se revisan los aspectos técnicos, las principales indicaciones en las patologías que suelen motivar ingreso en la Unidad de Cuidados Intensivos y las potenciales complicaciones de la plasmaféresis. Así mismo, se incluye una revisión de otras técnicas de depuración extracorpórea, tales como las columnas de fijación de endotoxinas y otros procedimientos que persiguen la eliminación de citoquinas o la inmunomodulación del proceso inflamatorio en el paciente crítico.
Abel et al.1 performed the first plasmapheresis procedure in 1914. In the 1970s, plasmapheresis was increasingly used to treat various conditions,2 but it was not until the 1990s that a consensus was reached about the specific but limited number of diseases for which it confers a definite benefit.3
In plasmapheresis, the plasma is separated from the blood and is processed to selectively eliminate some of its components. After processing, the plasma is reinfused. Plasma exchange is defined as the procedure in which the plasma is separated from the blood and replaced by a replacement fluid. In clinical practice, the terms plasmapheresis and plasma exchange are used synonymously, although in the vast majority of occasions, the plasma separated from the whole blood is eliminated and replaced with the same volume of another solution.
The exact mechanism through which plasmapheresis exerts its therapeutic effect is unknown, although it seems likely that plasmapheresis could work by eliminating pathologic substances from the plasma or decreasing their concentration. These harmful substances can include antibodies, immunocomplexes, monoclonal proteins, cryoglobulins, complement components, lipoproteins, toxins bonded to proteins, and other, unknown substances.
IndicationsPlasmapheresis has been used to treat diverse pathologies, especially in the fields of neurology, hematology, and rheumatology, although the grade of evidence for these treatments varies. The American Society for Apheresis (ASFA)4 periodically revises the indications for plasmapheresis and classifies them according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria.5Table 1 shows the Grade I indications (first-line therapy) and the Grade II indications (established second-line therapy). Table 2 lists the most relevant pathologies that could require therapeutic plasmapheresis in critical care patients, as well as the modality, clinical context, category, and grade of recommendation.
Category I–II ASFA indications for therapeutic plasma exchange.
| Category I ASFA: Indications for therapeutic plasma exchange (first-line therapy) |
| Acute inflammatory demyelinating polyradiculoneuropathy/Guillain–Barre syndrome |
| ANCA-associated rapidly progressive glomerulonephritis (Granulomatosis with polyangiitis; and microscopic polyangiitis) |
| Anti-glomerular basement membrane disease (Goodpasture's syndrome) |
| Chronic inflammatory demyelinating polyradiculoneuropathy |
| Focal segmental glomerulosclerosis (Recurrent in transplanted kidney) |
| Hyperviscosity in monoclonal gammopathies (Symptomatic/Prophylaxis for rituximab) |
| Liver transplantation (Desensitization, ABOi LD) |
| Myasthenia gravis (Moderate-severe/Pre-thymectomy) |
| N-methyl-d-aspartate receptor antibody encephalitis |
| Progressive multifocal leukoencephalopathy associated with natalizumab |
| Renal transplantation, ABO compatible (Antibody-mediated rejection/Desensitization, LD) Renal transplantation, ABO incompatible (Desensitization, LD) |
| Thrombotic microangiopathy, complement mediated (Factor H autoantibodies) |
| Thrombotic microangiopathy (ticlopidine drug associated) |
| Thrombotic thrombocytopenic purpura |
| Wilson's disease (Fulminant) |
| Category II ASFA: indications for therapeutic plasma exchange (established second-line therapy) |
| Acute disseminated encephalomyelitis |
| Autoimmune hemolytic anemia (severe cold agglutinin disease) |
| Cardiac transplantation (desensitization) |
| Catastrophic antiphospholipid syndrome |
| Cryoglobulinemia (symptomatic/severe) |
| Familial hypercholesterolemia (homozygotes with small blood volume) |
| Hashimoto's encephalopathy: Steroid-responsive encephalopathy associated with autoimmune thyroiditis |
| Hematopoietic stem cell transplantation, ABO Incompatible (Major HPC, Marrow/Major HPC, Apheresis) |
| Lambert–Eaton myasthenic syndrome |
| Multiple sclerosis (acute CNS inflammatory demyelinating) |
| Myeloma cast nephropathy |
| Neuromyelitis optica spectrum disorders (Acute) |
| Mushroom poisoning |
| Paraproteinemic demyelinating neuropathies/chronic acquired demyelinating polyneuropathies (IgG/IgA; IgM) |
| Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (exacerbation) |
| Renal transplantation, ABO incompatible (antibody medicated rejection) |
| Systemic lupus erythematosus (severe) |
| Vasculitis (HBV-PAN) |
| Voltage-gated potassium channel antibodies |
ASFA, American Society for Apheresis; CNS, central nervous system; HBV-PAN, hepatitis B-polyarteritis nodasa; LD, living donor; HPC, hematopoietic progenitor cells.
Indications for plasmapheresis in critical patients.
| Indications for plasmapheresis in critical patients | Category/grade | Method | Replacement fluid and dose | Factors eliminated | |
|---|---|---|---|---|---|
| Thrombotic thrombocytopenic purpura60 | This systemic thrombotic disease mostly affects small vessels. It is caused by decreased activity of the metalloprotease ADAMTS13 in plasma, which is responsible for the fragmentation of high molecular weight multimers of the Von Willebrand factor. Plasmapheresis has enabled the high mortality to be reduced to <10%. | I/1A | Plasmapheresis must be started urgently, preferably within 4–6h after diagnosis. It is done daily until a response is achieved (platelet count>150×109/L, LDH in normal range, increased hemoglobin, and disappearance of signs and symptoms), which usually takes 7–8 days. | Fresh frozen plasma. Plasma volume treated: 1–1.5. | Enables the elimination of antibodies against ADAMTS1361 and the replacement of this metalloprotease through the contents of the fresh frozen plasma. |
| Hemolytic-uremic syndrome (HUS)62 | HUS is characterized by microangiopathic (Coombs-negative) hemolytic anemia, thrombocytopenia, and acute kidney damage. The typical type is related with Shiga-toxin-producing E. coli. Atypical types are related with genetic mutations and polymorphisms involving complement-regulating proteins causing endothelial damage. Eculizumab has been associated with improvements in renal function and the interruption of plasma therapy.63 | I/2C: Anti-Factor H autoantibodies. III/2C: Mutations affecting complements. III/1C: Membrane cofactor protein mutations. | TPE for atypical HUS should be started urgently. After urgent TPE, continue with 5 sessions daily, then 5 sessions per week for 2 weeks, and finally 3 sessions per week for 2 weeks, evaluating the outcome on the 33rd day. | Plasma or albumin. Plasma volume treated: 1–1.5. | Elimination of circulating mutant antibodies or complement regulators, and replacement of the absent or defective regulators. |
| Waldenstrom's macroglobulinemia (monoclonal gammopathy).64 | Lymphoplasmacytic lymphoma associated with the production of more than 3g/dl immunoglobulin monoclonal IgM (protein M) in the plasma, which results in increased blood viscosity. | I/1B: Symptomatic. I/1C: Prophylaxis for rituximab. | Start in function of symptoms. Neurologic deterioration (stupor or coma) in the absence of intracranial bleeding requires urgent TPE. Daily until symptoms disappear (1–3 procedures), then every 1–2 weeks in function of symptoms. | Albumin. Plasma volume treated: 1–1.5. | Decrease in IgM (30–50%), associated with decreased plasma viscosity and increased capillary flow. Should be administered together with chemotherapy to reduce IgM production. |
| Guillain–Barré syndrome65 | This acute inflammatory demyelinating polyradiculoneuropathy courses with flaccid paralysis affecting the peripheral motor and sensory nerves. | I/1A: First line. III/2C: If done after IgIV | Compared to isolated support measures, TPE can accelerate motor recovery, decrease time under mechanical ventilation, and hasten improvement. For axonal involvement, TPE reportedly yields greater improvement than IgIV. 5–6 sessions on alternate days for 7–14 days. | 5% albumin. Plasma volume treated: 1–1.5. | Autoimmune disease mediated by antibodies against the myelin in peripheral nerves. |
| Acute disseminated encephalomyelitis (ADEM)66 | This acute monophasic demyelinating inflammatory disease that affects the white matter of the CNS normally occurs after a viral or bacterial infection or vaccination. | II/2C: Corticoids are considered the first-line treatment. | TPE should be considered in patients with severe ADEM that does not respond to corticoid treatment and in those in whom corticoid treatment is contraindicated. 3–6 sessions on alternate days. | 5% albumin. Plasma volume treated: 1–1.5. | Transient autoimmune response against myelin or other autoantigens. Acts by eliminating the presumptive autoantibodies generated as well as by immunomodulation. |
| Myasthenia gravis (MG)67 | Autoimmune disease characterized by weakness and fatigue on repetitive physical activity. The causal antibody is generally directed against the acetylcholine receptor (anti-AChR) on the surface of the postsynaptic motor terminal, but MG can also be caused by other antibodies. | I/1B: Moderate-Severe. I/1C: Pre-thymectomy. | Especially indicated in myasthenic crises, in the perioperative period in thymectomy, or as an adjuvant to immunotherapy. Can be more efficacious than IgIV in patients with MuSK68 | 5% albumin. Usually 5 procedures done between 7 and 14 days. | Eliminates circulating antibodies and is effective in both seropositive patients (anti-AChR) and seronegative patients (other antibodies). |
| ANCA-associated rapidly progressive glomerulonephritis (Granulomatosis with polyangiitis; and microscopic polyangiitis)69 | There is rapid loss of renal function with the histologic finding of crescent formation in over 50% of glomeruli. GPA, more often associated with c-ANCA, and MPA, more often associated with p-ANCA, are related systemic vasculitides, with ANCA positivity and similar outcomes. | I/1A: Dialysis dependence I/1B: DAH | The current management is combination therapy with high-dose corticosteroids and immunosuppressive drugs (cyclophosphamide and rituximab).70 Trials suggest that TPE is most beneficial in patients with dialysis-dependency (at presentation) and offers no benefit over immunosuppression in milder disease.71 RCTs of TPE in patients with RPGN and DAH have not been conducted. However, retrospective case series reported effective management of DAH in GPA/MPA.72 | 5% albumin; plasma when DAH present. Plasma volume treated: 1–1.5, Daily or every other day | Removes disease-associated molecules and therefore interrupts antineutrophil antibody (ANCA)-associated vasculitis. |
| Anti-glomerular basement membrane disease (Goodpasture's syndrome)73 | It is mediated by anti-glomerular basement membrane (anti-GBM) antibodies directed against a domain of the a3 chain of Type IV collagen, causing activation of the complement cascade, resulting in tissue injury due to a classic Type II reaction. Only alveolar and GBM are affected; therefore, symptoms include crescentic or rapidly pro gressive glomerulonephritis (RPGN) and diffuse alveolar hemorrhage (DAH). | I/1B: Dialysis independence I/1C: DAH | Treatment includes the combination of TPE, cyclophosphamide, and corticosteroids. It is critical that TPE be implemented early in the course of anti-GBM. Several series have demonstrated that most patients with creatinine less than 6.6mg/dL recover renal function with treatment. | Albumin; in the setting of DAH, plasma should be used for part or whole of the replacement fluid. Plasma volume treated: 1–1.5, Daily or every other day. | Rapid reduction in anti-glomerular-basement-membrane (GBM) antibody levels. |
| Cryoglobulinemia74 | Cryoglobulins are immunoglobulins that reversibly precipitate below body temperature. The aggregates of cryoglobulins can deposit on small vessels and cause damage by activating complement and recruiting leukocytes. Cryoglobulinemia is associated with a wide variety of diseases. Severe symptoms include glomerulonephritis, neuropathy, and systemic vasculitis. | II/2A: Severe/symptomatic | Management is based on the severity of symptoms and treating the underlying disorder. Double or cascade filtration and cryoglobulinapheresis have also been used to treat cryoglobulinemia. | 5% albumin, Plasma volume treated: 1–1.5. Every 1–3 daily. | Removes cryoglobulins efficiently. |
DAH, diffuse alveolar hemorrhage; GPA, granulomatosis with polyangiitis; MPA, microscopic polyangiitis; RCT, randomized control trial; TPE, therapeutic plasma exchange.
For most indications, the goal is to exchange from 1 to 1.5 times the volume of plasma, which is usually estimated with the following formula:
Methods of separationThe methods used to separate the plasma from the blood can be divided into centrifugation and filtration. Centrifugation is the older method, based on the separation of cellular elements from the plasma by rapid spinning, in which centrifugal force separates the different components according to their density, size, and molecular weight. This method has the advantage that there is no upper limit to the molecular weight of the substances to be separated out. It makes it possible to perform cytapheresis, in which cells of interest can be removed for therapeutic purposes or for later donations. The main drawback of centrifugation is the risk of thrombocytopenia. Moreover, it requires anticoagulation with citrate, so it can lead to hypocalcemia. Centrifugation is the method used by blood banks; it requires sophisticated difficult-to-transport equipment that limits its use in therapeutic apheresis in critical care environments.
In filtration, the cellular components of blood are separated from the plasma by passing the blood through a filter with large pores (0.2–0.7μm) that extracts molecules weighing up to 3millionDa. The mechanism of separation consists of applying pressure to transfer the blood across a synthetic membrane that is highly permeable due to the large size of its pores. This membrane is the central element of an extracorporeal circuit, similar overall to those used in intensive care units (ICU) for other purification treatments such as continuous renal replacement therapy (CRRT) or extracorporeal albumin dialysis with the molecular adsorbent recirculating system (MARS®). This approach requires a central venous catheter and anticoagulation with heparin. The advantages of filtration include the low risk of thrombocytopenia and the possibility of eliminating more plasma in less time. This approach also enables double filtration or cascade filtration in which the first filter separates the plasma, which is in turn passed through a second filter that has the capacity to selectively separate out certain molecules through filtration or adsorption.
Vascular accessVascular access and blood flow through the extracorporeal circuit are fundamental for the success of the procedure. Vascular access can vary depending on the plasmapheresis technique, the condition being treated, and/or the duration of the treatment. In plasmapheresis by intermittent centrifugation and short-term procedures, peripheral venous accesses that provide blood flow of 50–90ml per minute can be used. In acute processes, the most frequently used accesses are temporary central venous catheters that provide blood flow of at least 70ml per minute, making it possible to complete the procedure in 3–4h. In conditions that require chronic treatment with plasmapheresis, permanent external vascular access or arteriovenous fistulas can be used. For plasmapheresis procedures indicated in critical patients by plasma filtration, where renal dysfunction and other organ failures are probably also present, temporary central venous catheters with a double lumen are the first choice for venous access, and they can also be used for other extracorporeal support techniques.
AnticoagulationApheresis procedures require anticoagulation to prevent clots from forming in the extracorporeal circuit. Citrate, unfractionated heparin, and hirudin are all used for anticoagulation, although unfractionated heparin has long been the anticoagulant of choice for filtration plasmapheresis in ICUs. It is usually administered with an initial intravenous bolus of 40–60UI/kg followed by continuous infusion of 20UI/kg/h to maintain the activated partial thromboplastin time (aPTT) between 180 and 220s throughout the treatment.6 These doses are usually higher than those required for CRRT because a significant portion of the heparin is extracted together with the plasma. It is important to note that the loss of coagulation factors derived from the negative balance between the plasma extracted and the replacement fluid can result in a greater anticoagulant effect than might otherwise be predicted. In patients with a high risk of bleeding, the dose of anticoagulants must be considerably lower. Other authors consider that the administration of anticoagulants during plasmapheresis by membrane filtration is unnecessary.7 No published studies have looked at the risk to benefit ratio of the common practice of circuit anticoagulation, therefore further research should be conducted. In recent years, regional anticoagulation with citrate has been proposed as a new therapeutic strategy to maintain the permeability of the extracorporeal circuits, preventing early clotting and minimizing the systemic effects in the patient.8
Replacement fluidsThe characteristics of the replacement fluid will depend on the type of disease for which the treatment is being done. The volume of plasma extracted is replaced with a replacement solution with an appropriate electrolyte composition and colloid osmotic activity. The volume of replacement fluid must always be the same as that of the effluent obtained. In adults, the effluent can be replaced with crystalloid solutions only if the volume extracted is less than 1000ml. When a larger volume is extracted, it is essential to use colloids, with 4–5% human albumin being the colloid of choice. For this purpose, 20% human albumin is diluted with crystalloids or polyelectrolyte solutions. Currently, pasteurized liquid plasma proteins have the advantage of being sold in 500ml bottles, which are more economical and do not need to be manipulated; for these reasons, they have practically supplanted diluted 20% albumin as a replacement fluid.9 In patients with thrombotic thrombocytopenic purpura, coagulation factor deficiencies, or immune deficiencies, fresh frozen plasma must be used. Supplementation with intravenous immunoglobulin after plasmapheresis has been advocated to counteract progressive immunoglobulin depletion, but this provides only transient increases in levels, and is of questionable benefit.
Table 3 lists the advantages and disadvantages of the different replacement fluids.
Advantages and disadvantages of replacement fluids.
| Replacement fluid | Advantages | Disadvantages |
|---|---|---|
| Crystalloid solutions | Inexpensive | Do not maintain oncotic pressure. |
| No side effects | ||
| No risk of infection | ||
| Synthetic expanders | Inexpensive | Short half-life Depletion of plasma proteins |
| No side effects | ||
| No risk of infection | ||
| 5% albumin (pasteurized liquid plasma proteins) | Low incidence of side effects | |
| No risk of infection | Sometimes causes hypotension or nausea | |
| It is stable at room temperature and can be given without regard to blood type | Depletion of plasma proteins | |
| Iso-oncotic with plasma | ||
| Fresh frozen plasma | No depletion of plasma proteins | Expensive |
| Provides iso-oncotic coagulation factors | Risk of transmitting infections | |
| Allergic reactions/side-effects | ||
| ABO compatibility | ||
Plasmapheresis is an extracorporeal purification technique that has many indications with different grades of evidence. It is generally well tolerated and safe. The rate of complications ranges from 5% to 12% (Table 4). The most common symptoms are paresthesias, muscle cramps, hypotension, and urticaria and other anaphylactoid reactions. Most complications are mild (i.e., they do not require intervention) or moderate (i.e., they require intervention, but the plasmapheresis treatment can be completed). Severe complications (i.e., those that require plasmapheresis treatment to be discontinued) represent only 0.8% of cases.10 Although eight deaths have been reported in the more than 15,000 plasmapheresis treatments done,11 many of these occurred in patients with severe disease and the plasmapheresis procedures were not in themselves the precipitating cause. In the most recent literature, no deaths related to the technique have been detected.10,12,13
Complications of plasmapheresis 12,13
| Symptoms | Percentage (%) |
|---|---|
| Urticaria | 0.7–12 |
| Paresthesias | 1.5–9 |
| Muscle cramps | 0.4–2.5 |
| Nausea | 0.1–1 |
| Hypotension | 0.4–4.2 |
| Chest pain | 0.03–1.3 |
| Arrhythmias | 0.1–0.7 |
| Anaphylactoid reactions | 0.03–0.7 |
| Bronchospasm | 0.1–0.4 |
| Seizures | 0.03–0.4 |
| Cerebrovascular ischemia | 0.03–0.1 |
| Pulmonary edema/Respiratory failure | 0.2–0.3 |
| Myocardial ischemia/Infarct/Shock | 0.03–1.5 |
| Pulmonary embolism | 0.1 |
| Metabolic alkalosis | 0.03 |
| Hepatitis | 0.7 |
| Hemorrhage | 0.06–0.2 |
| Hemolysis | 0.01 |
| Related with vascular access | |
| Thrombosis/Hemorrhage | 0.02–0.7 |
| Infection | 0.06–0.3 |
| Pneumothorax | 0.04–0.1 |
| Mechanical | 0.08–4 |
The citrate administered as an anticoagulant for plasmapheresis bonds with calcium and can lead to symptoms of hypocalcemia. Likewise, fresh frozen plasma contains a high proportion of citrate,14 so if it is administered as a replacement fluid, it can cause the same effect. Administering albumin as a replacement fluid can also lead to hypocalcemia due to direct calcium sequestration.14 The symptoms include paresthesias around the mouth and extremities, dizziness, muscle cramps, nausea, and vomiting. Severe cases can lead to prolongation of the QT interval, arrhythmias, chest pain, seizures, and hypotension. To reduce the incidence of these complications, intravenous calcium can be administered prophylactically and/or the amount of citrate perfused can be reduced.15 Although there are many guidelines for the administration of intravenous calcium,11,16,17 one simple approach is to administer a 10ml bolus of 10% calcium gluconate every hour during the plasmapheresis procedures.14
Coagulation disorders and other hematologic complicationsDepletion coagulopathyAfter a plasmapheresis session, the serum levels of most coagulation factors decrease by about 60% when albumin is used as the replacement fluid. These levels are recovered in two phases: During the first 4h after the plasmapheresis session, there is a rapid increase that depends on the reestablishment of an equilibrium between the extravascular and intravascular compartments; in the following days, there is a slower increase that depends on the resynthesis of the coagulation factors.18 The prothrombin time (PT) increases by 30% and the aPTT doubles immediately after treatment. The aPTT returns to the normal range within 4h after the plasmapheresis session, and the PT returns to the normal range within the following 24h.15 The depletion of coagulation factors is more pronounced when 3–5 session are done in the same week, in which case total recovery can take several days.18 Despite the depletion of coagulation factors, the incidence of bleeding is low. To minimize the risk of bleeding, when 5 plasmapheresis sessions with albumin as the replacement fluid are done in a short time, it is recommended to administer 500–1000cc of fresh frozen plasma as the replacement fluid at the end of the session. This approach is more useful in patients with a greater risk of bleeding, such as those who have just undergone surgery or renal biopsy, those who need to have an intravascular catheter implanted or exchanged, or those whose underlying disease implies an increased risk of bleeding (Goodpasture syndrome or Wegener's granulomatosis).13,15
ThrombocytopeniaThrombocytopenia during plasmapheresis can be caused by multiple factors. It is most common when centrifugation is used instead of filtration. It can also be caused by the direct loss of platelets in the plasma extracted or by plasma filter clotting.15 If heparin is used for anticoagulation, we should always consider the possibility of heparin-induced thrombocytopenia.19
HemolysisThe incidence of hemolysis is very low; it is estimated at <0.01% of all treatments.12 When centrifugation is used for plasmapheresis, hemolysis can occur when the system is unduly primed with a hypotonic fluid. When filtration is used for plasmapheresis, hemolysis can occur when the pressure across the membrane is high. When the pressure surpasses 50mmHg, a plateau is reached in the filtration of the plasma and the increase in pressure across the membrane is not accompanied by an increase in the transfer of masses, increasing the risk of hemolysis.20
ThrombosisWhen albumin is used as the replacement fluid, the levels of antithrombin III (AT-III) drop. AT-III levels 24h after the session are 85% of the initial levels and can need 48–72h to recover completely.18 The incidence of thrombotic events is very low, but cases of pulmonary embolism, myocardial infarction, and ischemic stroke have been reported.15
Complications due to the replacement fluidsAdministering fresh frozen plasma as a replacement fluid can give rise to anaphylactic reactions such as fever, stiffness, urticaria, pruritus, bronchospasm, hypotension, and laryngeal edema.14 Anaphylactic reactions to albumin are much rarer; they can be associated with the formation of antibodies to polymerized albumin or they can develop in patients on angiotensin-converting enzyme (ACE) inhibitors.15 Most anaphylactic reactions are mild to moderate; only 0.1% of cases are classified as severe.13 Due to the relatively high incidence of anaphylactic reactions, patients who need plasmapheresis with massive fluid replacement with fresh frozen plasma (e.g., those with thrombotic thrombocytopenic purpura) are often pretreated with 50mg of intravenous diphenhydramine.21 In patients with previous reactions to fresh frozen plasma in whom fresh frozen plasma must be used as the replacement fluid (e.g., those with thrombotic thrombocytopenic purpura), prophylaxis can be administered, with 50mg oral prednisone 13h, 7h, and 1h administered before the plasmapheresis session, 50mg oral diphenhydramine administered 1h before the session, and 25mg ephedrine administered 1h before the session.22 If a severe reaction occurs, with refractory hypotension, severe bronchospasm, or laryngeal edema, the usual medical treatment and ICU support for anaphylactic shock must be administered. ACE inhibitors must be suspended at least 24h before any plasmapheresis procedure.23
Risk of infectionWhen albumin is the replacement fluid, the risk of an infection associated with plasmapheresis is due to the depletion of immunoglobulins (Ig). Replacing plasma with albumin results in a 60% decrease in Ig levels, and multiple plasmapheresis sessions in short time periods will lead to a drop in Ig levels that can persist for several weeks.21 Given that the depletion of Ig can worsen the patient's ability to fight infection, it is recommendable to restore the normal Ig levels with the intravenous infusion of 400mg/kg in patients who develop severe infections in the period after plasmapheresis.15 When fresh frozen plasma is the replacement fluid, the risk of an infection associated with plasmapheresis is due to viral transmission. The estimated risk of transmission is 1– 2 for every million units transfused for the human immunodeficiency virus and for the hepatitis C virus, and 1 for every 200,000–500,000 units transfused for the hepatitis B virus.24
Transfusion-related acute lung injuryTransfusion-related acute lung injury (TRALI) is characterized by the development of acute respiratory failure with non-cardiogenic pulmonary edema, often accompanied by hypotension that appears abruptly during the transfusion of blood products or within hours after the procedure. It is caused by the presence of antibodies (Ab) in the fresh frozen plasma infused and their reaction with antigens (Ag) in the patient's white blood cells. The Ag–Ab complex results in neutrophil activation and cytokine release, leading to increased endothelial permeability. There are only a few reports on development of this severe complication in small case series, therefore, the frequency and risk factor for TRALI in critically ill patients during plasmapheresis still remain to be determined in large prospective studies.25
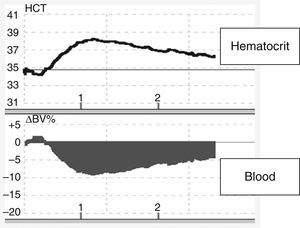
HypotensionThe incidence of hypotension varies (Table 4). Various factors can cause hypotension in plasmapheresis, including inadequate volume of replacement fluid (Figure 1), vasovagal episodes, anaphylactic reactions to different substances (replacement fluids, presence of anti-IgA antibodies in patients with IgA deficit, biocompatibility of membranes used in the plasma filter, sensitivity to ethylene oxide), arrhythmias induced by hypocalcemia and/or hypokalemia, generation of bradykinins in patients on ACE inhibitors, bleeding (related to the underlying disease, to the use of heparin as an anticoagulant, or to depletion coagulopathy), TRALI, pulmonary embolism, cardiovascular collapse, or factors related to the underlying disease, such as autonomic dysfunction in patients with Guillain–Barre syndrome.15
Changes in blood volume in plasmapheresis with albumin replacement fluid using an online volume monitor. Variation in blood volume (Crit-Line®). Starting plasmapheresis in a patient with a myasthenic crisis and replacement with 5% albumin solution. There is an abrupt drop in blood volume (about 10%), probably mediated by the transfer of fluids from the vascular space to the interstitial space due to the drop in the colloid osmotic pressure.
Commercial solutions containing 5% albumin contain less than 2mmol/l potassium. After a plasmapheresis session with albumin replacement fluid, serum potassium levels decrease by 25%, and this can lead to problems in patients with a history of arrhythmias and in those being treated with digoxin. To avoid hypokalemia in these patients, 4mmol of potassium can be added for every liter of 5% albumin.15
Metabolic alkalosisThe development of metabolic alkalosis is a very uncommon complication in patients undergoing plasmapheresis, although the risk of this complication increases when citrate is used as an anticoagulant and/or when fresh frozen plasma is administered, and when the patient has renal failure.14
Decreased levels of cholinesterase in plasmaLevels of cholinesterase in plasma decrease by 50% after a single plasmapheresis session with albumin replacement, and this can lead to prolonged apnea after the use of succinylcholine or other anesthetic agents that depend on serum cholinesterase for metabolism. Replacing cholinesterase with fresh frozen plasma is a treatment option in these clinical situations.15
Reactions to the biocompatibility of the membrane and to ethylene oxidePoor biocompatibility of the membrane used in the plasma filter can cause hypotension, dyspnea, and chest pain. These symptoms can also arise in patients who are sensitive to ethylene oxide, which is used as a sterilizing agent. With the use of more biocompatible filters and correct filter priming, the incidence of these complications is very low.15
Complications related with vascular accessComplications related with vascular access represent 1% of all complications. These include thrombosis, hemorrhage, infections, and pneumothorax.
Complications related with the elimination of drugsFactors that favor the elimination of drugs during plasmapheresis include greater bonding of the drug to proteins (>75%), lower volume of distribution (<0.3L/kg), and a shorter time between the administration of the dose and the start of plasmapheresis. Whenever possible, drugs should be administered after plasmapheresis.26
Other extracorporeal purification techniques in critical patientsIn this section, we review other extracorporeal purification techniques, such as endotoxin removal columns and other procedures that aim to eliminate cytokines or to affect the immunomodulation of the inflammatory process in critical patients.
Endotoxin removal columnsThe incidence of sepsis and septic shock is high in critical patients. Despite continual improvements in the management of sepsis and septic shock, these entities remain among the principle causes of death in the ICU. The pathophysiology of sepsis and septic shock involves several mediators released by the leukocytes, macrophages, and endothelial cells, such as cytokines, lysosomal enzymes, nitric oxide, and substances produced by oxidative stress. In infections due to gram-negative bacteria, these mediators are released in response to the endotoxin produced by the germs. Endotoxin is a component of the gram-negative bacteria's external membrane and one of the main causes of septic shock in patients with abdominal, urinary, or biliary infections. The concentration of endotoxins in the plasma is high in these infections, and the concentration correlates with prognosis and mortality.27 There is a system for detecting endotoxin in plasma based on the oxidation of neutrophils (measured by oxidation with luminol, which emits light) after it has been marked when a complex composed of endotoxin and a specific anti-endotoxin antibody is detected.28 Since this system was introduced, various studies have established different levels of endotoxemia. In patients with sepsis and septic shock induced by gram-negative bacilli, values above 500pg/ml (>0.6EU/ml) are considered significant.27
There are currently different extracorporeal devices used to eliminate endotoxins from plasma by hemoperfusion/adsorption. These treatments are based on the use of adsorbents made up of resins or carbons capable of eliminating endogenous and exogenous toxins by combining with them.
Polymyxin B hemoperfusionLike polymyxin E (colistin), polymyxin B belongs to the group of cationic polypeptide antibiotics. Both have efficacious antimicrobial activity against gram-negative bacilli; however, nephrotoxicity and neurotoxicity are important limitations for both. Polymyxin B is characterized by strong bonding with circulating endotoxin (1:1). Polymyxin B has been incorporated into polystyrene and polypropylene fibers in a device (Toraymyxin®) that has a high capacity for the adsorption of endotoxin. Since the drug does not enter the systemic circulation, its secondary effects are not problematic. Hemoperfusion treatment with polymyxin B takes 2h (saturable mechanism), and it is recommended to carry out two treatments in two consecutive days. Treatment should be started early in cases of elevated endotoxemia. The first studies of this device done in Japan, where it was invented, found decreased levels of endotoxin in plasma, improved hemodynamics, and a decrease in mortality. In 2005, the first European multicenter randomized prospective study was published.29 This study included 35 patients (17 in the polymyxin B group and 18 controls) with septic shock secondary to intraabdominal infection. A single session of hemoperfusion improved the cardiac index but did not significantly reduce the levels of endotoxin in plasma, SOFA scores, ICU stays, days on mechanical ventilation, or 28-day mortality. In 2007, a meta-analysis30 of 28 publications (9 randomized controlled trials, 7 non-randomized parallel studies, and 12 pre-post design studies) including a total of 1425 patients, of whom 978 had received treatment with polymyxin B, found that Toraymyxin® treatment was associated with a 21.2pg/ml decrease in the concentration of endotoxin (95% CI: 17.5–24.9pg/ml; p<0.001), a 19mmHg increase in mean arterial pressure (95% CI: 15–22mmHg; p<0.001), a 1.8μg/kg/min decrease in the dose of vasoactive amines (95% CI: 0.4–3.3μg/kg/min; p=0.01), a 32-unit increase in the Pa02/Fi02 ratio (95% CI: 23–41 units; p<0.001), and a halving of the 28-day mortality risk (RR 0.53; 95% CI: 0.43–0.65; p<0.001). However, in general, the methodological quality of the studies included was poor (Jadad scale<3) due to the low number of patients included, incorrect randomization, and/or lack of double blinding. Two years later, the EUPHAS study was published.31 In this multicenter randomized controlled trial, patients with septic shock secondary to abdominal infection who underwent emergency surgery were randomized within 6h of the intervention to receive either conventional treatment (30 patients) or conventional treatment plus polymyxin B hemoperfusion (34 patients) administered in two 2-h sessions in two consecutive days. The results showed improved mean arterial pressure with a reduction in the need for vasopressors, improved SOFA score, and a decrease in 28-day mortality (53% in the conventional treatment group vs. 32% in the polymyxin B group; adjusted HR 0.36: 95% CI 0.16–0.8). The ethics committee stopped the study early after the intermediate analysis showed decreased mortality in the treatment group. The major limitations were that the trial was not blinded, endotoxin activity was not measured, and likelihood ratios was used instead of Fisher's test, the preferable approach to estimating mortality. In 2013, Zhou et al.32 published a meta-analysis of the efficacy of blood purification treatments in patients with sepsis that showed a positive effect on survival in patients receiving hemoperfusion with polymyxin B. However, these results were not corroborated in another study based on propensity score matching, which found no improvement in survival in surgical patients with septic shock secondary to abdominal infection.33 Another randomized controlled trial, ABDOMIX,34 published in 2015, studied patients with septic shock due to peritonitis who required surgical intervention. A large number of patients were included in each arm (119 treated with hemoperfusion with polymyxin B vs. 113 controls), but no differences in 28-day mortality were found between groups. However, many patients received only one treatment and there were many cases of filter clotting, so we can deduce that polymyxin B was inadequately administered in a large proportion of patients in the treatment group. Another randomized controlled trial, EUPHRATES,35 is currently recruiting patients with septic shock and high endotoxin levels (EAA>0.6) in 50 ICUs in the USA and Canada. Its results will probably provide valuable information about the efficacy of hemoperfusion with polymyxin B. Until the results are available, following the indications proposed by Candel et al.,36 hemoperfusion with polymyxin B should be considered in patients with septic shock due to abdominal or urinary infections with gram-negative bacteria that do not improve within 6–12h after appropriate resuscitation and standard active and aggressive treatment.
LPS adsorber (Alteco Medical AB)This device has two porous polyethylene discs covered with a synthetic peptide that has a high capacity for the adsorption of endotoxins. To date, most publications about this device have been observational studies reporting some positive results but including few patients.37,38 Yaroustovsky et al.39 compared the LPS Adsorber versus hemoperfusion with polymyxin B in a small number of patients with sepsis due to gram-negative bacteria and found no differences in the outcomes achieved with the two approaches. In another study, Adamik et al.40 observed that the LPS adsorber effectively eliminated endotoxins in patients with septic shock, resulting in significant improvements in hemodynamic parameters and organic dysfunction, although without repercussions in the ICU stay or mortality. Further studies are necessary to determine the efficacy of this device.
Oxiris (Gambro–Hospal–Baxter)This polysulfone and polyacrylonitrile (AN-69) filter has ample adsorption of inflammatory cytokines and endotoxin, but clinical experience is limited given the scant number of studies published.41
MATISSE-Fresenius systemBased on endotoxin's affinity for human albumin, this system incorporates albumin in a polymethacrylate filter. Although the in vitro studies were promising, the clinical studies have focused mainly on safety and tolerance and have yet to be able to demonstrate clinical efficacy.42
Other extracorporeal purification strategies targeting cytokine eliminationHigh volume hemofiltrationSince Ronco et al.43 found improved survival in critical patients with acute renal failure treated with high rates of ultrafiltration (≥35ml/kg/h), there has been a trend toward using high filtration volumes in patients with severe sepsis/septic shock with the aim of affecting the levels of circulating cytokines, the systemic inflammatory state, and the hemodynamic situation.
Later studies found benefits with higher ultrafiltration rates, so after the Pardubice consensus conference,44 high volume hemofiltration (HVHF) was redefined as continuous high volume treatment with an effluent rate between 50 and 70ml/kg/h for 24h per day or intermittent treatment with an effluent rate between 100 and 120ml/kg/h for 4–8h followed by hemofiltration at conventional doses.
On the other hand, it has been postulated that applying HVHF in the early phases of sepsis could eliminate or reduce the peak levels of proinflammatory and anti-inflammatory cytokines. This elimination of cytokines from the bloodstream would create a gradient that would favor the extraction of these cytokines from the tissues, thus limiting damage to organs.45,45,46 In a meta-analysis, Lehner et al.47 point to the possible usefulness of HFHV pulses>50ml/kg/h in reducing the levels of circulating cytokines, and some of the studies included also found a greater decrease in the doses of vasoactive amines.
Nevertheless, other authors48,49 found no benefits in survival or in renal function with doses greater than 25–30ml/kg/h. A recent multicenter clinical trial that randomized patients to receive either HVHF (70ml/kg/h) or standard hemofiltration (35ml/kg/h) found no significant differences in mortality, improvements in hemodynamics or organ dysfunction, length of mechanical ventilation, time requiring CRRT or recovery of renal function, ICU or hospital stay, or adverse effects attributable to the technique.50 These findings are in line with those obtained in another later systematic review and meta-analysis.51
In summary, the heterogeneity of the studies hinders their inclusion in reviews and meta-analyses, so few of these types of studies have been done. Not enough evidence has been published to recommend the systematic use of HVHF at the reported doses in septic patients with acute renal failure.
Coupled plasma filtration and adsorptionTreatment with coupled plasma filtration and adsorption (CPFA) comprises 3 phases: (1) Plasma filtration. The patient's blood is passed through a filter that allows the components of plasma to cross the membrane while the cells are returned to the patient. (2) Purification. Next, the plasma passes through an absorbent cartridge composed of a resin that enables the absorption of inflammatory mediators and endotoxins, and then the treated plasma is returned to the circulation. (3) Hemofiltration or hemodialysis to eliminate water and low-molecular-weight toxins. This is made possible by a second filter.
Studies done in vitro have shown the efficacy of CPFA in adsorbing interleukins and TNF-α.52 They have also observed a decrease in the need for vasoactive amines in patients with septic shock,53 and CPFA has proven superior to HVHF in eliminating inflammatory mediators in septic patients with multiple organ failure.54 A recent prospective multicenter study was stopped for futility after the investigators were unable to find a decrease in mortality in patients with septic shock treated with CPFA.55 Nevertheless, a subgroup analysis showed that hospital mortality decreased in patients with greater volumes of plasma treated, a finding that was also suggested in another study in patients with septic shock and multiple organ failure.56 Evidence for CPFA in sepsis is sparse; studies are heterogeneous, include few patients, and suffer from various methodological shortcomings, so further research is required.
High cutoff membranesMembrane cutoff is defined as the mean value of the molecular weight of molecules with a sieving coefficient of 0.1. Clinically, this point is defined by the molecular weight of the largest molecule that passes through the membrane (≥60.000Da). Morgera et al.57 found that hemofiltration with high cutoff membranes was superior to conventional hemofiltration in the elimination of IL-6 and IL-1ra and in the reduction of doses of noradrenalin in septic patients with acute renal failure. Later reviews found a significant improvement in hemodynamic parameters and in oxygenation indices in septic and non-septic patients.58 Using convection techniques with this type of membranes has proven to be an efficient way to reduce the levels of circulating cytokines, although these techniques are associated with high albumin washout. The exclusive use of diffusion seems to be the most appropriate option for increasing cytokine elimination and reducing excessive albumin loss.59
Finally, Table 5 describes other extracorporeal depurative techniques with limited clinical experience.
Other extracorporeal depurative techniques with limited clinical experience.
| Cytosorb | DALI® | CTR column | Renal assist device (RAD) |
|---|---|---|---|
| Cytosorb is composed of porous polystyrene divinylbenzene material that does not neutralize endotoxin but does reduce the levels of circulating cytokines (IL-1ra, IL-6, IL-10, IL-8, IL-1α) in animal models and in humans with severe sepsis or septic shock. It has shown short-term mortality benefits, although in studies with few patients.75 | This device enables the direct adsorption of lipoproteins and LDL cholesterol in patients with hypercholesterolemia refractory to conventional medical treatment.76 | Created by modifying the cellulose pores of an adsorption column and combining them with a hydrophobic organic ligand, the CTR column makes it possible to mobilize cytokines and other molecules (enterotoxins) with molecular weights between 5000 and 50,000Da. In studies in rats, the CTR column decreased the inflammatory response after endotoxin injection and also reduced mortality.77,78 | Experiments demonstrated that human renal tubule cells could be isolated and incorporated into a hemofilter to make a device containing more than 109cells. In phase I and phase II clinical trials, these bioartificial kidneys have shown significant clinical effects on the recovery of renal function and on survival, as well as an acceptable safety profile. The data also suggest that this device could also decrease morbidity and mortality thanks to its ability to alter the proinflammatory response in patients with renal failure.79,80 |
Plasmapheresis is the procedure through which macromolecules are removed from plasma for therapeutic purposes. The clinical benefits are based on the elimination of pathologic substances or on the replacement of abnormal components of plasma. The clinical indications are periodically revised by the American Society for Apheresis. The most important diseases in the ICU that could require plasmapheresis are thrombotic microangiopathies, hyperviscosity syndromes, Guillain-Barré syndrome, acute disseminated encephalomyelitis, myasthenia gravis, rapidly progressive ANCA-positive glomerulonephritis, anti-glomerular basement membrane antibody disease, cryoglobulinemia, and kidney transplantation. Plasmapheresis with plasma filters is well known in ICUs today. It is done with extracorporeal circuits adapted to the usual CRRT monitors. Plasmapheresis is relatively safe, and complications are usually minor. The estimated mortality is less than 0.1% of all procedures.
Nowadays, there are various devices that eliminate endotoxin and can be used as adjuvant treatment for septic shock caused by gram-negative bacilli. The most widely studied of these is polymyxin B hemoperfusion; although its indication awaits the results of a large multicenter study, it can be considered as a rescue treatment in carefully selected patients in whom septic shock does not improve within 6–12h of optimal treatment. Other strategies that target the elimination of cytokines or the immunomodulation of the inflammatory process have not demonstrated a clear clinical benefit, so their routine use outside clinical trials is not recommended.
FundingThere has been no funding for this writing.
Conflict of interestsThe authors declare that they have no conflict of interest.