Hyponatremia is the most prevalent electrolyte disorder in Intensive Care Units. It is associated with an increase in morbidity, mortality and hospital stay. The majority of the published studies are observational, retrospective and do not include critical patients; hence it is difficult to draw definitive conclusions.
Moreover, the lack of clinical evidence has led to important dissimilarities in the recommendations coming from different scientific societies.
Finally, etiopathogenic mechanisms leading to hyponatremia in the critical care patient are complex and often combined, and an intensive analysis is clearly needed. A study was therefore made to review all clinical aspects about hyponatremia management in the critical care setting. The aim was to develop a Spanish nationwide algorithm to standardize hyponatremia diagnosis and treatment in the critical care patient.
La hiponatremia es el trastorno electrolítico más prevalente en las Unidades de Cuidados Intensivos. Se asocia a un aumento de la morbilidad, mortalidad y estancia hospitalaria. La mayoría de los estudios publicados hasta el momento son observacionales, retrospectivos y no incluyen pacientes críticos, lo que dificulta la extracción de conclusiones sólidas. Además, debido a la escasa evidencia científica de calidad, incluso las recomendaciones realizadas por distintas sociedades científicas recientemente publicadas difieren en aspectos importantes como son el diagnóstico o el tratamiento de la hiponatremia.
Los mecanismos etiopatogénicos en los pacientes críticos suelen ser complejos. Sin embargo, hay que profundizar en ellos para llegar al diagnóstico más probable y a la pauta de tratamiento más adecuada. Todo ello, ha motivado la realización de esta revisión práctica sobre aspectos útiles en el abordaje de la hiponatremia en las Unidades de Cuidados intensivos, con el objetivo de homogeneizar el manejo de esta entidad y disponer de un algoritmo diagnóstico a nivel nacional.
Hyponatremia (HN) is the most frequent electrolytic disorder in the Intensive Care Unit (ICU). It therefore seems reasonable to develop a document addressing HN in the critical patient from a practical perspective. Two important guides have been published to date,1,2 though some of their recommendations are not applicable to the critically ill due to the type of disease involved and the severity of the patient condition (e.g., water restriction in neurocritical patients).3 Furthermore, the guides present controversial points particularly as refers to treatment, since the American guides indicate vaptans for the management of euvolemic and hypervolemic HN, while the European guides do not indicate them in any case.4
The present study was therefore carried out to offer a practical review of useful aspects in dealing with HN in Spanish ICUs, with the aim of clarifying, homogenizing and providing a common algorithm for the management of this disorder.
Methodology used to produce the documentDrafting of this document was proposed by its coordinators, and it was developed by a group of Spanish intensivists chosen by the coordinators, with experience in the management of critical patients in different scenarios (neurocritical, cardiac, postsurgical, hepatic and multiple disorders), and forming part of the different working groups of the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias [SEMICYUC]), in collaboration with Otsuka Pharmaceutical. The document received the scientific endorsement of the SEMICYUC.
The document was developed on the basis of three phases and meetings. The first meeting involved presentation of the questions raised by the coordinators to the members of the group of specialists. The items were discussed, and consensus was reached among all the members, with assignment of the questions among the participants. It was agreed that each question should be reviewed by two experts, and the literature was distributed following an initial global search conducted by the coordinators. This search comprised a PubMed review of the available scientific literature (in English and in Spanish) covering the period from 1 January 2000 to 31 December 2017, and including observational studies (prospective and retrospective), clinical trials, meta-analyses and reviews. Those studies not involving humans were initially excluded. The literature search terms used were hyponatremia «and»: algorithm, classification, critical care, diagnosis, differential diagnosis, etiology, euvolemia, heart failure, hypervolemia, hypovolemia, incidence, liver cirrhosis, neurocritical care, physiopathology, prognosis, recommendations, syndrome of inappropriate ADH (antidiuretic hormone) secretion, surgery, symptoms, treatment, vasopressin, vaptans, wasting salt syndrome.
Posteriorly, and according to the criterion of each expert, additional references were selected and added based on the abovementioned articles or following a second literature search where considered necessary.
The second meeting was used to submit the answers, define the key concepts related to each question, and introduce the opportune changes based on consensus among all the experts. The last meeting in turn served to discuss the writing and extent of the draft, which was reviewed and approved by all the signing participants.
Thus, the conclusions and practical recommendations of this document are largely fundamented upon the experience and opinions of the authors, and supported by a comprehensive review of the current literature.
Definition of hyponatremiaHyponatremia is defined as a plasma sodium concentration ([Na+]p) of under 135mEq/l.5 However, the risk threshold for starting treatment depends on the background disease involved. In this respect, the threshold in neurocritical patients is considered to be [Na+]p<140mEq/l,6 versus [Na+]p<130mEq/l7 in patients with liver failure, and [Na+]p<130mEq/l8 in patients with heart failure.
It is important to take into account that the clinical diagnosis in the critical patient is more difficult to establish than in other scenarios, due to the previous treatments and background disease involved. Furthermore, HN in these patients may present with clinical manifestations similar to those of the disorders giving rise to hyponatremia.9
Incidence and prognosis of hyponatremia in the critical patientIn hospitalized patients, the incidence of HN is close to 30% (when defined as <135mEq/l),10 but it is difficult to establish the impact of HN in the ICU, due to the scarcity of studies made with this purpose in mind. In any case, the reported incidence is between 12 and 34%, depending on the series (Table S1 of supplementary material). Although there are studies that have evaluated the association between HN and hospital stay, quality of life or mortality, quality issues prevent us from establishing whether there is a causal relationship or not (Table S1 of supplementary material). Different hypotheses have been proposed to explain the association, such as the notion that the high mortality rate observed among hospitalized patients with HN could be more an expression of the severity of the background disease than of the direct impact of HN as such – since patients with extremely low sodium values present lesser mortality.11 Another hypothesis is that very low plasma sodium values are detected earlier, and are therefore quickly treated, whereas sodium values in a more moderate range might be regarded as unimportant – thereby resulting in delayed treatment that may worsen the morbidity–mortality figures.12
Physiopathology of hyponatremiaFrom the physiopathological perspective, subtle increments in osmotic pressure (Osmp) (1–2%) are detected by the hypothalamic osmoreceptors, which in turn stimulate the thirst center, with the synthesis of antidiuretic hormone (ADH) or vasopressin-arginine.5 This hormone acts upon the collecting tubules, and water is reabsorbed via the type 2 aquaporins (AQ2) (water channels) to normalize Osmp (280–296mOsm/kg)13 and increase urine concentration. There are other stimuli more potent than Osmp that also release ADH (e.g., a decrease in effective circulating volume [ECV], stress, pain, nausea or drugs14). In this way, dilute urine (osmolality<100mOsm/kg) with low urinary potassium and sodium ([Na++K+]u<[Na+]p) is indicative of the inhibition ADH.15 The simultaneous determination of the plasma and urinary concentration of Na+, K+ and of osmolality, as well as the estimation of total body water and volemia, are key elements for establishing the etiology of HN. Table 1 provides an orientative summary of the most common clinical scenarios and the behavior of the parameters that help to distinguish them, though some cases may differ from those described (diuretics) or may not be interpretable (renal failure).
Interpretation of laboratory test values in hypotonic hyponatremia (Osmp<280mOsm/kg).
| Total body water | Volemia (ECV) | ADH secretion | ADH effect | [K+]+[Na+]u (free water excretion) | Osmu (mOsm/kg) | [Na+]u | Examples | Treatment |
|---|---|---|---|---|---|---|---|---|
| Hypervolemic | High | High, non-osmotic | Water retention>sodium | [K+]+[Na+]u>[Na+]p(decreased) | High(>Osmp) | Variable | Cirrhosis, CHF, nephrotic syndrome | Correction ECV |
| Normovolemic | Normal | High, non-physiological | No pathological water excretion | [K+]+[Na+]u>[Na+]p(decreased) | High(>100) | High | SIADH | Treatment SIADH |
| Normal | No sufficient water excretion | [K+]+[Na+]u<[Na+]p(elevated, insufficient) | Low(<100) | Low | Polydipsia | Water restriction | ||
| Hypovolemic | Low | High, osmotic | Water retention>sodium | [K+]+[Na+]u>[Na+]p(decreased) | High(>100) | Low | Dehydration | Hydration |
ADH: antidiuretic hormone; CHF: congestive heart failure; [K+]+[Na+]u: sum of urinary concentration of sodium and potassium; [Na+]u: urinary concentration of sodium; Osmp: plasma osmolality; Osmu: urine osmolality; SIADH: syndrome of inappropriate ADH secretion; ECV: effective circulating volume.
Acute HN can cause brain edema as a consequence of the osmotic gradient generated by hypoosmolarity and that causes water to penetrate into the glial cells.16 There are mechanisms allowing the brain to adapt to HN (outflux of inorganic ions [K+ and Cl−]17 thanks to the Na-K-ATPase pump18 and of osmotic metabolites), and which come into effect in the presence of HN for over 48h,19–21 reducing the brain edema.22 Some individuals are characterized by poorer adaptation to HN,23 such as women of child-bearing age and patients with hypoxemia, since estrogens and hypoxemia block the Na-K-ATPase pump, preventing water outflux from the intracellular compartment.24
On the other hand, having established the brain adaptation mechanisms, rapid correction of HN would give rise to a hypertonic extracellular environment15 that can cause cell dehydration and structural neuronal damage25,26 known as cerebral osmotic demyelination syndrome (ODS) – the consequences of which can range from attention deficit to coma and death.27,28 Factors favoring the development of ODS are malnutrition, hypopotassemia, alcoholism, cirrhosis and very low natremia levels, among others.1,29
Practical concepts referred to the critical patient with hyponatremiaQuestion one. How is hyponatremia classified?Practical recommendations- •
Hyponatremia can be classified according to natremia, tonicity, volemia, symptoms and/or speed of onset.
- •
It is considered important to individualize the risk threshold of [Na+]p according to the patient clinical context in order to start treatment.
- •
It is preferable to assume as chronic any presentation of hyponatremia if we do not know its speed of onset.
Due to the complexity of the critical patient with hyponatremia, its classification should be based on a series of concepts – the most widely used being natremia,30 tonicity,13 volemia,31,32 symptoms33 and/or speed of onset 22,34(Table 2).
Classification of hyponatremia according to different criteria.
| According to plasma concentration of sodium | ||
|---|---|---|
| Natremia | [Na+]p (mEq/l) | Analytical techniques |
| Mild | 134–130 | Recommended for measurement of [Na+]p:•Direct measurement•Always the same technique•Selective ion flux electrode |
| Moderate | 129–125 | |
| Severe | <125 | |
| According to tonicity | ||||
|---|---|---|---|---|
| Tonicity | Osmp (mOsm/kg) | Degree of osmolality | Example | It is advisable to measure Osmp rather than calculate it |
| Hypotonic | <280 | Hypoosmolar | SIADH | |
| Isotonic | 280–290 | Isosmolar | Hyperlipidemia | |
| Hyperproteinemia | ||||
| Hypertonic | >290 | Hyperosmolar | Hyperglycemia | |
| Mannitol | ||||
| According to symptoms | |
|---|---|
| Degree of severity | Symptoms |
| Mild | No altered consciousness: headache, asthenia, weakness, attention deficit, memory or gait alterations, slowed mental reaction |
| Moderate | Altered consciousness: nausea, vomiting, disorientation, delirium, confusion, drowsiness, weaning difficulties |
| Severe | Neurological dysfunction: stupor, seizures, coma, brain herniation, death, non-cardiogenic lung edema |
| According to speed of onset | |||
|---|---|---|---|
| Onset time | Associated symptoms | If not known, it is advisable to assume hyponatremia as chronic, since treatment will be less aggressive, in order to avoid risk of osmotic demyelinating syndrome (ODS) | |
| Acute | <48h | More frequent neurological symptoms (greater brain edema) | |
| Chronic | >48h | Attention deficit, altered balance, osteoporosis and bone fractures, falls | |
We must discard possible pseudohyponatremia or isotonic HN,35 which constitutes a laboratory artifact found in the presence of high blood lipid or protein concentrations, where a relative increase is detected in the solid phase of plasma that can be avoided by using direct selective ion flux techniques.35 On the other hand, when plasma contains osmotically active particles (hyperglycemia, mannitol, sorbitol or contrast media) that increase plasma tonicity, water leaves the intracellular compartment, reducing [Na+]p. These are cases of hypertonic HN known as HN.36 It is important to detect these scenarios, since specific treatment is only required in the case of hypotonic or hypoosmolar HN.
One of the most important classifications is based on the presence or absence of symptoms.
The current tendency is to consider that all HN presentations have symptoms, though these may go unnoticed because they are very subtle or are masked by the background disease process, particularly in elderly patients, where an increased incidence of falls, osteoporosis, attention deficits, fractures and gait instability have been documented.37 However, in the critical patient, it is still practical to consider HN as either symptomatic or asymptomatic according to the presence or absence of neurological symptoms, which are the factors deciding the urgency of treatment.
The risk threshold for starting the treatment of hyponatremia depends on the background disease and the clinical context – the criterion being more strict in the case of neurocritical patients.3,38,39 If the speed of onset is not known, and there are no serious neurological symptoms attributable to HN, it is preferable to assume the latter as being chronic, in order to minimize the risk of ODS.24,40
Question two. What is the importance of volemia evaluation in the hyponatremic critical patient?Practical recommendations- •
Try to obtain an approximate and precise assessment of volemia in the patient with hyponatremia in order to secure a correct etiological diagnosis.
- •
Combine different methods for the evaluation of volemia and the obtainment of a differential diagnosis of hyponatremia.
- •
Avoid using only clinical criteria for assessing total body water and ECV in the critical patient, particularly in distinguishing between normo- versus hypovolemic patients.
Patients with HN can present hypo-, normo- or hypervolemia.31 In the critical patient, precise assessment of volemia and body water is very complex, and analytical or clinical parameters32 alone are not enough, since several causes of HN may coexist, along with specific factors that alter the exploration. In order to obtain a global view and avoid potential errors, a comprehensive evaluation is advisable – not only parametric assessment of volemia – taking into account the clinical elements, time course and volemia estimators. Traditionally, extracellular volume has been used as a reflection of ECV, and has been the parameter evaluated first in order to obtain a physiopathological interpretation.9 However, ECV is the variable that conditions the mechanisms that favor the development of HN, and is therefore the key to correct diagnosis. In this document we use the terms ECV (effective circulating volume or volemia) versus total body water in order to lessen ambiguity. The estimation of ECV is not easy41–44 and requires both the evaluation of clinical aspects and the use of instrumental methods (Table 3). The estimation is more complex when edematous conditions coexist with low and ineffective ECV (congestive heart failure, nephrotic syndrome or cirrhosis), since these scenarios are characterized by stimulation of the non-osmotic secretion of ADH and of the renin-angiotensin-aldosterone system,45 with the consequent decrease in excretion of free water and the appearance of HN.46Table 3 details the methods available for assessing effective volemia, though we are aware of the difficulties involved. Although the clinical history is crucial, in cases of doubt we can use hemodynamic monitoring to complement the diagnosis, though caution is required when using this strategy in the context of HN.
Methods for estimating hydration status in the critical patient.
| Methods | Hypovolemia | Normovolemia | Hypervolemia | Advantages | Limitations | |
|---|---|---|---|---|---|---|
| Non-instrumental | ||||||
| Clinical history | Renal and extrarenal losses.Negative balance | Neutral balance | Cirrhosis, CHF, nephrotic syndrome, fluid therapy. Positive balance | Always indicated | Not objective | |
| Physical examination | Diminished ocular tone, mucocutaneous dryness, weight below basal | No data of hypo- or hypervolemia.No weight changes | Edemas, ascites, pleural effusion, weight gain | Always indicated | Not objective | |
| Static instrumental | ||||||
| Bioimpedance | Low body water percentage | Water, fat and lean mass balanced | High body water percentage | Noninvasive | No experience in ICUAltered if hyponatremia | |
| Ultrasound | IVCd | <12mm (Δresp>50%) | bAn intermediate value is not evaluable | >20mm (Δresp<50%)A high value is exceptional in hypovolemia, but does not imply hypervolemia | NoninvasiveBasic ultrasound | Learning curve.Interfered by MV and AW |
| E/e’ ratio | <8Low values suggested non-elevated pressures, though a low value does not imply hypovolemia | b | >15High values are associated to high ventricular filling pressures, more frequent in hypervolemic patients | Noninvasive | Also, only if LVEF>40%.Advanced ultrasound | |
| E/A ratio | <1 (E<50cm/s)Low values suggested non-elevated pressures, though a low value does not imply hypovolemia | b | >2 (TD<150ms)High values are associated to high ventricular filling pressures, more frequent in hypervolemic patients | Noninvasive | Also, only if LVEF<40%.Advanced ultrasound | |
| CVP (mmHg) | <5 | b | >12 | General use | Invasive.Useful only in extreme values | |
| PCP (mmHg) | <5 | b | >15 | Gold standard | ||
| ITBVI | <850ml/m2 | b | >1000ml/m2 | Precise static variable | ||
| GEDI | <600ml/m2 | b | >800ml/m2 | Most precise static variable | ||
| Dynamic instrumental | ||||||
| SVV | >10% | b | b | Predict response to volume.Scantly invasive | Do not estimate volemia.Limited to CMV in SR | |
| VPP | >13% | b | b | |||
| ΔVPV LVOT | >12% | b | b | Also, advanced ultrasound | ||
| Raising of legs | Increase of SVa>10% | b | b | Also validates in arrhythmias and spontaneous ventilation | Do not estimate volemia | |
GEDI: Global End Diastolic Volume Index; ITBVI: Intrathoracic Blood Volume Index; mmHg: millimeters of mercury; PCP: pulmonary capillary pressure; CVP: central venous pressure; Δresp: respiratory variation; SR: sinus rhythm; TD: left ventricular filling flow deceleration time; AW: acoustic window; IVCd: diameter of the inferior vena cava; MV: mechanical ventilation; CMV: controlled mechanical ventilation; VPP: variation of pulse pressure; SV: systolic (stroke) volume; ΔVPV LVOT: variation of peak flow velocity of the left ventricular outflow tract; SVV: stroke volume variation.
General objectives of the treatment of HN
- •
Adjust the treatment of HN to the severity of the symptoms, the duration of the condition, and the underlying cause and physiopathology.
- •
Prescribe individualized treatment based on the risk of over-correction versus the risk of brain edema.
- •
Start treatment early in the case of acute or severe HN.
- •
Avoid inadequately rapid correction of natremia, since structural neurological damage may result.
- •
Avoid the use of HN correction formulas, due to the high risk of over-correction.
Specific objectives of treatment. Monitoring of HN
- •
Optimize oxygenation and correct the potassium levels as part of the hyponatremia management plan.
- •
Avoid the use of hypotonic fluids in the event of severe or symptomatic HN.
- •
Use 3% hypertonic saline solution (HSS) when the symptoms are severe and adjust its administration to the weight of the patient (0.5–2ml/kg) according to the desired correction rate.
- •
Follow a treatment and monitoring protocol that combines natremia elevation with ideal correction time.
- •
Monitor the treatment of HN with data referred to Na+ in blood and urine.
In treating HN it is essential to take a series of general objectives into account, considering each case, in order to avoid delays in management or excessively rapid correction of the condition.21,34,47–49
Individualization allows us to adopt a short- to middle-term management protocol that adequately combines natremia elevation and ideal correction time (Table 4). Likewise, monitoring of the critical patient with HN must be suited to the established management plans.
Treatment of hyponatremia.
| Management of hyponatremia | Objective | Ideal Na+ increase | Maximum Na+ increase | Recommended rate of increase [Na+]p | Monitoring |
|---|---|---|---|---|---|
| Acute, severe | Control of neurological symptoms | 6mmol/l in 6h8mmol/l in 24h | 8mmol/l in 24h(general)12mmol/l in 24h(neurocritical)6mmol/l in 24h(risk ODS) | 2h: 0.5–2mmol/l6h: 2–5mmol/l24h: 6–8mmol/l48h: 12–14mmol/l | [Na+], [K+] plasma and urine every 2h in the first 6h.[Na+], [K+] plasma and urine every 4h until first 24hHourly diuresis |
| Acute, not severe | Correction of [Na+] to >120mmol/l | 6–8mmol/l every 24h | 8mmol/l in 24h | 24h: 6–8mmol/l48h: 12–14mmol/l | [Na+], [K+] plasma and urine every 8h.Diuresis every 8h. |
| Chronic | Correction of [Na+] to >120mmol/l | 4–6mmol/l every 24h | 6mmol/l in 24h | 24h: 4–6mmol/l48h: 12mmol/l | [Na+], [K+] plasma and urine every 8h.Diuresis every 8h. |
h: h; mmol/l: millimoles per liter; K+: potassium; Na+: sodium; p: plasmatic; ODS: osmotic demyelinating syndrome.
We consider that hypoxemia and hypopotassemia must be taken into account in the management plan, as patients with hyponatremia and hypoxemia have a poorer prognosis,24 since hypoxemia can alter solute extrusion by blocking the Na-K-ATPase pump and thus delay the resolution of brain edema.50 Likewise, there have been reports of ODS with slow natremia correction in patients with hypopotassemia. In this regard, it has been speculated that the reduced Na-K-ATPase pump concentration in the endothelial membrane in situations of hypopotassemia may predispose to neuron damage due to the osmotic stress associated to the correction of natremia.51,52
With regard to specific treatment, we can use an infusion of 3% HSS in boluses or in perfusion.6,45,53
While a common practice, the use of formulas for calculating the correction of natremia may prove hazardous due to the high risk of over-correction or the provision of large volumes that would be prohibitive in patients with heart failure.54 These formulas regard the patient as a closed system and ignore the renal response of the subject to such infusion – thereby possibly offering inexact information.55
Question four. What is the most appropriate treatment for hyponatremic patients according to the type of natremia involved?Practical recommendations- •
Use 3% HSS in hyponatremic encephalopathy, independently of the cause.
- •
Administer 0.9% saline solution (SS) in hypovolemic HN, with close monitoring of aquaresis (water excretion without electrolyte loss) in order to avoid over-correction.
- •
Treat the triggering cause of hypervolemic HN.
- •
Vaptans can be used in hypervolemic HN refractory to first line treatment.
- •
Do not use vaptans in severe or hypovolemic HN.
- •
Diuretics can be used as adjuvant treatment in hypervolemic and normovolemic HN.
For the correction of HN we can use water restriction (WR), NaCl capsules, saline solution (normal or hypertonic),21 diuretics (furosemide), urea, vaptans and fludrocortisone.1,2,9,56 The choice of one treatment or other should be based on the underlying cause and physiopathology, the desired correction rate, and the associated comorbidities (Table 5). Loop diuretics may be useful as coadjuvants to the treatments started in euvolemic or hypervolemic HN.56
Treatment of hyponatremia according to associated disease.
| First line | Second line | Not recommended | |
|---|---|---|---|
| CHF | Specific treatmentWater restriction3% HSS (on point basis, if water overload allows)Furosemide if high Osm u13 | Vaptans in case of neurological symptoms or refractory to treatment or impossibility of first line treatment63,64,86–88 (not approved [off-label] in Summary of Product Characteristics in Spain) | 3% HSS in case of important water overload due to risk of worsening CHF |
| Cirrhosis | 3% HSS in case of important water overload due to risk of worsening edema-ascites decompensation | ||
| Cranial hypertension | 3% HSS89 | Hydrocortisone/fludrocortisone | Water restriction due to difficulty in intubated patient and risk of SWS90 |
| Postsurgery | Control of factors that trigger SIADH | Fluid therapy adjusted to input and losses balance | Fluid therapy excessive or with hypotonic fluids.Vaptans if suspected SWS |
| SIADH | Water restrictionCapsules of NaCl3% HSSFurosemide if high Osm u75 | Vaptans is refractory or impossibility of first line treatment (approved in Spain)57,61,91 | Lithium,56 demeclocycline,92 urea93 |
| Hypovolemia | 0.9% saline solution94,95 | Packed red cells if bleeding | Fluid therapy with hypotonic fluids, vaptans58 |
CHF: congestive heart failure; NaCl: sodium chloride; Osm u: urinary osmolality; SIADH: syndrome of inappropriate ADH secretion; SWS: salt-wasting syndrome; 3% HSS: 3% hypertonic saline solution.
The vaptans, as V2 receptor antagonists, increase free water excretion, reducing Osmu and incrementing natremia.57 In 2006, two randomized clinical trials known as the Study of Ascending Levels of Tolvaptan in hyponatremia (SALT-1 and SALT-2) respectively compared the efficacy of tolvaptan versus placebo in 225 and 223 patients with euvolemic and hypervolemic HN – the latter being severe in at least 50% of the cases in both cohorts. These studies reported an increase in serum sodium concentration from the start until day 4 of treatment, and from the start until day 30 in all the patients treated with an initial daily dose of 15mg, which could be incremented conditioned to the observed increase in serum sodium.58 In Spain, tolvaptan has only been approved for the management of syndrome of inappropriate antidiuretic hormone secretion (SIADH)59 refractory to first line therapeutic measures, despite the scant supporting evidence, or when such measures cannot be implemented.60 It is advisable to start the treatment in the hospital setting and not to simultaneously apply WR or administer 3% HSS, due to the risk of over-correction. For this same reason, adequate fluid supply must be ensured in patients with alterations of the thirst mechanism (intubated or sedated individuals).
We consider that it might be of benefit to reduce the starting dose to 7.5mg in critical patients. In some cases the administration of a single dose has been evaluated, followed by the decision to either repeat administration or introduce continuous therapy (off-label use not contemplated in the Summary of Product Characteristics).61 If correction is too fast, the drug will have to be suspended, or the dose will have to be lowered. Reported side effects have been thirst, pollakiuria, dehydration or orthostatic hypotension.58
The hypervolemic hyponatremia usually seen in patients with heart failure, liver cirrhosis and nephrotic syndrome requires specific treatment of the background disease, which among other aspects will help to increase free water clearance and thus improve HN. However, some patients are refractory to such treatment, and although vaptans are not indicated in Spain for hyponatremia of this kind, in some cases they may be used on an off-label basis. In this regard, in patients with heart failure, the joint administration of tolvaptan and standard treatment for heart failure – including loop diuretics – was seen to result in short term improvement of the global situation and of HN, though without benefits in terms of mortality.62,63 Likewise, patients with decompensated liver cirrhosis and refractory ascites showed a good response to tolvaptan, with an increase in urine volume and natremia, and improvement of ascites.64
Hypovolemic hyponatremia is characterized by the depletion of extracellular volume, and the administration of 0.9% saline solution suffices to correct this situation.65 Vaptans would not be indicated in this type of HN or in acute symptomatic HN. The latter requires urgent management to increase natremia, and the indication in this context is 3% HSS, for although there are no data in the literature supporting the use of vaptans in this type of HN, the onset of tolvaptan action occurs after 2h, and we therefore would not obtain the immediate effect we seek.
Tolvaptan likewise has no indication in patients with hypothyroidism or adrenal gland insufficiency.66
Question five. Is it important to study the causes of secondary syndrome of inappropriate antidiuretic hormone secretion in the critical patient?Practical recommendations- •
Study the causes that may trigger secondary SIADH, and consider that primary SIADH is an exclusion based diagnosis.
- •
In order to diagnose SIADH, the following criteria must be met: [Na+]p<135mEq/l, Osmp<275mOsm/kg, Osmu>100mOsm/kg, [Na+]u>30mmol/l with adequate Na+ supply, glomerular filtration rate (GFR)>60ml/min, normal thyroid and adrenal gland function, euvolemia, absence of diuretics and absence of physiological ADH stimulation (surgery, pain, thoracic, lung or brain involvement).
- •
A diagnosis of SIADH without correct assessment of secondary causes may result in an incorrect diagnosis and inadequate treatment.
Syndrome of inappropriate antidiuretic hormone secretion occurs due to non-physiological ADH release (in the pituitary gland or on an ectopic basis) or as a result of increased ADH activity secondary to genetic alterations of its receptors. Antidiuretic hormone reduces the renal excretion of free water with normal excretion of Na+, giving rise to euvolemic hypoosmolar HN. The causes of SIADH include neoplasms, central nervous system (CNS) disorders, lung disease, drugs, and transient and genetic factors (mutation of the V2 receptor gene).
Although the diagnostic criteria of SIADH have been clearly defined, it is important to remember that the diagnosis is established on an exclusion basis, and that the first step is to confirm the existence of normovolemia – something that may prove very difficult in the critical patient. The second step is to check that all the validated biochemical criteria are met, and we know that this is not done in routine practice. A recent Italian study has reported that less than half of the surveyed physicians used such parameters to establish the diagnosis.67 Furthermore, in patients with subarachnoid hemorrhage, it has been seen that 70% of all situations of normovolemic HN are attributable to SIADH and 10% to cortisol defects68 – hence the importance of remembering that we must have all the criteria in order to establish a correct diagnosis, since the approach to therapy will differ accordingly.
Question six. What is the appropriate treatment for correcting hyponatremia in patients with SIADH?Practical recommendations- •
Avoid using of WR, urea, lithium or demeclocycline for the treatment of SIADH.
- •
Vaptans can be used for the treatment of SIADH when water restriction is not feasible and there are no serious neurological symptoms.
- •
Natremia should be checked 6–8h after the administration of vaptans, in order to adequately adjust the next doses.
The treatment of SIADH should start by controlling the physiological stimuli that release ADH. Water restriction and the supply of solutes (NaCl capsules, proteins) are to be considered in patients with mild symptoms or a chronic syndrome onset,56 while the administration of 3% HSS can be decided in cases of severe symptoms and an acute syndrome onset .69
Water restriction remains the first line of treatment in the recently published guides on the management of HN due to SIADH, despite the recognized lack of randomized controlled trials demonstrating its efficacy or safety. The results of a hyponatremia registry showed fluid restriction to be no better than no treatment in patients with SIADH.70 Furthermore, in critical patients, WR is difficult to apply, since fluids, intravenous antibiotics or nutritional support are essential in such cases. Likewise, several days are usually needed in order for the measure to prove effective, and WR is poorly tolerated and not effective in all patients. The American guides1 have documented a series of parameters that can predict a poor response to WR: high Osmu (>500mOsm/kg), diuresis<1500ml/day [Na++K+]u>[Na+]p and a slow natremia correction rate (<2mEq/l in 24–48h).13
Although urea has repeatedly been proposed as a treatment for HN due to SIADH, the supporting evidence is far from convincing, since the existing studies are few and heterogeneous, nonrandomized, with variable doses and administration intervals, and treatment has been studied in chronic HN.71 Likewise, the correction afforded by urea is unpredictable and can be associated with dehydration or over-correction, with increased plasma urea levels. Adequate experience with the use of urea is not available, and its administration therefore cannot be recommended. The same applies to lithium or demeclocycline, since the response obtained is variable, unpredictable, and the risk of renal toxicity is high. A recent systematic review found no evidence on their safety and efficacy,72 and only 3% of the physician used them in SIADH.70 The use of these drugs therefore cannot be recommended.73
Loop diuretics in SIADH may be useful over the short term provided Osmu is high (preferably>400mOsm/kg), by increasing free water clearance through the kidneys. However, we lack randomized studies supporting their use. Such drugs could be administered in patients with transient SIADH (i.e., secondary to pneumonia or drugs that can be suspended).60
The vaptans, as V2 receptor antagonists, appear to be a logical treatment option in SIADH, since on binding to these receptors AQ2 is not inserted in the collecting tubule, thereby increasing free water clearance or aquaresis. The SALT studies58 have demonstrated the effectiveness of tolvaptan in increasing natremia in a cohort of mixed patients (SIADH, hypervolemic HN) versus placebo even one month after treatment. The vaptans usually increase natremia by 5–7mEq/l at 24h post-administration, though the effect is variable. In this regard, the European guides do not indicate vaptan use in any type of HN – one of the stated reasons being the fact that the randomized clinical trials made have been sponsored by the drug industry. Although this is a serious consideration in medicine based on evidence, in our opinion it is better to rely on well-designed studies than on case series. It would be useful to have studies comparing vaptans with the measures considered to constitute first-line therapy. According to our criterion, vaptans would not be indicated in HN with serious neurological symptoms, due to their unpredictable response and insufficiently rapid effect.
Question seven. How to differentiate among SIADH, salt-wasting syndrome and adrenal insufficiency in hyponatremic patients? (Table S2 of the supplementary material)Practical recommendations- •
It is important to establish a differential diagnosis between SIADH and salt-wasting syndrome (SWS), since opposite treatment strategies apply to these two conditions (free water restriction versus fluid therapy).
- •
Evaluate volemia and diuresis rate in order to distinguish between SIADH and SWS. However, both syndromes may form part of a mixed disorder, and their presentation depends on the intensity of each mechanism.
- •
Avoid fluid loading and water restriction as a method for distinguishing between SIADH and SWS, since it may be counterproductive for the clinical course of the patient.
- •
Determine basal cortisol for the diagnosis of adrenal insufficiency (AI). In the presence of inappropriately low values, the ACTH stimulation test is useful for distinguishing between primary and secondary presentations.
- •
Administer 0.9% SS and intravenous glucose in the presence of hypoglycemia in patients with HN secondary to AI.
- •
Administer replacement therapy with hydrocortisone (stress or maintenance dose) and fludrocortisone (following diagnostic confirmation), in addition to ensuring measures against hyperpotassemia in patients with HN secondary to AI.
- •
It is important to monitor sodium in blood and urine, Osmu and the diuresis rate after starting treatment with glucocorticoids, since they can cause important aquaresis with the risk of over-correction.
- •
The presence of HN secondary to hypothyroidism is very infrequent and can only be regarded as a cause of HN if the condition is clearly serious.
In SWS of cerebral origin, HN is of a hypovolemic hypoosmolar nature resulting from neurological dysfunction secondary to brain damage. Its definition is currently not clear,60 its diagnosis is exceptional,68 and there are doubts about the underlying physiopathogenic mechanism involved.74 Natriuresis gives rise to a decrease in ECV that stimulates ADH.75 Proximal tubule alterations favor the excretion of uric acid, Na+ and phosphorus.76 The condition is usually associated with hypouricemia, but this would not be a good marker for distinguishing between SIADH and SWS. Nevertheless, after correcting HN, the fractional excretion of uric acid may help to differentiate between the two disorders, since its value would remain elevated in the case of SWS (>12%). The treatment is based on fluid therapy to normalize ECV.76
Adrenal gland insufficiency occurs due to a deficit in adrenal hormone synthesis. In severe cases, the production of cortisol and aldosterone may be unable to meet the demands (stress), giving rise to HN. In some cases, neurological damage (traumatic brain injury or neurosurgery) gives rise to an alteration in ACTH release and secondarily to an alteration in cortisol output (secondary AI).68
Question eight. How should hyponatremia be treated in patients subjected to extrarenal filtration techniques?Practical recommendations- •
We do not recommend extrarenal filtration techniques (EFTs) for the correction of HN.
- •
Such techniques are a risk factor for the rapid correction of hyponatremia.
- •
We suggest avoiding citrate as anticoagulant if the patient presents severe HN.
- •
In order to avoid the rapid correction of natremia in patients with severe HN requiring EFT for any other reason, we consider the following to be useful:
- ∘
Apply continuous extrarenal filtration techniques (CEFTs) instead of intermittent hemodialysis.
- ∘
Add water to the commercial solutions used in CEFT to adjust to patient natremia. Use the lowest clearance dose.
- ∘
Monitor natremia closely during CEFT.
Kidney disease is responsible for between 2 and 12% of all cases of HN. In patients requiring EFT and who suffer HN, although high uremia attenuates the effects of rapid [Na+]p elevation, over-correction caused by the technique may occur (depending on the dose used and the sodium of the fluids). An alternative for avoiding this is to add sterile water in the dialysis bags in order to keep the sodium in the solutions between 6 and 8mEq/l above the desired [Na+]p77–79 (Table 6) and apply a low and sustained dose over time.80
Water dose to be added to 5-liter bags of CEFT exchanger bath.
| Water to add (ml) | [Na+] final objective (mEq/l) | Bicarbonate final objective (mEq/l) |
|---|---|---|
| 150 | 136 | 34 |
| 250 | 133 | 33 |
| 500 | 127 | 32 |
| 750 | 122 | 30 |
| 1000 | 117 | 29 |
| 1250 | 112 | 28 |
mEq/l: milliequivalents per liter; ml: ml; Na+: sodium.
Source: Yessayan et al.79
The generalization of the use of citrate as anticoagulant in CEFT could represent a further source of problems in the management of HN, since hypernatremia is a complication that has been associated with its use. Furthermore, although they allow easy and safe citrate use, the automated systems now employed require a specific concentration of ions in the solutions, and these must not be manipulated. Therefore, although published evidence is lacking, the physiological bases and the mode of application of citrate make it impossible to guarantee a slow or controlled increase in natremia. We therefore recommend avoiding citrate as anticoagulant in the presence of severe HN, at least until further evidence becomes available.
Question nine. If over-correction occurs during the management of hyponatremia, how should it be treated?Practical recommendations- •
Reduce [Na+]p in the event of over-correction in order to avoid cerebral ODS.
- •
Use 5% glucose solution to reduce natremia in the presence of over-correction.
- •
Use desmopressin in cases of excessive Na+p elevation.
- •
The rate of correction must be controlled particularly in the presence of factors favoring over-correction: hypovolemia, hypoxemia, heavy beer intake, nausea, vomiting, pain, thiazides or serotonin reuptake inhibitors, adrenal insufficiency or CEFT, among others.
The monitoring of Osmu and of urine volume may help predict the risk of over-correction.81,82 If the intended limits are exceeded, and despite the scant available evidence, the guides recommend reducing [Na+]p again using water via the enteral route, 5% glucose solution (3ml/kg/h)47 and/or desmopressin (2–4μg i.v.)83 in order to avoid ODS. Desmopressin induces the reabsorption of water by the collecting tubules, reducing natremia, and has been shown to be effective – though in the context of retrospective studies and case series.84,85
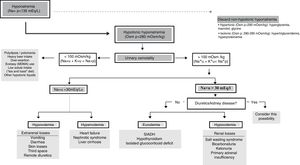
Algorithm for the diagnosis of hyponatremiaThe final objective of this panel of experts was to adopt a diagnostic algorithm of use in application to critical patients (Fig. 1).
In the presence of [Na+]p<135mEq/l, we must confirm genuine HN (Osmp<280mOsm/kg). At this point, if the patient has severe symptoms, urgent management is required. Posteriorly, we should evaluate Osmu, and if it is found to be <100mOsm/kg, we must discard a high provision of hypotonic solutions or insufficient intake of sodium or proteins (potomania, heavy beer intake, use of ecstasy [MDMA] accompanied by water, hypoproteic diets). A situation of [K++Na+]u<[Na+]p indicates that free water excretion is preserved. If Osmu is >100mOsm/kg or [K++Na+]u>[Na+]p, free water excretion may be decreased and urinary [Na+] should be assessed, since if it is found to be >30mEq/l we need to discard renal disorders or diuretic intake, while in the case of <30mEq/l we can assume that the kidneys compensate low ECV.
We must assess volemia, since diuretics, SWS or adrenal insufficiency are characterized by reduced volemia and SIADH, hypothyroidism or glucocorticoid deficit with normovolemia. Total body water can provide an orientation, for if it is found to be low, we must discard extrarenal water and sodium losses, while a high water total body water content (edemas) requires us to discard congestive heart failure, nephrotic syndrome or cirrhosis.
ConclusionsHyponatremia is common in the ICU, and in this setting, the signs and symptoms it causes are often masked by the disease condition for which the patient was admitted to the Unit, or by the characteristics inherent to patients of this kind (e.g., sedation). This makes it difficult to critically assess the impact of HN. On the other hand, the underlying etiopathogenic mechanisms in these patients tend to be complex, since in some cases the background disease itself is associated to the origin of HN, while in other cases it is integrated within the physiopathological response to aggression – thereby complicating correction of the problem.
Natremia depends on the balance between sodium and water input and losses, and in our patients this balance is often conditioned by our treatment decisions.
The threshold for defining hyponatremia depends on the clinical characteristics, and these in turn influence the need for correction and the speed or rate at which it can be applied.
Hyponatremia produces acute neurological dysfunction due to brain edema, but rapid correction of HN can give rise to neuronal damage (ODS). Management therefore must be adapted to the severity of the symptoms, the duration of the disorder and its cause, with individualized evaluation of the risk of over-correction.
Aspects apparently little related to HN and which form an important part of our clinical practice, such as the use of CEFT, may unexpectedly influence the evolution of HN or its correction.
Algorithms reduce the variability of clinical practice, but those available for the management of HN are not specifically targeted to the critical patient. This makes it desirable to have algorithms such as that proposed herein – though we must underscore that any algorithm should be complemented by the knowledge of the experienced intensivist.
The main limitation of this document is that the literature search was not systematic and is therefore not reproducible, since from the initial search we selected and added publications according to the individual criteria of the different members of the group of experts. On the other hand, the consensus-based recommendations of this document are largely fundamented on the experience and opinions of the authors, with no high-quality scientific evidence due to the scarcity of clinical trials and controlled studies that would have allowed evidence-based recommendations to be made in reference to most of the therapeutic issues raised. Therefore, the practical recommendations of this document lack a protocol with which to grade the supporting evidence; nevertheless, we feel that the recommendations can be useful in routine clinical practice, since they have been developed and agreed upon by physicians with great experience in dealing with patients with hyponatremia admitted to the ICU.
On the other hand, while the meetings held to develop the project were auspiced by Otsuka Pharmaceutical, in no way did this company influence our contributions and recommendations.
The document and resulting algorithm of this project seek to clarify and reduce variability in the management of these patients, and in the not too distant future we hope it will facilitate evaluation of the specific impact of hyponatremia in our patients. It is our wish for this document to promote the development of HN registries in Spanish ICUs, with a view to improving knowledge of the disorder among the critically ill.
Financial supportDevelopment of this document received financial support from Otsuka Pharmaceutical, which covered the expenses referred to transport of the members of the panel and the cost derived from the meetings. All the panel members declare having received payment for their participation, though not in relation to development of the manuscript – the editorial contents of which reside exclusively in its authors.
Conflicts of interestThe panel members declare the following conflicts of interest:
M.J. Broch-Porcar has received payment for conferences from Otsuka Pharmaceutical and Fresenius, and for participating in symposia organized by Otsuka Pharmaceutical.
J.M. Domínguez-Roldán has received payment for conferences from Integra Neurosciences, Otsuka Pharmaceutical and Masimo Corporation, and for participating in symposia organized by Otsuka Pharmaceutical.
B. Rodríguez-Cubillo declares having no conflicts of interest.
L. Álvarez-Rocha has received lecture payments from Pfizer and Otsuka Pharmaceutical.
M.A. Ballesteros-Sanz has received lecture payments from Otsuka Pharmaceutical and Astellas.
M. Cervera-Montes has received conference and lecture payments from Nestle, Abbot Nutrition, Fresenius Kabi, Pfizer, Philips and Covidien.
M. Chico-Fernández has received lecture payments from MSD, Octapharma, Behring and Otsuka Pharmaceutical, and has participated in the NOSTRA trial of GmpVasopharma.
J.H. de Gea-García has received lecture payments from Otsuka Pharmaceutical and Astra Zeneca.
P. Enríquez-Giraudo declares having no conflicts of interest.
A. García de Lorenzo y Mateos declares having no conflicts of interest.
R. Gómez-López has received payment for conferences from Astra Zeneca and Pfizer, and for scientific counseling from Cardiolife, Maquet, AstraZeneca and Otsuka Pharmaceutical.
R. Guerrero-Pavón declares having no conflicts of interest.
F. López-Sánchez declares having no conflicts of interest.
J.A. Llompart-Pou has received payment for conferences from Otsuka pharmaceutical.
S. Lubillo-Montenegro has received payment for conferences from Integra Neurosciences up until June 2014.
Z. Molina-Collado declares having no conflicts of interest.
P. Ramírez-Galleymore has received payment for conferences from Pfizer, MSD, Otsuka Pharmaceutical and Novartis and for participating in symposia organized by Otsuka Pharmaceutical.
M. Riveiro-Vilaboa declares having no conflicts of interest.
A. Sánchez-Corral declares having no conflicts of interest.
M.E. Herrera-Gutiérrez has received payment for conferences from Baxter, Fresenius and Otsuka Pharmaceutical.
Thanks are due to Dr. Alberto Tejedor for his collaboration and contributions.
Please cite this article as: Broch Porcar MJ, Rodríguez Cubillo B, Domínguez-Roldán JM, Álvarez Rocha L, Ballesteros Sanz MÁ, Cervera Montes M, et al. Documento práctico del manejo de la hiponatremia en pacientes críticos. Med Intensiva. 2019;43:302–316.