The objective of the study is to identify the risk factors associated with mortality at six weeks, especially by analyzing the role of antivirals and munomodulators.
DesignProspective descriptive multicenter cohort study.
Setting26 Intensive care units (ICU) from Andalusian region in Spain.
Patients or participantsConsecutive critically ill patients with confirmed SARS-CoV-2 infection were included from March 8 to May 30.
InterventionsNone.
VariablesVariables analyzed were demographic, severity scores and clinical condition. Support therapy, drug and mortality were analyzed. An univariate followed by multivariate Cox regression with propensity score analysis was applied.
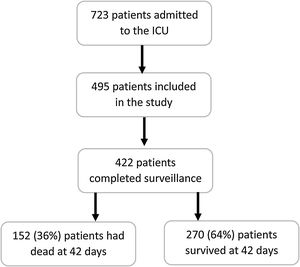
Results495 patients were enrolled, but 73 of them were excluded for incomplete data. Thus, 422 patients were included in the final analysis. Median age was 63 years and 305 (72.3%) were men. ICU mortality: 144/422 34%; 14 days mortality: 81/422 (19.2%); 28 days mortality: 121/422 (28.7%); 6-week mortality 152/422 36.5%. By multivariable Cox proportional analysis, factors independently associated with 42-day mortality were age, APACHE II score, SOFA score at ICU admission >6, Lactate dehydrogenase at ICU admission >470U/L, Use of vasopressors, extrarenal depuration, %lymphocytes 72h post-ICU admission <6.5%, and thrombocytopenia whereas the use of lopinavir/ritonavir was a protective factor.
ConclusionAge, APACHE II, SOFA>value of 6 points, along with vasopressor requirements or renal replacement therapy have been identified as predictor factors of mortality at six weeks.
Administration of corticosteroids showed no benefits in mortality, as did treatment with tocilizumab.
Lopinavir/ritonavir administration is identified as a protective factor.
Identificar los factores de riesgo asociados con la mortalidad a las seis semanas.
DiseñoEstudio prospectivo multicéntrico.
ÁmbitoSe incluyeron a 26 pacientes de la Unidad de Cuidados Intensivos (UCI) de Andalucía.
Pacientes o participantesPacientes ingresados en UCI por neumonía grave por SARS-CoV-2 del 8 de marzo al 30 de mayo de 2020.
IntervencionesNinguna.
Variables de interés principalesCaracterísticas demográficas, clínicas y escalas de gravedad. Se analizaron tratamientos de soporte, fármacos y la mortalidad.
ResultadosSe incluyeron 495 pacientes, 73 fueron excluidos por incompletos y 422 pacientes se incorporaron en el análisis final. La mediana de edad fue de 63 años, 305 (72,3%) eran hombres. La mortalidad en la UCI fue: 144/422 34%; mortalidad a los 14 días: 81/422 (19,2%); mortalidad a los 28 días: 121/422 (28,7%); mortalidad a las seis semanas 152/422 36,5%.
Los factores asociados con la mortalidad a los 42 días fueron la edad, APACHE II, SOFA > 6 y LDH al ingreso > 470 U/L, uso de vasopresores, necesidad de técnicas de reemplazo de la función renal, porcentaje de linfocitos a las 72 horas del ingreso en UCI < 6,5%, y trombocitopenia, mientras que el uso de lopinavir/ritonavir fue identificado como un factor protector.
ConclusionesLa edad, gravedad y fracaso orgánico junto con la necesidad de terapias de soporte fueron identificadas como factores predictores de mortalidad a las seis semanas.
La administración de corticoesteroides a dosis altas no mostró beneficios en la mortalidad, al igual que el tratamiento con tocilizumab, lopinavir/ritonavir se identificaron como un factor protector.
Past december, the first cases of respiratory infection caused by SARS-CoV-2 were documented in the Chinese city of Wuhan, observing as a percentage of patients developing the most serious clinical forms requiring respiratory support treatment with invasive mechanical ventilation.1 Already, in the first series of published patients it was found that between 10% and 20% of hospital admissions would develop Acute Respiratory Distress Syndrome (ARDS) and/or multiple-organ failure,2 charging special importance the role that intensive care units are occupying in the pandemic. Since then, the expansion of the epidemic has been global causing a serious unprecedented health crisis in the history of modern medicine. In the middle of October, more than 71 million cases are surpassed with more than one million and six thousand deaths documented by the World Health Organization.3 Andalusia is the Spanish Autonomous Community with the largest population in Spain, with 8.446.561 inhabitants. From March to May 2020, more than 6000 patients were admitted to hospital, exceeding 12% of ICU admissions. The Working Group on Infectious Diseases of the Andalusian Society of Intensive Medicine and Coronary Units (SAMIUC) has worked dynamically and continuously since the beginning of March, when the first patient was admitted in ICU occurred, by prospectively registering and monitoring ICU admitted cases with a fundamentally clinical objective so that the exchange of clinical information among the Andalusian ICU, in the form of a weekly newsletter, allowed to expand and share the limited clinical experience available at the beginning of the health emergency with the aim of improving the healthcare of critically ill patients infected by SARS-CoV-2. This study is the result of this collaborative project. It has been scarcely studied predictors of short term mortality 6 weeks after ICU admission now that many studies have been published with short-term mortality data and in many of them patients were still admitted in ICU.
The objective of the study is to identify the risk factors associated with mortality at six weeks, especially by analyzing the role of antivirals and immunomodulators in both the total cohort of patients and those who needed respiratory support with invasive mechanical ventilation.
MethodsThis is a prospective descriptive multicenter cohort study. Twenty-six intensive care units (ICU) participate in the study from Andalusian region in Spain.
Study populationConsecutive critically ill patients with confirmed SARS-CoV-2 infection admitted to the participants ICU were included from March 8 to May 30.
Inclusion criteria: Patients older than 18 years, acute infection by SARS-CoV-2 confirmed by RT-PCR and admitted to the ICU, and a potential surveillance time of 6 weeks at least.
Exclusion criteria: pregnant woman, patients with SARS-CoV-2 but admitted to ICU for other reason not related to COVID-19, and patients or relatives who declined to participate in the study.
Because of the nature and contact restrictions of this pandemic disease, the informed consent was obtained from the patient when it was possible or from the relatives by a phone call with or without a complementary mail, and it was registered in the medical history. The Ethical Committee of Investigation in Cádiz [SAM-COVUCI-2020-01] approved the study protocol.
Variables: We analyzed demographic, epidemiological, comorbidities collected in the electronic medical history, body mass index, time from onset of symptoms, microbiological diagnoses, defining bacterial coinfection as an acute bacterial infection at clinical onset presentation of viral pneumonia with an isolate from respiratory tract or blood culture and/or a positive pneumococcal urinary antigen, time from hospital admission to ICU admission, severity scores and clinical condition at ICU admission, laboratory determinations at ICU admission and 72h after, respiratory function at ICU admission and 72h after, need for support therapy in ICU (high flow oxygen, non-invasive and invasive ventilation, prone position therapy, extrarenal depuration, extracorporeal membrane oxygenation), drug therapy (chloroquine, antiviral, interleukin-6 inhibitors, steroids, antibiotics), and mortality (ICU, early at 14 days, 28 days and 6 weeks mortality [main outcome]).
Statistical analysisThe design of the analysis comprised an univariate followed by multivariate Cox regression with propensity score analysis for the whole population and for the group of intubated patients, and a complementary decision tree analysis for the whole population with 6-weeks mortality as the outcome variable.
A descriptive analysis was made using absolute frequencies for qualitative variables and mean with standard deviation (SD) or median with percentiles 25th and 75th (p25–p75) when appropriate. For the univariate analysis, we used Chi-square or exact Fisher test when appropriate for qualitative variables and Student t-test or Mann–Whitney U test for parametric or nonparametric variables.
All comparisons were two-tailed, and signification was established on p<0.05. Mortality related variables were determined using Cox proportional hazards multivariate analysis. We include those variables with p<0.20 in the univariate analysis and other that were considered to be clinically relevant for this study, like the drug therapy. Hazard ratios and 95% confidence intervals were calculated. Sample size calculation was made with the assumptions of a 50% mortality rate and 20 significant variables in the univariate analysis. With the requirement of 10 events for each analyzed variable, we calculated a simple size of 400 patients. Continuous variables significantly different in the univariate analysis were transformed in dichotomic variables using a ROC curve analysis, determining the cutoff point with maximum sensitivity and specificity. Missing values in these new variables were assigned to be negative values in order to facilitate the multivariate analysis, if the number of missing values was less than 10%. Because of the potential imbalances in the characteristics of treatment groups, we assessed the probability of receiving lopinavir/ritonavir by a logistic regression model to calculate a propensity score (PS). We included variables a p less than 0.20 in the univariate analysis. This approach was determined to minimize the risks of confounding by indication of this drug. The covariates finally included in this PS were APACHE II score and respiratory rate in the ICU. This PS was included as a covariate in the multivariate Cox regression analysis for 42-days mortality.
A classification of regression tree (CRT) analysis was also made to characterize risk groups for 42-days mortality, with a maximum of 5 levels, minimum parental level of 50 cases and minimum childsize of 10 cases, and a cross validation with 20 sample folds.
ResultsIn the study period, 723 were admitted in Andalusian ICU, 495 patients were enrolled in this study but 73 of them were excluded for incomplete data. Thus, 422 patients were included in the final analysis (Fig. 1). Median age was 63 years (54–71) and 305 (72.3%) were men. ICU mortality: 144/422 34%; 14 days mortality: 81/422 (19.2%); 28 days mortality: 121/422 (28.7%); 6-week mortality 152/422 36.5%. Table 1 compares demographics and clinical characteristics of those who died with those alive on day 42. As expected, those who died were significantly older and with higher APACHE II and SOFA scores. Moreover, level of consciousness (evaluated by GCS) was worse in those who were not alive at day 42.
Baseline characteristics and univariate analysis with 6 weeks mortality.
| n | Dead at 42 days(n=152) | Survivor at 42 days(n=270) | OR (CI 95%) | p | |
|---|---|---|---|---|---|
| Age [years], mean (SD) | 419 | 65.6 (11.0) | 59.4 (11.8) | <0.001 | |
| Age groups, n (%) | 419 | <0.001 | |||
| 18–45 years | 6 (4) | 35 (13) | 1 | ||
| 46–60 years | 34 (22.8) | 95 (35.2) | 2.09 (0.81–5.40) | ||
| 61–75 years | 85 (57) | 125 (46.3) | 3.97 (1.60–9.84) | ||
| >75 years | 24 (16.1) | 15 (5.6) | 9.33 (3.17–27.5) | ||
| Sex (male), n (%) | 420 | 118 (78.1) | 187 (69.5) | 1.57 (0.99–2.50) | 0.057 |
| Body mass index, mean (SD) | 356 | 29.1 (5.2) | 29.2 (5.4) | 0.859 | |
| COPD, n (%) | 422 | 9 (5.9) | 16 (5.9) | 1.00 (0.43–2.32) | 0.998 |
| Renal disease, n (%) | 422 | 21 (13.8) | 23 (8.5) | 1.72 (0.92–3.23) | 0.087 |
| Cirrhosis, n (%) | 422 | 7 (4.6) | 4 (1.5) | 3.21 (0.92–11.2) | 0.063 |
| Onco haematological disease, n (%) | 422 | 13 (8.6) | 17 (6.3) | 1.39 (0.66–2.95) | 0.387 |
| APACHE II score at ICU admission, mean (SD) | 410 | 16.4 (9.6) | 11.0 (5.0) | <0.001 | |
| SOFA score at ICU admission, mean (SD) | 398 | 6.6 (3.0) | 4.9 (2.7) | <0.001 | |
| GCS score at ICU admission, mean (SD) | 419 | 13.9 (3.2) | 14.8 (1.4) | <0.001 | |
| SOFA score 72h post-ICU admission, mean (SD) | 386 | 8.0 (2.7) | 5.7 (2.8) | <0.001 | |
| High flow oxygen, n (%) | 420 | 52 (34.4) | 143 (53.2) | 0.46 (0.31–0.70) | <0.001 |
| Non-invasive ventilation, n (%) | 420 | 12 (7.9) | 24 (8.9) | 0.88 (0.43–1.82) | 0.732 |
| High flow oxygen or non-invasive ventilation before intubation, n (%) | 407 | 54 (36.2) | 86 (33.3) | 1.14 (0.75–1.74) | 0.552 |
| Endotracheal intubation, n (%) | 422 | 146 (96.1) | 196 (72.6) | 9.19 (3.89–21.69) | <0.001 |
| Vasopressors, n (%) | 419 | 143 (94.7) | 190 (70.9) | 7.34 (3.43–15.68) | <0.001 |
| Days with vasopressors, median (p25–p75) | 314 | 7 (4–14) | 6 (3–10) | 0.027 | |
| Extrarenal depuration, n (%) | 415 | 33 (22.3) | 17 (6.4) | 4.22 (2.26–7.89) | <0.001 |
| Bacterial infection at presentation, n (%) | 416 | 24 (16.1) | 31 (11.6) | 1.46 (0.82–2.60) | 0.194 |
| Nosocomial infection, n (%) | 410 | 54 (36.7) | 95 (36.1) | 1.03 (0.68–1.56) | 0.902 |
| Days from ICU admission to VAP, median (p25–p75) | 101 | 9 (6–13) | 12 (8–19) | 0.005 | |
| Catheter related infection. n (%) | 415 | 27 (18.1) | 47 (17.7) | 1.03 (0.61–1.74) | 0.908 |
| Lopinavir/ritonavir, n (%) | 421 | 136 (90.1) | 254 (94.1) | 0.57 (0.27–1.19) | 0.131 |
| Remdesivir, n (%) | 421 | 2 (1.3) | 6 (2.2) | 0.518 | |
| Interferon β 1b, n (%) | 421 | 72 (47.7) | 130 (48.1) | 0.927 | |
| Chloroquine, n (%) | 410 | 119 (81.5) | 219 (83.0) | 0.712 | |
| Tocilizumab, n (%) | 410 | 64 (43.5) | 102 (38.8) | 0.347 | |
| Empiric antimicrobial therapy, n (%) | 420 | 140 (92.7) | 248 (92.2) | 1.08 (0.51–2.30) | 0.847 |
| Days of empiric antimicrobial therapy, median (p25–p75) | 377 | 6 (4–8) | 6 (4–8) | 0.314 | |
| Steroids therapy, n (%) | 420 | 99 (66.9) | 161 (59.9) | 1.36 (0.89–2.06) | 0.156 |
| Days from symptoms to hospital admission, median (p25–p75) | 417 | 7 (4–9) | 7 (4–9) | 0.379 | |
| Days from symptoms to microbiological diagnose, median (p25–p75) | 413 | 7 (4–10) | 7 (5–9) | 0.813 | |
| Days from hospital to ICU admission, median (p25–p75) | 412 | 1 (0–3) | 2 (1–4) | 0.020 | |
| Days from symptoms to therapy, median (p25–p75) | 398 | 7 (5–10) | 7 (5–10) | 0.961 | |
| ICU stay, median (p25–p75) | 421 | 13 (6–23) | 16 (8–31) | 0.012 |
APACHE: Age, Physiology, Chronic Health Evaluation; CI: confidence interval; COVID: coronavirus disease; COPD: chronic obstructive pulmonary disease; DAP: diastolic arterial pressure; ECMO: extracorporeal membrane oxygenation; ER: emergency room; FiO2: fraction of inspired oxygen; GCS: Glasgow Coma Score; ICU: Intensive Care Unit; MAP: mean arterial pressure; OR: odds ratio; PaO2: partial pressure of Oxygen; PEEP: positive end-expiratory pressure; SAP: systolic arterial pressure; Sat: saturation; SD: standard deviation; SOFA: Sequential Organ Failure assessment; VAP. ventilator-associated pneumonia.
The laboratory findings of all critically ill patients on day 1 and 3 are summarized in Table 2. The median PaO2/FiO2 ratio on day 1 was not statistically different in those who died compared with survivors, but it was significantly lower on day 3 in those who died. Percentage of lymphocytes, neutrophils/lymphocytes ratio, and platelets were statistically different on day 1 and day 3.
Laboratory findings of all critically ill patients on day 1 and 3 of ICU admission.
| At ICU admission | 72h post-ICU admission | |||||||
|---|---|---|---|---|---|---|---|---|
| n | Dead at 42 days(n=152) | Survivor at 42 days (n=270) | p | n | Dead at 42 days(n=152) | Survivor at 42 days (n=270) | p | |
| PaO2/FiO2, mean (SD) | 319 | 134.1 (74.7) | 140.6 (77.0) | 0.450 | 305 | 189.4(75.5) | 2089 (78.0) | 0.031 |
| Delta PaO2/FiO2 72–24h, median (p25–p75) | 298 | 10 (−42 to 67) | 30 (−32 to 81) | 0.253 | ||||
| Leucocytes [×109/L], mean (SD) | 418 | 11.2 (8.7) | 9.9 (6.0) | 0.104 | 393 | 11.8 (11.4) | 9.6 (6.5) | 0026 |
| Neutrophils [×109/L], mean (SD) | 418 | 9.6 (7.3) | 8.5 (5.5) | 0.086 | 390 | 9.7 (7.4) | 8.0 (5.2) | 0.014 |
| % Neutrophils [×109/L], mean (SD) | 416 | 86.4 (8.6) | 84.7 (8.1) | 0.046 | 390 | 84.7(13.1) | 81.9(8.8) | 0.015 |
| Lymphocytes [×106/L], mean (SD) | 409 | 637.5 (397.1–932.5) | 773.0 (490.0–996.0) | 0.663 | 383 | 595 (365–922) | 720 (479–1034) | 0.002 |
| % Lymphocytes, mean (SD) | 409 | 8.1 (5.4) | 9.7 (6.3) | 0.013 | 384 | 7.6 (6.4) | 10.3 (6.3) | <0.001 |
| Ratio neutrophils/lymphocytes, mean (SD) | 409 | 18.3 (19.0) | 13.9 (12.4) | 0.013 | 382 | 20.4 (22.4) | 12.3 (9.9) | <0.001 |
| Platelets [×109/L], mean (SD) | 418 | 229.1(108.1) | 252.1 (111.5) | 0.040 | 388 | 228.1 (102.1) | 290.2 (115.1) | <0.001 |
| Creatinine [mg/dL], mean (SD) | 412 | 1.26 (1.01) | 1.06 (0.76) | 0.020 | 397 | 1.61 (1.13) | 1.10 (0.92) | <0.001 |
| Urea [mg/dL], mean (SD) | 375 | 61.1(39.9) | 48.5 (36.5) | 0.003 | 353 | 80.4 (47.0) | 58.4 (40.0) | <0.001 |
| Lactate dehydrogenase [U/L], mean (SD) | 388 | 635.7(577.2) | 465.9 (195.2) | 0.001 | 353 | 532.8 (427.6) | 405.9 (172.6) | 0.002 |
| Creatine kinase [U/L], median (p25–p75) | 321 | 141 (69–281) | 104 (53–181) | 0.025 | 299 | 119 (47–329) | 90 (48–291) | 0.144 |
| Aspartate aminotransferase [U/L], median (p25–p75) | 385 | 54 (36–91) | 48 (33–78) | 0.074 | 359 | 47 (25–84) | 45 (29–70) | 0.794 |
| Alanine aminotransferase [U/L], median (p25–p75) | 366 | 39 (24–59) | 40 (27–64) | 0.325 | 337 | 37 (22–53) | 44 (28–68) | 0.018 |
| Procalcitonin [ng/mL], median (p25–p75) | 333 | 0.34 (0.17–0.94) | 0.23 (0.11–0.50) | 0.001 | 274 | 0.70 (0.26–2.07) | 0.27 (0.10–0.74) | <0.001 |
| C-reactive protein [mg/L], mean (SD) | 407 | 178.5 (109.9) | 165.0 (110.4) | 0.239 | 375 | 160.8 (131.5) | 114.4 (110.3) | <0.001 |
| Lactate [mmol/L], median (p25–p75) | 371 | 1.70 (1.15–2.85) | 1.33 (1.00–1.90) | 0.001 | 322 | 2.38 (2.65) | 2.0 (2.89) | 0.244 |
| Fibrinogen [mg/dL], mean (SD) | 296 | 702.8 (639.2) | 706.1 (730.2) | 0.969 | 260 | 626.0 (279.4) | 595.6 (256.5) | 0.383 |
| D-dimer [ng/mL], median (p25–p75) | 376 | 1723 (894–4113) | 1160 (660–2500) | 0.001 | 360 | 3619 (1600–16537) | 1934 (993–4662) | <0.001 |
Approximately, one-third of the patients were admitted to the ICU from the Emergency Department (138 patients; 32.7%) and the rest from the general ward. Median time from viral symptom onset to hospital admission was 86–11 days. Median time from hospitalization to ICU admission was 21–4 days. Three quadrants were involved on the chest X-ray in 78 patients (18.5%) and the four quadrants in 244 patients (57.8%). Microbiologically documented co-infection at ICU admission was diagnosed in 55 (13%) patients.
The majority of the patients were treated with lopinavir/ritonavir (n=390) and/or hydroxychloroquine (n=338). Remdesivir was used in a very small number of patients (n=8). Interferon beta-1b was used in 202 patients. High doses of corticosteroids (methylprednisolone≥2mg(kg/day). were administered in 260 (61.6%) patients whereas 166 patients received tocilizumab. Both drugs were used in 126 (29.9%) patients. Tables 1–4 of the supplementary material, show the comparison of different variables in patients treated and not treated with lopinavir/ritonavir, hydroxychloroquine, tocilizumab or Interferon beta-1b.
Overall, 342 (81%) patients were intubated. Vasopressors were used in 333 (78.9%) patients. Fifty (11.8%) patients required renal replacement therapy. One-hundred and forty-nine individuals (35.3%) developed at least one nosocomial infection in the ICU. The most frequent infections were: VAP (103 patients; 24.4%) and catheter-related bloodstream infections (74 patients; 17.5%).
By multivariable Cox proportional analysis, factors independently associated with 42-day mortality were age, APACHE II score, SOFA score at ICU admission >6, lactate dehydrogenase at ICU admission >470U/L, Use of vasopressors, extrarenal depuration, %lymphocytes 72h post-ICU admission <6.5%, and thrombocytopenia whereas the use of lopinavir/ritonavir was a protective factor (Table 3). Because of the noted imbalances in baseline characteristics and clinical situation at admission to the ICU, a Cox regression model was developed introducing the probability calculated by the propensity score in an attempt to ameliorate the impact of observed differences. This analysis also identified therapy with lopinavir/ritonavir as a protective factor for 6-week mortality. “Delta SOFA score 0–72h was significantly related to mortality in univarian analysis in all patients and mechanically ventilated patients. However, it is not independently associated with mortality at 42 days in both groups of patients (Table 6, supplementary material).”
Relationship between Delta SOFA score 0–72h and mortality.
| All patients | n | Dead at 42 days(n=152) | Survivor at 42 days (n=270) | OR (CI 95%) | p |
|---|---|---|---|---|---|
| Delta SOFA score 72h – ICU admission, mean (SD) | 272 | 1.8 (2.7) | 0.7 (2.6) | <0.001 | |
| Delta SOFA score 72h – ICU admission>0, n (%) | 422 | 78 (43.6) | 101 (56.4) | 1.76 (1.18–2.64) | 0.006 |
| Mechanically ventilated patients | n | Dead at 42 days(n=146) | Survivor at 42 days (n=196) | OR (CI 95%) | p |
|---|---|---|---|---|---|
| Delta SOFA score 72h – ICU admission, mean (SD) | 308 | 1.7 (2.8) | 0.9 (2.8) | <0.001 | |
| Delta SOFA score 72h – ICU admission>0, n (%) | 342 | 78 (51.3) | 74 (48.7) | 1.76 (1.18–2.64) | 0.006 |
In intubated patients (n=342), ICU mortality: 142/342 (41.5%); 14 days-mortality 77/342 (22.5%); 28 days mortality: 115/342(33.6%), 6-week Mortality 146/342 (42.7%). The median time from ICU admission to intubation was 0 days [0–1]). Table 4 depicts the clinical features of those patients who required invasive mechanical ventilation and their laboratory parameters on day 1 and 3 are shown in Table 5 (supplementary material). As in the entire group, those who died were significantly older and with higher APACHE II and SOFA scores at admission. No substantial imbalances in patients’ comorbidities were observed.
Multivariate Cox regression analysis of variables associated with mortality at 6 weeks with Propensity Score for lopinavir/ritonavir.
| Variables | All patients | Patients on Mechanical ventilation | ||
|---|---|---|---|---|
| HR (CI 95%) | p | HR (CI 95%) | p | |
| Age (years) | 1.04 (1.02–1.06) | <0.001 | 1.05 (1.03–1.07) | <0.001 |
| Cirrhosis | 2.37 (1.02–5.53) | 0.045 | ||
| APACHE II | 1.04 (1.02–1.07) | 0.002 | 1.03 (1.02–1.05) | <0.001 |
| SOFA score at ICU admission >6a | 1.43 (1.01–2.03) | 0.043 | ||
| Lactate dehydrogenase at ICU admission >470U/La | 1.57 (1.10–2.23) | 0.013 | ||
| First PEEP after intubation >12a | 2.05 (1.37–3.05) | <0.001 | ||
| PaO2/FiO2 24h post-intubation <160a | 1.67 (1.13–2.46) | 0.011 | ||
| SOFA score 72h post-ICU admission >6a | 1.73 (1.12–2.67) | 0.014 | ||
| %Lymphocytes 72h post-ICU admission <6.5%a | 1.61 (1.13–2.30) | 0.009 | ||
| Neutrophils/lymphocytes ratio 72h post-ICU admission >14a | 1.02 (1.01–1.03) | <0.001 | ||
| Platelets 72h post-ICU admission <150×109/La | 1.89 (1.18–3.03) | 0.012 | 2.53 (1.51–4.27) | <0.001 |
| D-dimer 72h post-ICU admission >3.400mg/La | 1.67 (1.14–2.47) | 0.009 | ||
| Use of vasopressors | 4.04 (1.73–944). | 0.001 | ||
| Extrarenal depuration | 1.85 (1.21–2.84) | 0.004 | 1.79 (1.13–2.82) | 0.011 |
| Use of lopinavir/ritonavir | 0.52 (0.29–0.93) | 0.029 | ||
| Propensity score | 0.586 | |||
APACHE II: acute physiology, age and Chronic Health Evaluation score; HR: hazard ratio; FiO2: fraction of inspired oxygen; ICU: intensive care unit; PEEP: positive end-expiratory pressure; SOFA: Sequential Organ Failure Assessment.
Binary variables calculated using ROC curves. Missing values treated as negative values. See Table 3s in Supplementary Material.
The median PaO2/FiO2 ratio on day 1 was 170 [115–236]. The use of ECMO was marginal in this series (only 4 patients; two died). Conversely, proning positioning was used in almost two-thirds of the patients (251/341; 73.6%). Tracheostomy was performed in 43.4% of the intubated patients (145/334).
By multivariable Cox proportional analysis, factors independently associated with 42-day mortality were age, cirrhosis, APACHE II score, First PEEP after intubation >12cmH2O, PaO2/FiO2 24h post-intubation <160, extrarenal depuration, neutrophils/lymphocytes ratio 72h post-ICU admission >14, D-dimer 72h post-ICU admission >3.400mg/L, and thrombocytopenia (Table 3). Of note, use of lopinavir/ritonavir was not included in this model as a protective factor of 1.01 (0.49–2.08) p=0.98.
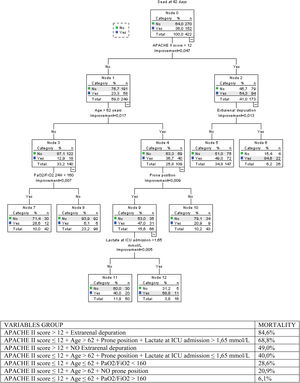
Classification of regression tree (CRT)A decision tree to depict the risk groups for 42-days mortality (Fig. 2) was built based on CRT analysis. It reached an overall percentage of correct classification of 69.7%. The established risk groups were on a range from 6.1% (APACHE II score≤12+Age≤62+PaO2/FiO2>160) to 84.6% (APACHE II score>12+Extrarenal depuration). Patients with APACHE II score>12 were ever classified with mortality at 6 weeks higher than 49%.
The decision tree based on CRT analysis reached an overall percentage of correct classification of 69.7%. The established risk groups were on a range from 6.1% (APACHE II score≤12+Age≤62+PaO2/FiO2>160) to 84.6% (APACHE II score>12+Extrarenal depuration). Patients with APACHE II score>12 were ever classified with mortality at 6 weeks higher than 49%.
Most published studies to date about critically ill patients with SARS-CoV-2 infection has been retrospective series of patients. This study, by its prospective and multicenter design, with more than 420 patients analyzed, representatively reflects the clinical profile and prognosis of the most severe form of clinical presentation of SARS-CoV-2 pneumonia. It seems transcendent to identify prognosis-related factors to optimize the use of health resources and guide better clinical management. We suggest using clinical tools for helping clinicians, such as the decision-making tree documented in the results in difficult decision-making. We were able to identify a subgroup of patients with very high mortality, there are few critical pathologies in which only with an APACHE II>12 and the need for extrarenal depuration therapies can be associated with a high mortality. This information may be useful to be considered in decision-making on limitation of life support treatment.
One third of patients admitted to ICU died, with higher mortality of 43% observed, in patients receiving mechanical ventilation. This mortality is below that described in a recent meta-analysis4 that included more than 14,000 patients in 44 studies, which estimates mortality in ventilated patients by a percentage greater than 80%. Although remarkable heterogeneity was observed in the selected studies, weaknesses in the follow-up of those patients still admitted, and most studies came from the Asian continent, where the published series point to a worrying high mortality in mechanically ventilated patients.5–8 The Spanish publications documented to date express short series of no more than 50 patients coinciding with the clinical profile, ventilatory requirements and prognosis described in our study.9–13 Published series of Italian patients from the Lombardy region14 document mortality similar to that observed in our study, and in the New York area, a study describes a mortality of 24.5% in mechanically ventilated patients, these results should be analyzed considering the limitation that at that time most continued receivinfg mechanical ventilation, 72.2% of patients, and had only been discharged from ICU a 3.3% of patients, so mortality can be expected to be higher.15 In the Seattle series, they documented 50% mortality and there are still being mechanically ventilated patients when they published it.16
In a novel way, lopinavir/ritonavir administration is identified as a protective factor. This finding deserves to be interpreted wisely, since there were no differences in the univariant analysis, turns out to be significant in the multivariate analysis of the total cohort of patients but not in the subgroup that received mechanical ventilation. This is the only study group up to our knowledge that provides this evidence. To control the potential weaknesses of an obervational study in an environment changing treatment guidelines, we have performed a propensity score analysis, which was included in the final model maintaining as a protective factor the treatment with lopinavir/ritonavir.
Of course, this finding should be endorsed in randomized studies, but it opens up a possibility of treatment in this entity with so few therapeutic options. The clinical trial of Cao B y cols17 conducted in 199 patients showed no benefits in treatment, but we must take into account that the population studied is notably far from the clinical profile of critically ill represented in our series. Only one patient was included in the defined category 6 who contemplates hospitalization with mechanical ventilation, ECMO or both treatments and only 15% within category 5 requiring high flow oxygen therapy or non-invasive ventilation. In addition, randomization occurred 13 days after the onset of symptoms, an average time significantly longer than documented in our series that was 8 days, it seems key as has happened in other infectious diseases for other causes that the administration of antibiotic or antiviral treatment achieves the best benefits as soon as possible are administered.18,19
Recently, RECOVERY study results about lopinavir/ritonavir has been published, conducted at 176 hospitals in the United Kingdom, this study does not demonstrate benefits in terms of survival, but we have to discuss the eligibility of participants with a high number of losses because they did not have treatment available, do not consider it appropriate or were receiving other treatments. In addition, the percentage of critically ill patients was very low and the days after the onset of symptoms when treatment was prescribed exceeded one week.20
Unfortunately, the newly demonstrated benefits in viral load reduction, clinical improvement and modulation of inflammatory response with combined administration of lopinavir/ritonavir with interferon and ribavirin21 barely anecdotally contemplated the inclusion of critically ill patients in their study.
We have not observed benefits in all other antiviral treatments used, hydroxychloroquine and interferon, although the use of remdesivir was minorityly used due to restrictions on its access to critical patients in the early months of the pandemic.
The benefits currently demonstrated22 were not seen in the subgroup of patients receiving mechanical ventilation although it should be noted that it was not the primary objective of the study. The need for clinical trials specifically targeting the critically ill with respiratory infection with SARS-CoV-2 infection has become a long-awaited necessity.
Administration of corticosteroids at high doses, the percentage of use of which was more than half of patients, showed no benefits in mortality, as did treatment with tocilizumab who received more than one third of patients. Although there are described clinical experiences that point to a potential benefit of tocilizumab in mechanically ventilated patients,23 we do not observe this effect, a significant limitation of the comparative study of Somers and cols is that the comparative groups were not homogeneous by noting significant differences in age and presence of chronic pathology prior to in addition to lower levels of D-dimer in the treated group. Pending the final publication of the results of the phase 3 COVACTA clinical trial, it appears that COVACTA has not demonstrated benefits in terms of survival.24
The use of corticosteroids in our patient series corresponds to the prior period of publication of the clinical trial that has associated the administration of dexamethasone at doses of 6mg with mortality benefits,25 a guideline that is far from that currently recommended was used. Research continues on the potential benefit that could be associated with higher doses of corticosteroid treatment.26 Our findings coincide with the lack of solid evidence currently supporting the widespread use of these treatments in critical patients.
During the recruitment period, empiricism capitalized on treatments of severe forms of the disease, eager to obtain new evidence from ongoing clinical trials,27 this has conditioned significant variability in clinical practice.28 Aware of this reality we present in a novel way a decision-making tree analysis based on the main factors related independently to mortality.
Age, severity at ICU admission measured by APACHE II and organic failure determined by a SOFA>value of 6 points, along with vasopressor requirements or renal replacement therapy have been identified as predictor factors of mortality at six weeks, in line with findings from other published series.5,7,8 With regard to laboratory tests, we must comment as a limitation that the levels of ferritin, whose role in severe SARS-CoV-2 infection is to be clarified,29 and interleukin-6,30 that we know have been linked to the inflammatory response and severity of the disease, were not collected in the database. Registration began in the early days of the pandemic, so most of the participating centres did not have the possibility of measuring them and the prospective design of the study conditioned that we had not incorporated it later. They have been identified as prognostic factors presenting with lymphopenia at admission and/or elevated levels of lactate dehydrogenase (LDH) related to increased mortality. The presence of lymphopenia at admission has been linked to the need to ICU admission,31,32 but there are also authors who associate it with greater severity, need for mechanical ventilation and increased mortality.33,34 Higher levels of LDH have been reported in the most severe forms of SARS-CoV-2 infection and have even correlated the drop in their levels with the negativization of the virus PCR and a better prognosis,35 although there are references linking high levels of LDH with the most severe forms of clinical presentation36,37 we present as an independent mortality factor that their admission levels are higher than 470U/L.
In the multivariate analysis carried out in the subpopulation of mechanically ventilated patients have been identified as independent mortality factors at six weeks age, severity measured by APACHE II upon ICU admission, history of cirrhosis, the need for renal replacement therapies and thrombopenia along with the persistence of organic failure at 72h of admission as measured by SOFA>6. In addition, to the severity of respiratory failure determined by PEEP needs within the first 24h after intubation and PaO2/FiO2 ratio less than 160. Mortality described in our series approaches, being somewhat higher, to the estimation of Berlin definition of respiratory distress,38 that established in moderate grades with PaO2/FiO2 between 100 and 200 mortality of 32%; 95% CI, 29–34% increasing to 45%; 95% CI, 42–48% in those classified as severe with PaO2/FiO2 less than 100.
With regard to laboratory tests, D dimer levels >3400mg/L and neutrophil/lymphocyte ratio >14–72h of ICU entry are identified as mortality factors. These findings at 72h of ICU admission express a lack of response to support treatments instituted when entering ICU and therefore partly a therapeutic failure associated with increased mortality.
The neutrophil/lymphocyte ratio has been used as an inflammatory state factor with prognostic value in various pathologies,39,40 not all of an infectious nature, although it is in sepsis that it has been identified as a prognostic factor,41 the fact that the most severe forms of clinical presentation of SARS-CoV-2 infection develop sepsis can justify our results that identify this marker at 72h with the highest mortality when it is >of 14. There are authors who have described this parameter as an independent mortality factor in respiratory infection due to SARS-CoV-2, especially in males,42 a recent meta-analysis43 identifies it as a prognostic factor in this disease.
In a novel way, we provide this finding in critical patients, aware that this is an inexpensive laboratory measure, accessible in most ICU, and reflecting the intensity of the inflammatory response and we have identified it as an independent mortality factor.
D dimer levels at hospital admission have been linked as a predictor of mortality,7,44,45 from an analytical point of view it remains to be explained what the cut-off points are, their need to be adjusted by age46 and it seems mandatory that the units of measurement and how to obtain it from the different studies be specified in order to be compared.47 High levels at 72h of admission were associated in our series to higher mortality.
The main limitation of the study derives from the timing of the study's design, which being at first of pandemic time, no variables that were later demonstrated to have predicted value were included in the study protocol. We do not have the levels of ferritin, interleukin 6 nor was treatment was collected with anticoagulant therapy. Other limitations were the lack of a standard definition of comorbidities and the definition used to classify bacterial co-infection, with the proposed criteria it is not possible to detect co-infection due to atypical bacteria. From the point of view of statistical analysis we acknowledge a limitation derived from the use of the transformation of quantitative variables into dichotomous variables to facilitate multivariate analysis, by the assumption of lost values as negative. Another limitation given the abruptness of the pandemic is the absence of unified criteria for ICU admission and drug use, which can make it difficult to interpret the data. However, we consider this to be currently the widest series of critical patients collected prospectively and highly representatively for the multicenter participation involving most of the ICU in our community, belonging to hospitals of different levels of care. Identifying mortality-related factors at six weeks in critically ill patients will help the clinician stratify risk and make decisions. The uncertainty of clinical practice in this new disease48 can be fought applying contingency plan for the intensive care services49 based on the documentation of clinical experiences such as the one we present, and from them with the design of research studies in well-designed critical patients aimed at solving challenges about treatment that remain unresolved today.
Authors’ contributionAE is the project coordinator, designed the study protocol and contacted the researchers. He managed approval by the Ethics Committee of Inquiry. AE has collected data from researchers and JLGG has performed statistical analysis. AE, JLGG and JG have written the contents of the manuscript. All researchers have participated in the inclusion of cases and have reviewed the version of the manuscript.
Ethics approvalBecause of the nature and contact restrictions of this pandemic disease, the informed consent was obtained from the patient when it was possible or from the relatives by a phone call with or without a complementary mail, and it was registered in the medical history. The Ethical Committee of Investigation in Cádiz [SAM-COVUCI-2020-01] approved the study protocol.
FundingNone declared.
Conflict of interestNone declared.
The study coordinator would like to thank Ms. Monica Saldaña on behalf of the SAMIUC Infectious Diseases Working Group for their help in designing the study protocol in the worst days of the pandemic.