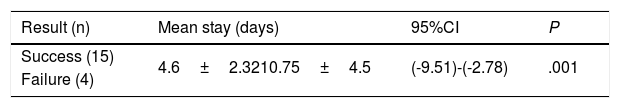High-flow nasal cannula (HFNC) therapy is used in the treatment of acute respiratory failure (ARF), and is both safe and effective in reversing hypoxemia. In order to minimize mortality and clinical complications associated to this practice, a series of tools must be developed to allow early detection of failure. The present study was carried out to: (a) examine the impact of respiratory rate (RR), peripheral oxygen saturation (SpO2), ROX index (ROXI = [SpO2/FiO2]/RR) and oxygen inspired fraction (FiO2) on the success of HFNC in patients with hypoxemic ARF; and (b) analyze the length of stay and mortality in the ICU, and the need for mechanical ventilation (MV).
MethodsA retrospective study was carried out in the medical-surgical ICU of Hospital de Montilla (Córdoba, Spain). Patients diagnosed with hypoxemic ARF and treated with HFNC from January 2016 to January 2018 were included.
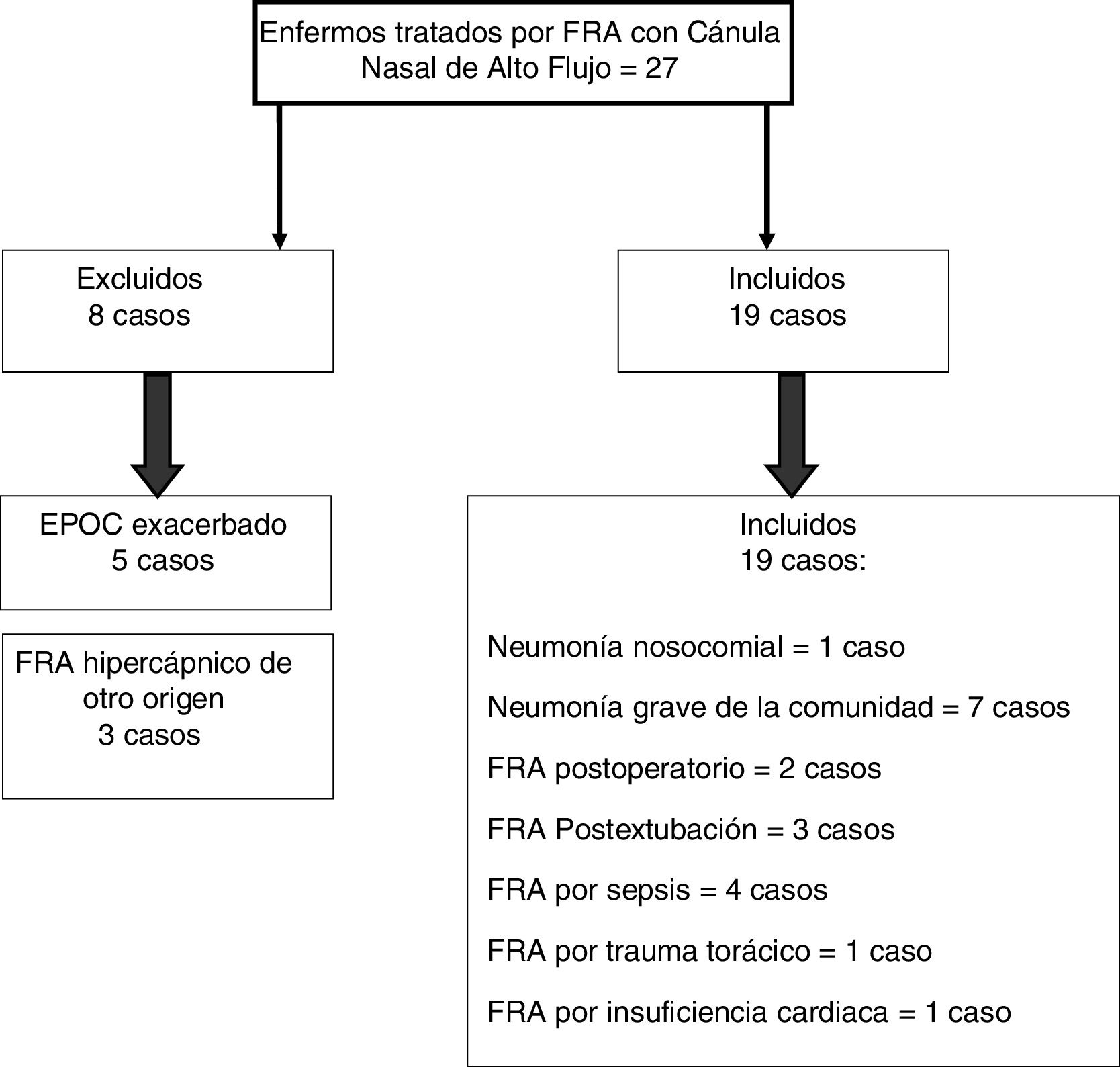
ResultsOut of 27 patients diagnosed with ARF, 19 (70.37%) had hypoxemic ARF. Fifteen of them (78.95%) responded satisfactorily to HFNC, while four (21.05%) failed. After two hours of treatment, RR proved to be the best predictor of success (area under the ROC curve [AUROC] 0.858; 95%CI 0.63–1.05; p=0.035). For this parameter, the optimal cutoff point was 29rpm (sensitivity 75%, specificity 87%). After 8h of treatment, FiO2 and ROXI were reliable predictors of success (FiO2: AUROC 0.95; 95%CI 0.85–1.04; p=0.007 and ROXI: AUROC 0.967; 95%CI 0.886–1.047; p=0.005). In the case of FiO2 the optimal cutoff point was 0.59 (sensitivity 75%, specificity 93%), while the best cutoff point for ROXI was 5.98 (sensitivity 100%, specificity 75%). Using a Cox regression model, we found RR<29rpm after two hours of treatment, and FiO2<0.59 and ROXI>5.98 after 8h of treatment, to be associated with a lesser risk of MV (RR: HR 0.103; 95%CI 0.11−0.99; p=0.05; FiO2: HR 0.053; 95%CI 0.005−0.52; p=0.012; and ROXI: HR 0.077; 95%CI 0.008−0.755; p=0.028, respectively).
ConclusionsRR after two hours of treatment, and FiO2 and ROXI after 8h of treatment, were the best predictors of success of HFNC. RR<29rpm, FiO2<0.59 and ROXI>5.98 were associated with a lesser risk of MV.
La terapia con cánula nasal de alto flujo (CNAF) se ha introducido recientemente en el tratamiento del fallo respiratorio agudo (FRA), siendo una técnica segura, confortable y eficaz que logra revertir la hipoxemia en estos pacientes. Es necesario disponer de herramientas que nos permitan detectar precozmente el fallo de este tipo de tratamiento para evitar el incremento de la mortalidad que puede conllevar. El objetivo primario de este estudio ha sido analizar el impacto que la frecuencia respiratoria (FR), la saturación periférica de oxígeno (SpO2), la fracción inspirada de oxígeno (FiO2) y el índice ROX (IROX = [SpO2/FiO2]/FR) tienen sobre el éxito de la CNAF en los pacientes con FRA hipoxémico. Los objetivos secundarios han sido analizar la estancia y mortalidad en la UCI y la necesidad de ventilación mecánica (VM).
Material y métodosSe trata de un estudio retrospectivo efectuado en una UCI polivalente del Hospital Comarcal de Montilla (Córdoba). Se incluyeron los pacientes tratados con CNAF por FRA hipoxémico desde enero de 2016 hasta enero de 2018.
ResultadosDesde enero de 2016 hasta enero de 2018 se trataron 27 pacientes con FRA, de los cuales, 19 (70,37%) presentaban FRA hipoxémico. De estos, 15 (78,95%) respondieron bien al tratamiento y 4 (21,05%) fracasaron. A las 2 horas de tratamiento la FR demostró ser el mejor predictor (área bajo la curva ROC [AUROC] 0,858; IC 95% 0,63 – 1,05; p=0,035). La FiO2 y el IROX fueron buenos predictores a las 8 horas de tratamiento (FiO2: AUROC 0,95; IC 95% 0,85 – 1,04; p=0,007), (IROX: AUROC 0,967; IC 95% 0,886 – 1,047; p=0,005). El mejor punto de corte de la FR a la segunda hora fue de 29 respiraciones/minuto (75% sensibilidad, 87% especificidad). El mejor punto de corte de la FiO2 a las 8 horas de tratamiento fue de 0,59 (75% sensibilidad, 93% especificidad). El mejor punto de corte para IROX a las 8 horas de tratamiento fue de 5,98 (100% de sensibilidad, 75% de especificidad). En el modelo de regresión de Cox, una FR<29 respiraciones/minuto a la segunda hora de tratamiento y una FiO2<0,59 e IROX>5,98, a las 8 horas de tratamiento, se asociaron a un menor riesgo de VM (FR: HR 0,103; IC 95% 0,11-0,99; p=0,05), (FiO2: HR 0,053; IC 95% 0,005-0,52; p=0,012) y (IROX: HR 0,077; IC 95% 0,008 – 0,755; p=0,028) respectivamente.
ConclusionesLa FR a la segunda hora de tratamiento y la FiO2 e IROX a las 8 horas de tratamiento fueron los mejores predictores de éxito del CNAF. Una FR<29 respiraciones/minuto (2ª hora), una FiO2<0,59 y un IROX>5,98 (8ª hora), se asociaron a un menor riego de VM.
Oxygen therapy is the first-line therapy for the management of hypoxemia in acute respiratory failure (ARF). It can be administered through low flow devices, nasal cannulas, fascial masks or masks with reservoir bag or high flow systems like Venturi mask. With low flow devices the fraction of inspired oxygen (FiO2) cannot be predicted and depends on several factors: the patient’s respiratory pattern, the flow peak, and the system used. With the Venturi mask, the FiO2 is more predictable as long as the flow supplied exceeds the patient’s flow demand. Recently, the high-flow nasal cannula (HFNC) has joined this therapeutic armamentarium. This system uses a mixture of heated and humidified air and oxygen with a flow rate that can be as high as 60L/min. In order to deliver this gas mixture nasal cannulas of different sizes or connectors for tracheotomy cannulas can be used.1–3
HFNCs deliver high oxygen flows (of up to 60L/min) with a FiO2 somewhere between 0.21 to 1. The gas heats up, becomes humidified, and is delivered to the patient via nasal cannulas. The favorable effects reported are oropharyngeal dead space lavage; positive end-expiratory pressure (PEEP effect); improved respiratory pattern; reduced respiratory rate (RR); less inspiratory effort; better tolerance and comfort; increased ratio of partial pressure arterial oxygen and fraction of inspired oxygen (PaO2/FiO2 ratio), favorable hemodynamic effects in heart failure, and delivery of a high flow rate to make the FiO2 more predictable.1–6 Also, it reduces driving pressure leading to less self-inflicted lung injury.7,8
Although the criteria that define success or failure in other respiratory support techniques have been properly described,9–13 there are not too many studies with HFNCs on this regard. Frat et al.14 conducted a prospective, observational clinical trial. They used HFNC and non-invasive mechanical ventilation (NIMV) sequentially in 28 patients with hypoxemic ARF. In this study, RRs>30breaths per minute within the first hour of treatment were associated with treatment failure. In a cohort study that included 191 patients with pneumonia, Roca et al.15 proposed the ROX index (ROXI) as predictor of successful HFNC oxygen therapy on the 2, 6, and 12-h mark after treatment.15
It is important to have tools for the early detection of any technique failures because late intubations are associated with higher mortality rates.16
Our study primary endpoint was to analyze the value of the different respiratory variables (RR, peripheral oxygen saturation [SpO2], FiO2, and ROXI) as predictors of successful HFNC oxygen therapy for the management of hypoxemic ARF. The study secondary endpoints were the ICU stay, the mortality rate at the ICU setting, and the need for mechanical ventilation (MV).
Material and methodsRetrospective study conducted at the polyvalent ICU of the Hospital de Montilla, Córdoba, Spain. We used a medical database with patients over 18 treated with HFNC oxygen therapy due to ARF from January 2016 through January 2018. Patients treated with HFNC oxygen therapy due to hypoxemic ARF were included in the study. The HFNC was indicated if the patient’s SpO2 was<94% breathing with a Venturi system with a FiO2 of 0.5 and a RR>25 breaths per minute. Patients with a past medical history of chronic obstructive pulmonary disease (COPD) or any other condition with hypercapnic respiratory failure different from COPD were excluded from the study. All patients with a PaCO2>45mmHg were excluded as well. The SpO2, RR, and FiO2 of all the patients were estimated at baseline and 2 and 8h after treatment. With these 3 parameters the ROXI was estimated at the 2 and 8-h mark after treatment.
HFNC oxygen therapy failure was defined as the need for intubation and MV support. The indications were lack of signs of improved work of breathing, gas exchange, the appearance of hemodynamic impairment or an altered level of consciousness.
Two different systems were used for the administration of the HFNC: AIRVO2 (Fisher & Paykel Healthcare, Auckland, New Zealand), and Optiflow (MR850 heater and humidifier with a RT202 delivery tube and a RT050/051 nasal cannula, Fisher & Paykel Healthcare, Auckland, New Zealand). The oxygen flow rate receive by all the patients was 60L/min, humidified and heated to 98.6°F, and a FiO2 to keep SpO2 between 94% and 98%.
The study was evaluated and approved by the ethics committee of the Province of Córdoba, Spain (record no. 282, ref. 4082).
Statistical analysisQuantitative variables were expressed as interquartile range or means ±standard deviation. Qualitative variables were expressed as frequencies and percentages. The Student’s t test was used to analyze the continuous variables. The Mann-Whitney test was used to analyze the difference between independent and paired variables. Also, the Shapiro-Wilk test was used to analyze the variance test for normality. Qualitative variables were compared using the chi-square test or Fisher’s exact test, when appropriate. To assess the predictive capabilities of the different respiratory variables (RR, SpO2, FiO2, and ROXI) on the success of the HFNC oxygen therapy a ROC (receiver operating characteristic) curve analysis was conducted of each of these variables to find the cut-off value associated with successful HFNC oxygen therapy. The area under the ROC curve (AUROC) was estimated to determine the probability of association between the cut-off value selected and a successful HFNC oxygen therapy. The analysis was considered valid with an AUROC ≥0.75. The cut-off value with the best sensitivity/specificity ratio was picked. The positive predictive value (PPV) and the negative predictive value (NPV) were both estimated. The possible correlation between the respiratory variable cut-off value—given in the analysis of the ROC curve—and the need for MV 96h after treatment, was analyzed using Cox logistic regression model. The result obtained in this model was analyzed again and adjusted for other covariables that may be indicative of confusion or interaction. Before measuring this association, an univariate analysis was conducted for each one of the covariable included in the model with P values<.2. The correlation between both cut-off values was studied using Cox logistic regression model with survival 96h after treatment. P values≤.05 were considered a statistically significant difference.
The statistical software package SPSS 15.0 for Windows was used for statistical analysis.
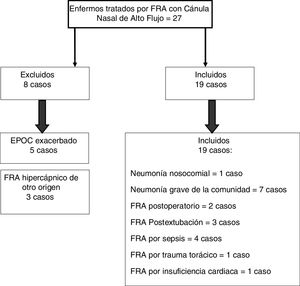
ResultsFrom January 2016 through January 2018, 27 patients with ARF were treated with HFNC oxygen therapy. A total of 19 of these patients (70.37%) showed hypoxemic ARF (Fig. 1).
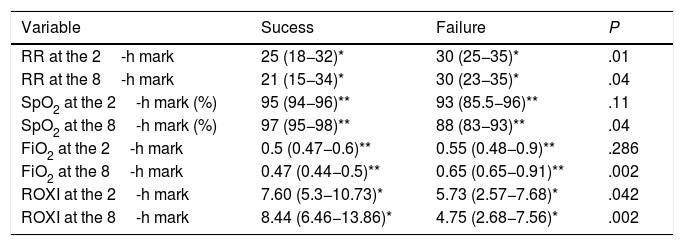
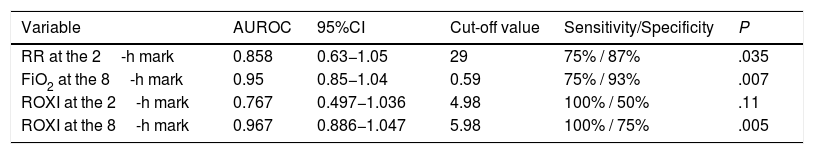
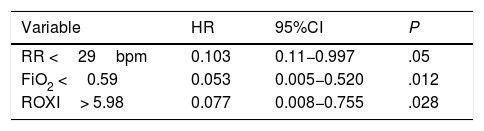
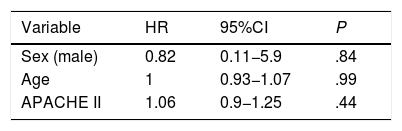
The HFNC oxygen therapy failed in a 4 (21.05%) out of the 19 patients treated with HFNC oxygen therapy. Actually, these 4 patients (21.05%) required intubation and had to be connected to a mechanical ventilator (3 patients with pneumonia [75%] and 1 patient with sepsis [25%]). The remaining 15 patients (78.95%) responded well to the therapy. No statistically significant differences were found regarding age, sex or baseline levels of RR, SpO2, and the APACHEII score between patients with a favorable response to the therapy and those in whom the HFNC oxygen therapy failed (Table 1). In general, and without making any distinctions between success and failure, the HFNC oxygen therapy reduced the RR significantly at the 2nd hour of treatment (baseline RR, 30 [28–35]; RR at the 2-h mark, 25 24–30; 95%CI, 3.51–7.96; P<.001), and the SpO2 increased (baseline SpO2, 89% [85–91]; SpO2 at the 2-h mark, 95% [93–96]; 95%CI, from ―8.79 to ―2.89; P=.001). Table 2 shows the results of RR, SpO2, FiO2, and ROXI after 2 and 8h of treatment in patients in whom the therapy succeeded and in those in whom it did not. Patients with SpO2 levels > 94% (the SpO2 target in our study) after 8h of treatment had lower chances of failing compared to those with SpO2 levels < 94% (OR=0.024; 95%CI, 0.001−0.497; P=.016). We analyzed the results of the ROC curve for the different respiratory variables if AUROC ≥0.75. The best predictor after 2h of treatment was RR (AUROC, 0.85; 95%CI, 0.63−1.05; P=.035). Both the FiO2 and the ROXI were good predictors after 8h of treatment (FiO2 = AUROC 0.95; 95%CI, 0.85−1.04; P=.007, as well as ROXI: AUROC, 0.967; 95%CI, 0.886−1.047; P=.005) (Table 3). The analysis of the ROC curve of RR after 2h of treatment with HFNC oxygen therapy gave us a cut-off value of 29 breaths per minute. However, the cut-off values obtained from the FiO2 and the ROXI after 8h of treatment were 0.59 and 5.98, respectively (Table 3). The PPV and the NPV for the RR were 60% and 93%, respectively. The PPV of the FiO2 was 75%, and the NPV was 93%. The PPV of ROXI was 93% and the NPV, 75%. The correlation between the risk among the different cut-off values obtained from RR, FiO2, and ROXI and the need for MV was studied using the Cox logistic regression model (Table 4). Patients with RRs<29breaths per minute after 2h of treatment had a significantly lower risk of needing MV (HR=0.103; 95%CI, 0.11−0.997; P=.05). The same thing happened with patients who required a FiO2<0.59 after 8h of treatment (HR=0.053; 95%CI, 0.005−0.520; P=.012) and with those with ROXIs>5.98 after 8h of treatment (HR=0.077; 95%CI, 0.008−0.755; P=.028). The univariate analysis of the possible covariables that may have contributed to confusion and/or interaction (sex-male, age, and APACHEII score) showed P values >.2 in each and every one of the covariables studied, which is why they were not included in the Cox logistic regression model (Table 5). The mean hospital stay was significantly shorter in patients in whom the therapy succeeded (success, 4.6±2.32days; failure, 10.75±4.5days; 95%CI, from ―9.51 to ―2.78; P=.001). Mortality risk was significantly higher in patients in whom treatment failed (OR=8.5; 95%CI, 2.31−31.24; P=.035) (Table 6).
Values of RR, SpO2, FiO2, and ROXI at the 2- and 8-h marks.
| Variable | Sucess | Failure | P |
|---|---|---|---|
| RR at the 2-h mark | 25 (18−32)* | 30 (25−35)* | .01 |
| RR at the 8-h mark | 21 (15−34)* | 30 (23−35)* | .04 |
| SpO2 at the 2-h mark (%) | 95 (94−96)** | 93 (85.5−96)** | .11 |
| SpO2 at the 8-h mark (%) | 97 (95−98)** | 88 (83−93)** | .04 |
| FiO2 at the 2-h mark | 0.5 (0.47−0.6)** | 0.55 (0.48−0.9)** | .286 |
| FiO2 at the 8-h mark | 0.47 (0.44−0.5)** | 0.65 (0.65−0.91)** | .002 |
| ROXI at the 2-h mark | 7.60 (5.3−10.73)* | 5.73 (2.57−7.68)* | .042 |
| ROXI at the 8-h mark | 8.44 (6.46−13.86)* | 4.75 (2.68−7.56)* | .002 |
Values are expressed as mean with interquartile range.
ROC curves of RR (at the 2-h mark), FiO2 (at the 8-h mark), and ROXI (at the 2- and 8-h marks).
| Variable | AUROC | 95%CI | Cut-off value | Sensitivity/Specificity | P |
|---|---|---|---|---|---|
| RR at the 2-h mark | 0.858 | 0.63−1.05 | 29 | 75% / 87% | .035 |
| FiO2 at the 8-h mark | 0.95 | 0.85−1.04 | 0.59 | 75% / 93% | .007 |
| ROXI at the 2-h mark | 0.767 | 0.497−1.036 | 4.98 | 100% / 50% | .11 |
| ROXI at the 8-h mark | 0.967 | 0.886−1.047 | 5.98 | 100% / 75% | .005 |
Mean stay and mortality rate at the ICU of successful and failed patients.
| Result (n) | Mean stay (days) | 95%CI | P |
|---|---|---|---|
| Success (15) | 4.6±2.3210.75±4.5 | (-9.51)-(-2.78) | .001 |
| Failure (4) |
| Mortality rate of successful patients compared to failed patients | ||||
|---|---|---|---|---|
| Result (n) | Deaths, n (%) | OR | 95%CI | P |
| Success (15) | 0 (0) | 8.5 | 2.31−31.24 | .035 |
| Failure (4) | 2 (50) | |||
In our study, RRs < 29 breaths per minute after 2h of treatment with HFNC or a FiO2 < 0.59 and a ROXI>5.98 at the 8-h mark were associated with a successful technique and with less need for MV. It is interesting to use scoring tools to predict the success and failure of this type of respiratory support since delays in intubation and MV can lead to higher mortality rates as seen in the study conducted by Kang et al.16 These authors showed that patients in whom HFNC oxygen therapy failed and who required earlier intubations (within the first 48h of their study) had a lower mortality rate compared to those who were intubated later in time (48h after starting HFNC oxygen therapy). One of the effects seen when treating ARF with HFNC is a lower RR.7,17 Roca et al.15 saw a significant reduction of RR after 2h of treatment (RR, 25 [22–28]) in patients in whom the HFNC oxygen therapy succeeded compared to patients in whom the treatment failed (RR, 28 [22–32], P=.023). In our study, RR after 2h of treatment was significantly lower in patients in whom treatment succeeded, 25 (18−32), compared to the RR of patients in whom treatment failed, 30 (23−35), P=.01. Frat et al.14 obtained similar results in a study conducted among 28 patients with hypoxemic ARF. A total of 23 of these patients showed acute respiratory distress syndrome (ARDS)-like criteria. All patients received sequential treatment with HFNC and NIMV. After 1h on HFNC oxygen therapy, patients with RRs>30 breaths per minute had more chances of being intubated and failing to therapy (sensitivity, 94.1%; specificity, 87.5%). Maggiore et al.18 compared HFNC with oxygen therapy with the Venturi system in a group of patients intubated and ventilated for over 24h and then extubated with a PaO2/FiO2 ratio≤300. The saw a significant reduction of RR in patients after 1h on HFNC oxygen therapy (P<.01).
At 8 hours of treatment, the patients we received a FiO2<0.59 had more chances of success compared to those who received a FiO2>0.59. The general rule in all the studies conducted so far is to start with a high FiO2 of 1 to achieve a certain saturation target and then reduce it based on the patient’s response. We started treatment with a FiO2 high enough to achieve a SpO2 of 94% to 98% following the recommendations established by the British clinical guidelines on the administration of oxygen therapy. Given the gradual reduction of PaO2 that comes with age, the ideal levels of PaO2 can be calculated by subtracting the product of age by 0.3–100mmHg (ideal PaO2=100mmHg ― 0.3 ×age). In terms of SpO2 this may be extrapolated to levels of between 94% and 98% in most situations. The upper limit proposed in these guidelines (98%) is based on the idea of avoiding the deleterious effects of hypoxemia while the lower limit of 94%, close to the lower limit of normal, guarantees that the SpO2 remains > 90% when small drops of SpO2 occur.19 The HFNC achieves a more stable and predictable FiO2 compared to conventional oxygen therapy by supplying high oxygen flow above the patient’s flow rate, thus avoiding dilution with room air and a better rate of oxygenation. Another factor influencing oxygenation is the PEEP effect achieved with the HFNC oxygen therapy, which is proportional to the flow rate used.17,20 A few authors report an improved PaO2, but not an improved PaO2/FiO2 ratio, which is attributed to a higher FiO2 rather than to the PEEP effect of the HFNC oxygen therapy.14 Other authors actually do confirm a better PaO2/FiO2 ratio after 24h on HFNC oxygen therapy.18 This increase of PaO2 and the PaO2/FiO2 ratio can be attributed to an alveolar recruiting effect induced by PEEP.21 The response of PaO2 to the higher levels of the FiO2 depends on the mechanism responsible for hypoxemia. If the latter is mainly due to low ventilation/perfusion ratios, hypoxemia will improve after increasing FiO2. On the other hand, if the shunt is the mechanism responsible, the response will be minimum.22 This may explain the lack of an improved PaO2/FiO2 ratio seen in the study conducted by Frat et al.14 that included a considerable number of patients with ARDS. The 4 patients of our study in whom treatment failed needed a higher FiO2 and a lower SpO2 after 2 and 8h on therapy compared to patients who responded well to treatment (successful FiO2 at the 8-h mark: 0.47 [0.44−0.5]; failed FiO2 at the 8-h mark: 0.65 [0.65−0.91]; P=.002; successful SpO2 at the 8-h mark: 97% [95−98], failed SpO2 at the 8-h mark: 88% [83−93]; P=.04). Three of these 4 patients (75%) had serious community acquired pneumonias, a condition that can start with a considerable shunt percentage. Reske et al.22 correlated the PaO2/FiO2 ratio with the shunt percentage. With a PaO2/FiO2 ratio=200 the minimum shunt was 21.3% (it could go up to 48.4%). With a PaO2/FiO2 ratio of 100mmHg the shunt went from 36% to 66.3%. It is well-known that when the shunt fraction is > 30%, the PaO2 response to an increasing FiO2 is minimum. Also, delivering a high FiO2 rate may increase even more the ventilation/perfusion mismatch contributing to worse oxygenation.23,24 It is obvious that the 4 patients of our study in whom treatment failed must have had high shunt percentages given the greater need for lower FiO2 and SpO2, which eventually may explain the lack of response to the HFNC oxygen therapy.
The SpO2 improved significantly with the HFNC oxygen therapy, and response was evident 2h after treatment. At that time, no significant differences were found between the patients in whom treatment succeeded compared to those in whom it failed. Nonetheless, differences were actually seen at the 8-h mark. Also, patients who maintained a SpO2 > 94% (the saturation target in our study) at the 8-h mark had lower chances of failing. Roca et al.15 found a significantly higher SpO2 in patients in whom treatment succeeded after 2h of treatment (97% [95–99] compared to 96% [94–98] of the patients in whom treatment failed, P=.032). We estimated the ROXI after 2 and 8h of treatment, and it was significantly higher in both, in patients in whom treatment succeeded (at the 2-h mark, 7.60 [6.58–10] compared to 5.73 [3.09–7.46], P=.042; at the 8-h mark, 8.44 [7.92–11.38] compared to 4.75 [3.01–7.04], P=.002). Roca et al.15 found statistically significant differences between patients who succeeded and those who failed at the 2-h mark. Actually, this difference was very significant at the 6, 12, and 24-h mark (P<.001). In our series, the cut-off value estimated with the ROC curve at the 8-h mark (5.98) was associated with less need for MV in the Cox regression model used (HR=0.077; 95%CI, 0.008−0.755; P=.028). Roca et al.15 also found a weaker correlation between the risk of MV in patients with ROXIs ≥4.88 from the 2nd hour of treatment. In this same study, the authors calculated the ROXI associated with failed HFNC oxygen therapy at the 2, 6, and 12-h mark (2.85, 3.47, and 3.85, respectively). How should these results be interpreted? Most of the patients are intubated 12h into treatment. Therefore, if a patients’ ROXI at the 12-h mark is >4.88, chances are lower if his ROXI is <3.85. What happens between these 2 values, 3.85 and 4.88? This is what the authors call gray zone. Patients in this gray zone should be managed with extreme care and controlled more often. If the ROXI tends to go up, then the chances of success go up as well. If not, the chances of intubation are higher. The levels of ROXI estimated in our patients are different from those obtained by Roca et al.15 We think it may have to do with the lower number of patients included, the different control times, and the different conditions treated in our series. The progression of ARF in postsurgical patients can be different from the progression of ARF due to severe pneumonia. Therefore, we could the suggest the possibility of the existence of different ROXI values based on the cause of ARF. The Berlin Definition of ARDS assesses the severity of the ROXI using the PaO2/FiO2 ratio calculated with a minimum PEEP of 5cmH2O (severe with a PaO2/FiO2 ratio <100mmHg, moderate with a PaO2/FiO2 ratio between 100mmHg and 200mmHg, and mild with a PaO2/FiO2 ratio between 200mmHg and 300mmHg.)25 The PEEP reached with the HFNC, at 50L/min, can go up to 3.81mmHg±1.33mmHg with the mouth closed,20 very close to the PEEP of 5cmH2O used as the definition criterion of ARDS. Our study included 4 patients with hypoxemic ARF with bilateral pulmonary infiltrates due to sepsis (Fig. 1). Three of these 4 patients (75%) had a good response to treatment but 1 (25%) failed and had to be intubated due to hemodynamic impairment at the 4-h mark. The SpO2/FiO2 ratio of the 3 patients who had a good response to the HFNC oxygen therapy was > 188, while the SpO2/FiO2 ratio of the patient who failed was 133.84 when he was intubated. This patient survived and was eventually discharged from the hospital. These patients may have been diagnosed with ARDS since such infiltrates were not due to heart failure of volume26 overload. However, we cannot confirm this since we did not know their actual PEEPs. The ARDS included in the de novo hypoxemic ARDS of recently published clinical guidelines is a condition where evidence does not recommend the use of non-invasive respiratory support.27 Recently, it has been proven that the HFNC oxygen therapy reduces driving pressure, suggestive that this type of support can reduce pulmonary stretching and tensions and self-inflicted lung injury.7,28 In patients with mechanically ventilated ARDS, it has been confirmed that driving pressure is a strong predictor of survival and authors recommend it should be kept ≤13cmH2O.29,30 The observational study conducted by Messika et al.31 included 45 patients diagnosed with ARDS treated with HFNC oxygen therapy. A total of 18 of these patients (40%) required intubation and MV. Nine of these patients (50%) died at the ICU. Thew saw that the PaO2/FiO2 ratio of the patients in whom treatment failed and who were intubated was lower from the beginning of the treatment compared to those who did not require MV (115.3 [84–177.1] vs 145.3 [97.5–223.5]; P=.26). At the 12-h mark, the patients in whom treatment failed had a PaO2/FiO2 ratio that was significantly lower compared to those who were not intubated (91.5 [64–129.5] vs 124 [93–217]; P=.02). The authors assumed that the pressures generated by the HFNC at 60L/min met the criteria required to diagnose and assess the severity of ARDS based on the Berlin Definition. Antonelli et al.32 saw that patients on NIMV due to hypoxemic ARF with a PaO2/FiO2 ratio ≤146mmHg after 1h of treatment had more chances of failing. It was surprising to see that the patients from the study conducted by Messika et al. who were intubated had lower PaO2/FiO2 ratios at the 12-h mark compared to the values proposed by Antonelli as an indicator of failure. The study conducted by Messika was commented by Medina and Alapont.33 In their manuscript, these authors claim that the mortality rate of ARDS goes from 20% to 60%, and that there is a correlation between the level of PEEP, the PaO2/FiO2 ratio, and mortality rate. Thus, in intubated and ventilated patients with a PEEP>10cmH2O, if the PaO2/FiO2 ratio is <150, mortality rate is as high as 60.3%. Many intensivists do not agree with continuing NIMV in patients with ARDS with PaO2/FiO2 ratios< 175 after 1h of treatment. These authors do not believe in treating patients diagnosed with ARDS with PaO2/FiO2 ratios <200, that is, moderate-to-severe ARDS with HFNC oxygen therapy. They believe that if a patient with ARDS is on NIMV and a PaO2/FiO2 ratio>175 cannot be reached, then the patient should be intubated. The PaO2/FiO2 ratio only assesses oxygenation. The ROXI proposed by Roca et al.15 combines oxygenation (SpO2/FiO2) and work of breathing (WOB). We believe that this index can be useful to decide if a patient should be intubated and on MV.
Study limitationsWe found two limitations in our study. The first one was the small size of the sample used and the fact the study was conducted in one single center only. The analysis of the risk correlation between patients with a RR <29 breaths per minute at the 2-h mark, a FiO2 <0.59, and a ROXI >5.98 at the 8-h mark and the need for MV give us the following HRs: 0.103 for RR, 0.053 for FiO2, and 0.077 for the ROXI. However, the interval between the minimum and maximum values of the 95%CI is large. This aspect of the statistical analysis is indicative of how low the precision of the test really is, probably due to the small size of the sample.
The second limitation was the different type of conditions included in our study. Roca et al.15 only included patients with pneumonia and concluded that a ROXI>4.88 after 2h of HFNC oxygen therapy was associated with the success of this technique. The different design of our study may explain the disparity of results seen between studies.
ConclusionsA RR<29 breaths per minute at the 2-h mark, a FiO2 < 0.59, and a ROXI>5.98 at the 8-h mark were associated with successful HFNC therapies in the management of hypoxemic ARF.
Failed HFNC therapies are associated with longer hospital stays, higher mortality rates at the ICU setting, and a higher need for MV.
More prospective studies with larger samples are needed before confirming these preliminary results.
FundingThe authors declared that they have received no funding to conduct this study.
AuthorsRafael Artacho Ruiz: study coordination, database review, reference search, statistical analysis.
Belén Artacho Jurado: reference search, writing of the manuscript, statistical analysis.
Francisco Caballero Güeto: reference search, writing of the manuscript, database review.
Ángela Cano Yuste: statistical analysis.
Ignacio Durbán García: reference search, writing of the manuscript, database review.
Francisco García Delgado: reference search, writing of the manuscript, database review.
José Antonio Guzmán Pérez: reference search, writing of the manuscript, database review.
Manuel López Obispo: reference search, writing of the manuscript.
Isabel Quero del Río: reference search, writing of the manuscript.
Francisco Rivera Espinar: reference search, writing of the manuscript, database review, statistical analysis.
Emilio del Campo Molina: reference search, writing of the manuscript, database review.
Conflicts of interestNone declared.
We wisk to thank Dr. José María Guzmán Jiménez from the UW School of Aquatic and Fishery Sciences, University of Washington, United States for his collaboration writing this manuscript into English.
Please cite this article as: Artacho Ruiz R, Artacho Jurado B, Caballero Güeto F, Cano Yuste A, Durbán García I, García Delgado F, et al. Predictores de éxito del tratamiento con cánula nasal de alto flujo en el fallo respiratorio agudo hipoxémico. Med Intensiva. 2021;45:80–87.