To analyze measures referred to venous thromboembolic prophylaxis in critically ill patients.
DesignAn epidemiological, cross-sectional (prevalence cut), multicenter study was performed using an electronic survey. Comparison of results with quality indexes of the Spanish Society of Intensive Care Medicine, the American College of Chest Physician guidelines and international studies.
SettingIntensive Care Units (ICUs) in the Community of Madrid (Spain).
PatientsAll patients admitted to the ICU on the day of the survey.
Variables of interestGeneral aspects of venous thromboembolic prophylaxis and protocols used (risk stratification and ultrasound screening). A descriptive analysis was performed, continuous data being expressed as the mean or median, and categorical data as percentages.
ResultsA total of 234 patients in 18 ICUs were included. Eighteen percent (42/234) received no prophylaxis, and 55% had no contraindication to pharmacological prophylaxis. Of the 192 patients receiving prophylaxis, 84% received pharmacological prophylaxis, 14% mechanical prophylaxis and 2% combined prophylaxis. Low molecular weight heparin was the only pharmacological prophylaxis used, with a majority use of enoxaparin (17 of 18 ICUs). In patients with mechanical prophylaxis (31/192), antiembolic stockings were the most commonly used option (58%). Pharmacological prophylaxis contraindications were reported in 20% of the patients (46/234), the most frequent cause being thrombocytopenia (28% of the cases). Fifty percent of the ICUs used no specific venous thromboembolic prophylaxis protocol.
ConclusionsPharmacological prophylaxis with low molecular weight heparin was the most frequently used venous thromboembolic prophylactic measure. In patients with contraindications to pharmacological prophylaxis, mechanical measures were little used. The use of combined prophylaxis was anecdotal. Many of our ICUs lack specific prophylaxis protocols.
Analizar la utilización de medidas de profilaxis de enfermedad tromboembólica venosa en el paciente crítico.
DiseñoEstudio epidemiológico, transversal (corte de prevalencia) y multicéntrico realizado mediante encuesta electrónica. Comparación de resultados con índices de calidad de la Sociedad Española de Medicina Intensiva, guías del American College of Chest Physicians y registros internacionales.
ÁmbitoUnidades de Cuidados Intensivos (UCI) de la Comunidad de Madrid.
PacientesTodos los pacientes ingresados en UCI el día de la realización de la encuesta.
Variables de interésAspectos generales de profilaxis de enfermedad tromboembólica venosa y utilización de protocolos. Análisis descriptivo expresado como media o mediana para variables cuantitativas y porcentajes para variables cualitativas.
ResultadosSe incluyeron 234 pacientes de 18 UCI. El 18% (42/234) no recibía ninguna profilaxis; un 55% de ellos no tenía contraindicación para profilaxis farmacológica. De los 192 pacientes con profilaxis, en el 84% fue farmacológica, en el 14% mecánica y en el 2% combinada. Las heparinas de bajo peso molecular fueron los únicos fármacos usados (enoxaparina en 17 de 18 UCI). En pacientes con profilaxis mecánica (31/192) las medias de compresión graduada fueron las más utilizadas (58%). El 20% de los pacientes (46/234) presentaba contraindicación para profilaxis farmacológica, con trombocitopenia como causa más frecuente (28%). La mitad de las UCI no utilizaba un protocolo específico de profilaxis.
ConclusionesLa profilaxis farmacológica con heparinas de bajo peso molecular fue la medida preventiva de enfermedad tromboembólica venosa más utilizada. Considerando los pacientes con contraindicación para profilaxis farmacológica, los sistemas mecánicos de profilaxis fueron poco utilizados. El uso de profilaxis combinada fue anecdótico. Hubo ausencia de protocolos específicos de profilaxis en muchas de nuestras UCI.
Venous thromboembolic disease (VTED), which comprises both deep venous thrombosis (DVT) and pulmonary embolism, is one of the most common avoidable complications in hospitalized patients.1 The association of multiple risk factors (previous chronic diseases, severity of the condition leading to admission, the use of mechanical ventilation, immobility, invasive procedures, etc.) causes critically ill patients to be particularly vulnerable to VTED.2 In this respect, a number of studies have demonstrated a high incidence of VTED in patients admitted to Intensive Care Units (ICUs), with the observation of DVT in 10–28% of the individuals who do not receive prophylaxis,2,3 and of pulmonary embolism in 7–27% of the necropsy studies in patents who have died of any cause.4 The use of both pharmacological and non-pharmacological prophylactic measures has been able to lower the incidence of DVT to 5–10%.2,5,6 The utilization of such prophylactic measures is therefore regarded as a quality index of particular relevance in the critically ill.7
The latest clinical practice guides of the American College of Chest Physicians (ACCP 2012) offer concrete recommendations for the prevention of VTED in different diseases and types of patients, including the critically ill.8–11 The heterogeneity and complexity of the patients admitted to the ICU (with multiple and different conditions leading to admission, previous chronic diseases predisposing to VTED, the presence of factors that increase the risk of bleeding, the use of medical apparatuses and devices, techniques and drugs routinely used in these Units, etc.) make the application of these recommendations complex and difficult, to say the least. To this problem we in turn must add the possible increase in mortality rate associated to a lack of prophylaxis or the use of inadequate prophylaxis, and which varies according to the type of disease involved, but can range from 8% in the septic patient to 15% in polytraumatized individuals.12
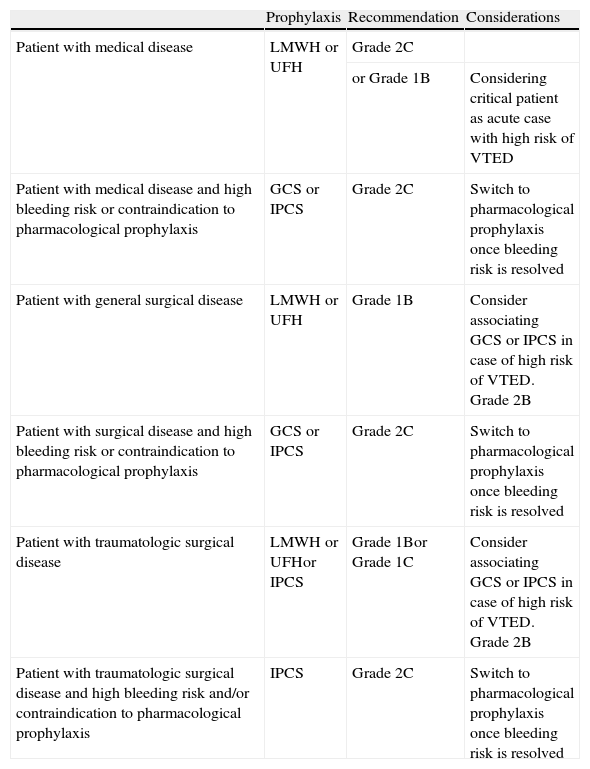
A series of measures are currently available for the prevention of VTED in the critical patient (Table 1). Pharmacological (drug) prophylaxis with both unfractionated heparin (UFH) and low molecular weight heparin (LMWH) has been shown to be the most effective preventive measure in both medical disease (grade 2C recommendation) and in general surgical or traumatologic cases with a high risk of VTED (grade 1B recommendation). The utilization of other drugs administered via the oral route, such as direct thrombin and factor Xa inhibitors, has not been tested to date in critical patients–their only possible indication being patients with orthopedic trauma of the hip or knee (fundamentally the placing of prostheses), with good oral tolerance, and who require admission to the ICU for some reason. Mechanical prophylaxis involving both graduated compression stockings (GCS) and intermittent pneumatic compression systems (IPCS) would be indicated in the management of patients with medical diseases and contraindications to pharmacological prophylaxis, or with a high bleeding risk (grade 2C recommendation), as well as in patients with non-traumatic (grade 2C recommendation) or traumatic surgical disease (grade 1C recommendation). Mechanical prophylaxis is moreover recommended in addition to pharmacological prophylaxis (i.e., as combined prophylaxis) in surgical patients with a high risk of suffering VTED (grade 2B recommendation).8–11,13
Recommendations referred to venous thromboembolic disease prophylaxis in the critical patient.
| Prophylaxis | Recommendation | Considerations | |
| Patient with medical disease | LMWH or UFH | Grade 2C | |
| or Grade 1B | Considering critical patient as acute case with high risk of VTED | ||
| Patient with medical disease and high bleeding risk or contraindication to pharmacological prophylaxis | GCS or IPCS | Grade 2C | Switch to pharmacological prophylaxis once bleeding risk is resolved |
| Patient with general surgical disease | LMWH or UFH | Grade 1B | Consider associating GCS or IPCS in case of high risk of VTED. Grade 2B |
| Patient with surgical disease and high bleeding risk or contraindication to pharmacological prophylaxis | GCS or IPCS | Grade 2C | Switch to pharmacological prophylaxis once bleeding risk is resolved |
| Patient with traumatologic surgical disease | LMWH or UFHor IPCS | Grade 1Bor Grade 1C | Consider associating GCS or IPCS in case of high risk of VTED. Grade 2B |
| Patient with traumatologic surgical disease and high bleeding risk and/or contraindication to pharmacological prophylaxis | IPCS | Grade 2C | Switch to pharmacological prophylaxis once bleeding risk is resolved |
VTED, venous thromboembolic disease; LMWH, low molecular weight heparin; UFH, unfractionated heparin; GCS, graduated compression stockings; IPCS, intermittent pneumatic compression system.
The application of these recommendations in the critical patient setting is highly variable, as reflected by different international studies published over the last 10 years.14–17 No descriptions of the situation in Spain are available to date.
The present study aims to analyze the frequency of use of the different measures for the prevention of VTED in patients admitted to ICUs in the Community of Madrid (Spain), and to compare the findings with the quality indexes of the Spanish Society of Intensive Care Medicine (SEMICYUC),7 the clinical practice guides of the ACCP 2012,8–11 and the data found in the different international studies.14–17
Patients and methodsA multicenter, cross-sectional epidemiological study was made involving a sample composed of all the patients admitted to different ICUs in the Community of Madrid, with the purpose of analyzing the frequency of use of the different VTED prophylactic measures proposed for application in critical patients. To this effect a prevalence cut was made of the prophylactic measures employed, performed on a single day of a concrete week.
A list was prepared of the hospitals belonging to the public health system of Madrid, with the addition of private hospital centers with polyvalent ICUs of similar characteristics. The heads of the different Units were contacted by telephone and invited to participate. In the case of those centers that agreed to take part in the study, a physician in charge of conducting the survey was assigned, and a contact e-mail address was established. Each physician in charge of conducting the survey received a first e-mail with information on the most relevant aspects of the study. The definitive electronic survey was then sent, with instructions to return it to us via e-mail once completed. The survey was considered adequate for collecting the required data, since an instrument of similar characteristics had been used in a recent study,17 and it had moreover been adopted without problems by physicians belonging to our Department and who did not participate in the project. The collected information was entered in a Microsoft Excel spreadsheet and subsequently analyzed using the SPSS version 18 statistical package.
In accordance with the clinical practice guides of the ACCP of the year 2008 and revised in 2012,1,8–11 all the patients admitted to the ICU were considered to be at risk of developing VTED, and were thus candidates for some type of prophylaxis (specific recommendation 2C for the critical patient or recommendation 1B considering the critical patient as an acute patient with a moderate or high risk of developing VTED). Likewise, all the Units were considered to require a specific VTED prevention protocol (grade 1A recommendation of the clinical practice guides of the ACCP 2008 and 100% proposition according to the critical patient quality indexes of the SEMICYUC), including assessment and risk stratification for the appropriate adjustment of prophylaxis.
The prevalence cut was performed in the week from 16 to 23 March 2012. We included all the patients admitted in each Unit on the concrete day on which the survey was carried out.
The main data included in the survey were the following:
- 1.
Unit size and occupation: number of available beds and number of admitted patients.
- 2.
Type of patient according to the disease leading to admission:
- a.
Medical disease: no surgery was required to resolve the condition.
- b.
Surgical disease: non-traumatologic surgical measures were required to resolve the condition (both emergency and programmed surgery with posterior complications).
- c.
Polytraumatism: high-energy multiple trauma conditions. Major burn cases were also included.
- a.
- 3.
Information on the VTED prophylactic measures used:
- a.
Use or not of some type of prophylaxis.
- b.
Type of prophylaxis used:
- -
Pharmacological prophylaxis: UFH or LMWH (direct thrombin and factor Xa inhibitors were excluded, since they currently have no indications in the critical patient).
- -
Mechanical prophylaxis: GCS or IPCS.
- -
Combined prophylaxis: UFH or LMWH added to some mechanical prophylactic system.
- -
- c.
Existence of contraindications to pharmacological prophylaxis and the reasons for contraindication: thrombocytopenia, coagulopathy, recent major surgery, high bleeding risk, or others. Each Unit cataloged the contraindications of their patients according to their own criteria.
- d.
Patients subjected to systemic anticoagulation therapy.
- a.
- 4.
Information on the use of a specific VTED protocol by the Unit.
- 5.
Information on the adjustment of VTED prophylaxis according to risk scales.
- 6.
Information on the use of deep venous system ultrasound as a VTED screening method.
The only exclusion criterion for the definitive analysis was established after the data from each Unit had been obtained, and consisted of the exclusion of those patients receiving treatment with heparin at anticoagulation doses.
Following review of the project and protocol, the Clinical Research Ethics Committee of Hospital General Universitario Gregorio Marañón approved conduction of the study.
Due to the characteristics of the study, only a descriptive analysis of the patients was carried out. Quantitative variables were reported as the mean (standard deviation) in the case of a normal distribution, and as the median (interquartile range) in the case of a non-normal distribution. Qualitative variables in turn were reported as a proportion or percentage.
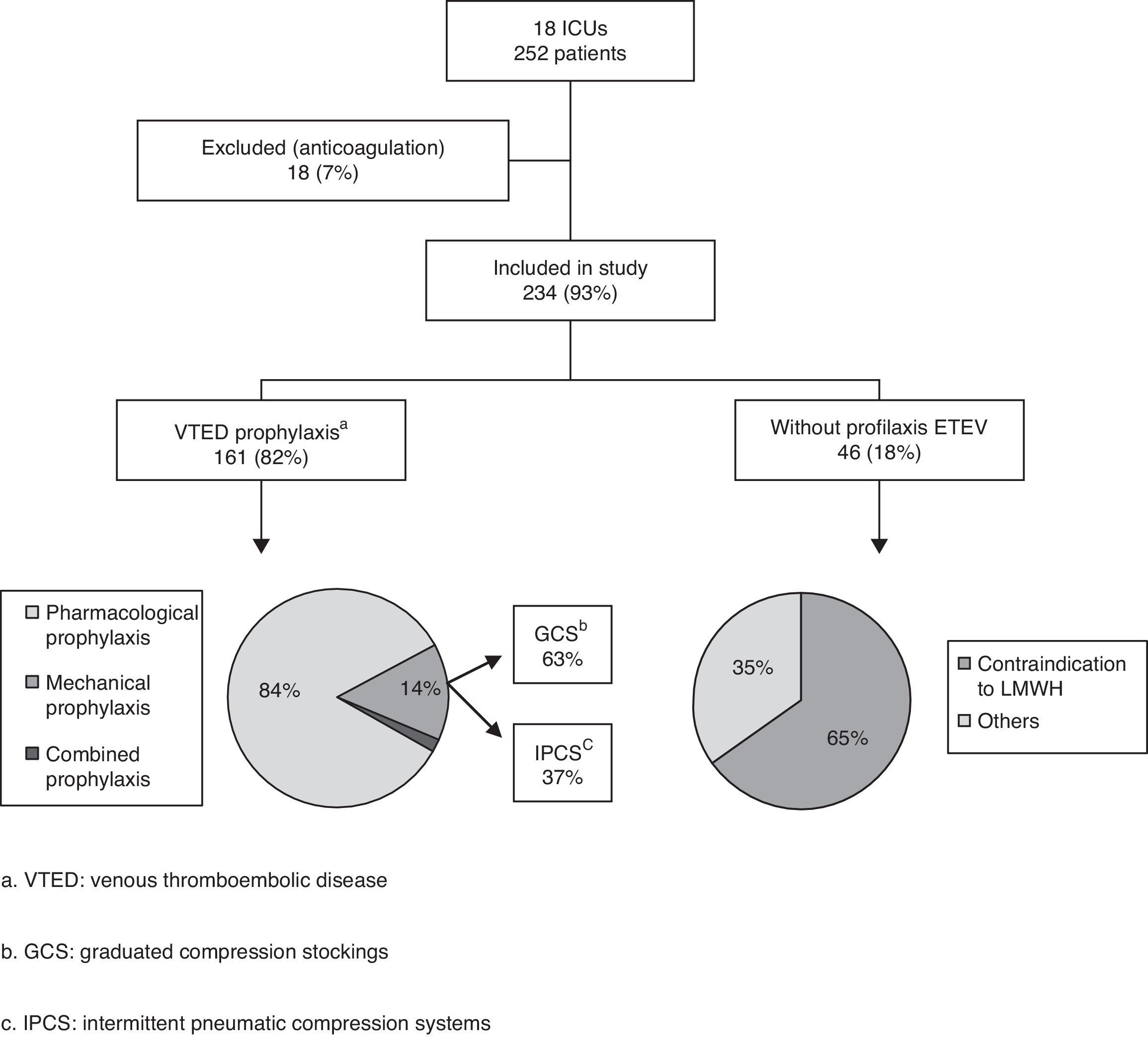
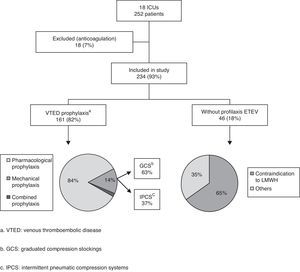
ResultsA total of 26 of the ICUs contacted by telephone agreed to participate in the study. Of these, 18 (17 public and one private center) finally completed the survey (69.2%) (see Annex). Out of a total of 302 critical patient beds, with a median of 14 beds per Unit (8–20), we collected data on 252 patients (10 patients per Unit; 7–17), representing an occupation rate of 83% of the total available beds. Sixty percent of the patients presented medical diseases (153/252), 32% surgical diseases (81/252), and 8% polytraumatisms (18/252). A total of 234 patients were included in the definitive analysis, since 18 were excluded because systemic anticoagulation had been used (7%).
On the day of their inclusion in the study, 82% of the patients (192/234) were receiving some type of VTED prophylaxis: pharmacological prophylaxis in 84% of the cases (161/192), mechanical prophylaxis in 14% (27/192), and both types of prophylaxis (combined prophylaxis) in only four patients (2%) (Fig. 1).
In the 165 patients receiving pharmacological prophylaxis, including the four with combined prophylaxis, LMWHs were the only drugs used – particularly enoxaparin, which was administered in 17 of the 18 Units (94%). One of the Units used nadroparin in addition to enoxaparin. In the remaining Unit, dalteparin was the LMWH used.
Of the different mechanical prophylactic measures (including the measures used in the 4 patients with combined prophylaxis), GCS were the most commonly used option (58% of the patients; 18/31). IPCS were used in only 13 patients.
Contraindications to the use of pharmacological prophylaxis were documented in 20% of the patients (46/234), with a median of one patient per Unit (0–4). More than one reason for not providing drug prophylaxis was recorded in 19 patients (41%). Of the patients with contraindications to heparin, 27 (58%) were subjected to mechanical prophylaxis, while the rest of the patients received no prophylactic measures. The causes of contraindication to pharmacological prophylaxis were the following (in decreasing order of frequency): thrombocytopenia 28% (18/65), recent bleeding 26% (17/65), high bleeding risk 21% (14/65), recent major surgery 14% (9/65), and coagulopathy 11% (7/65).
Eighteen percent of the analyzed patients (42/234) received no prophylactic measures. Of these individuals, 19 (45%) belonged to the group of patients with contraindications to pharmacological prophylaxis, while 23 patients had no contraindications. More than one patient received no prophylactic measures in 11 of the 18 Units (61%), with a median of two patients per Unit (0–4).
Lastly, it should be mentioned that one-half of the Units (9/18) had no specific VTED prophylaxis protocol, and in only one of them were VTED risk scales used to adjust prophylaxis. Four of the total ICUs (22%) used clinical ultrasound to screen for DVT in patients at a high risk of developing VTED.
DiscussionThis study included 15 of the 20 ICUs belonging to the public health network (75%)(3 critical patient Units of the same hospital center responded independently), together with the polyvalent ICU of a private center, in the Community of Madrid. Considering both the number of participating Units and the number of patients studied (252 subjects), the results obtained can give us a good idea of the prevalence of VTED prophylactic measures among the critical patients of our Community, and the findings can probably be extrapolated to the situation found in the rest of the country.
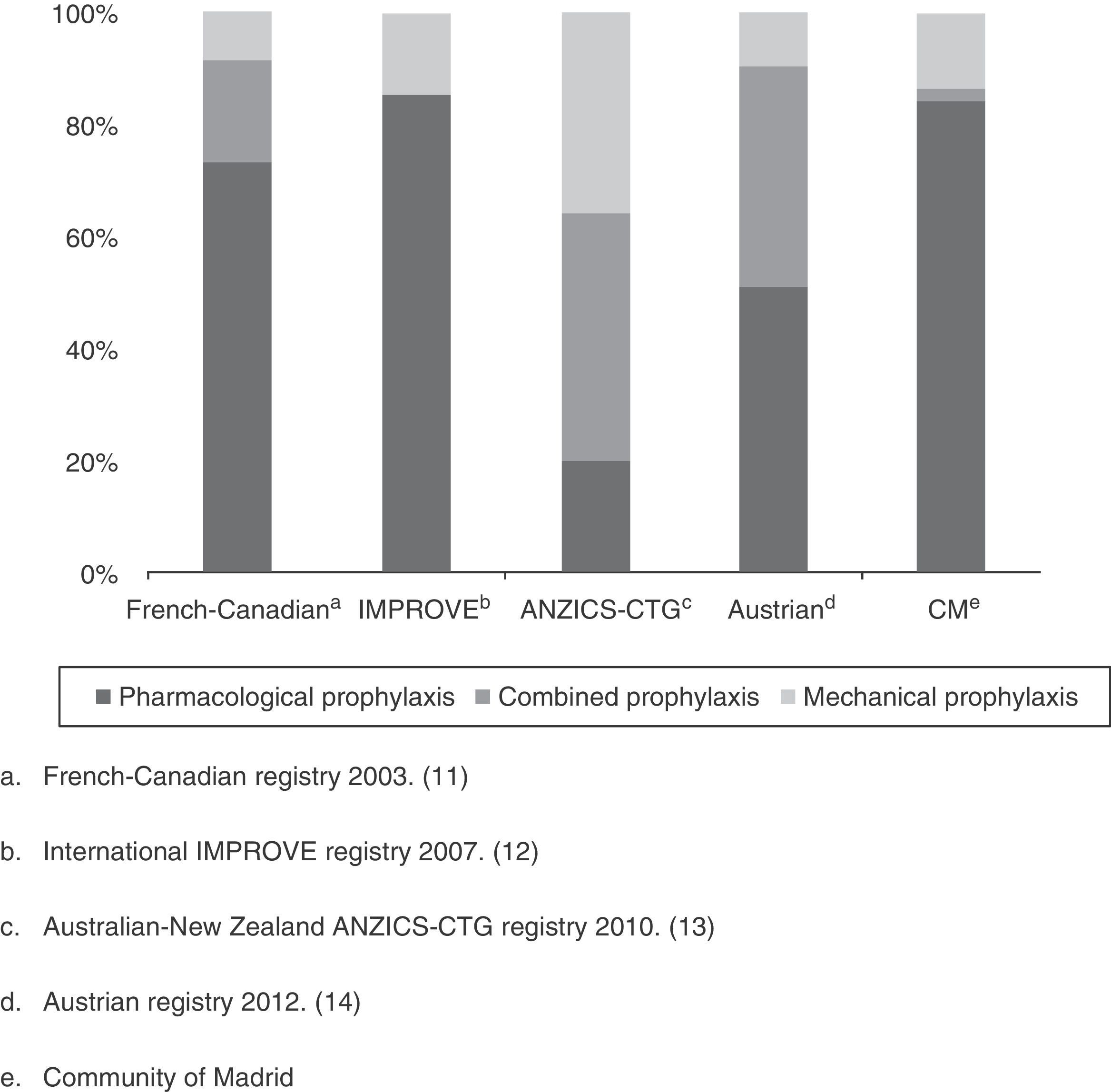
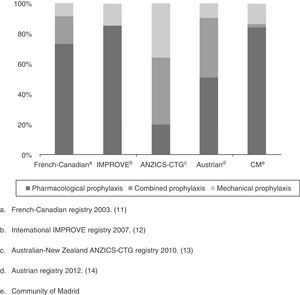
As a first appraisal of the results obtained, it should be mentioned that while the percentage of patients with some VTED prophylactic measure is apparently high (82%), it falls short of the 90% rate proposed by the SEMICYUC quality indexes for the management of critical patients in Spain,7 or the levels proposed by the clinical practice guides of the ACCP 2012.8 Although the level of evidence 2C (grade 1B recommendation when regarding critical patients as acute patients with a high risk of VTED) and 1B is meant for surgical patients (general or traumatologic), these guidelines advise the use of prophylactic measures in the critical patient. On examining the situation by Units, over one-half (60%) presented percentage prophylaxis rates lower than those recommended. This was more apparent in the larger Units (88% in the case of ICUs with more than 10 beds versus only 25% of the Units with fewer than 10 beds). The described panorama is not very different from that reported in other countries referred to the use of VTED prevention measures. In effect, with the exception of the study conducted in Austria in the year 2012,17 which included 52 ICUs with 502 patients and reported a compliance rate of 95% (though the authors described a large percentage of inadequate prophylaxis, taking into account the lack of anti-Xa factor monitoring), the rest have all reported compliance rates that fall short of the recommendations of both the SEMICYUC and the ACCP: 87% in the French-Canadian registry of 2003,14 77% in the international IMPROVE registry of 2007,15 and 86% in the Australian-New Zealand ANZICS-CTG registry of 201016 (Fig. 2).
In concordance with the current recommendations on preventive measures in the critical patient, pharmacological prophylaxis–in the absence of contraindications–was the most widely used strategy.8–11,13 The great variability in the use of the different heparins (UFH and LMWH) described in the literature reflects the lack of scientific evidence of the superiority of one type of heparin over the rest,18 except as refers to the greater reduction in the incidence of pulmonary embolism following prophylaxis with LMWH (dalteparin) versus UFH described in a recent clinical trial.5 In our Units the exclusive use of LMWH could be related to simpler administration (once a day) and the fact that in most cases routine testing is not needed to adjust the drug. This practice is consistent with the way things are commonly done in other European countries such as France and Austria–in contraposition to the more frequent use of UFH in countries such as the United States, Canada, New Zealand and Australia. The recent publication of the results of the PROTECT multicenter trial5 has not been able to clarify the situation, since after randomizing almost 4000 patients to either LMWH (5000IU s.c. of dalteparin once a day) or UFH (5000IU s.c. twice day), the incidence of DVT (as assessed by ultrasound) was found to be similar in both groups (5.1% and 5.8%, respectively). Although as mentioned above it is true that there appears to be a decrease in the incidence of pulmonary embolism in the group treated with dalteparin (1.3% versus 2.3%; HR 0.51; 95%CI 0.30–0.88), this reduction was not associated to a decrease in mortality. Little can be commented on the more frequent use in our study of enoxaparin as LMWH (16 of the 18 Units), since to date no clinical trials have compared the efficacy of the different types of LMWH, and there are no concrete recommendations in this respect.8–11,13
In relation to this subject, an important limitation of our study is the fact that the enoxaparin dose administered was not recorded, its activity was not monitored, and the type of patient receiving the drug was not documented–specifically as refers to the subgroup of patients with impaired renal function (creatinine clearance≤30ml/min). The utilization of repeated enoxaparin doses for prophylaxis in this subgroup of patients (which is the practice found in most of the Units in our study) could lead to drug accumulation over a number of days, and although this does not seem to increase the risk of bleeding episodes, it would oblige us to reduce the usual dose. In contrast, the administration of two daily doses for anticoagulation purposes does increase bleeding risk. There appears to be no such accumulation in the case of nadroparin, dalteparin and tinzaparin, and dose adjustment therefore would not be needed when using these agents.19,20 However, both in patients with renal failure and in other patient subgroups such as neurocritical cases, patients requiring vasopressor drugs, or individuals with generalized edema or anasarca, the need to measure the anti-factor Xa levels in order to assess the correct LMWH dosage remains subject to discussion, due to the lack of evidence of a correlation between such levels and the development of symptomatic or asymptomatic VTED.21–26
Mechanical prophylaxis as recommended in patients with a high bleeding risk or contraindications to pharmacological prophylaxis (grade 2C recommendation in medical or general surgical patients and grade 1C recommendation in traumatologic surgery patients) was little used in our study, representing about 14% of the total patients receiving some type of prophylaxis and a little over 50% of those with a concrete indication for the use of mechanical prophylaxis.8–11,13 The use of this type of prophylaxis, as reported in the different international registries, varies from 14% of all patients (31% among those with a specific indication for such measures) in the French-Canadian study of 2003, with a higher utilization rate in Canada,14 to 36% of all patients (70% among those with a specific indication for such measures) in the ANZICS-CTG study of 2010.17 The poor acceptance of this type of prophylactic strategy could be related to the lack of clinical trials in critical patients supporting its use, and to the absence of cost-effectiveness analyses. We consider it important to underscore the great variability in the use of the different mechanical prophylactic devices, with a predominance of GCS over IPCS–though the latter might be more effective in preventing DVT in certain patient subgroups such as neurocritical cases, polytraumatized patients or high-risk surgical patients.8–11,27,28
On the other hand, the use of combined prophylaxis (drugs and mechanical measures simultaneously) has been indicated in critical patients with a high risk of suffering VTED.1,10,11 Although our study did not require specification of the risk factors for DVT among the patients, and we therefore were unable to stratify them according to risk, the use of this form of prophylaxis was infrequent (2%). On examining the literature, the use of combined prophylaxis in critical patients is seen to have varied over the last 10 years, increasing from a little over 10% in the French-Canadian study of 2003 to about 40% in the Australian-New Zealand study of 2010 and the Austrian publication of 2012.14,16,17 Two recent metaanalyses by Kakkos et al.,29,30 with results that appear favorable to the use of combined prophylaxis versus single-mode prophylaxis (pharmacological or mechanical) in patients with a high risk of developing VTED–fundamentally surgical patients (OR 0.16; 95%CI 0.07–0.34 for DVT in the former, and OR 0.31; 95%CI 0.23–0.43 for DVT with OR 0.34; 95%CI 0.23–0.50 for pulmonary embolism in the latter)–appear to offer a reasonable argument for increased use of combined prophylaxis while in wait of clinical trials specifically centered on critical patients. Furthermore, according to the clinical practice guides of the ACCP 2012,10,11 the recommendation in surgical patients (traumatologic or otherwise) with a high risk of suffering VTED is to combine pharmacological prophylaxis with intermittent pneumatic compression for the duration of hospital stay, based on 2C level of evidence. In our study, up to 40% of the patients were surgical or polytraumatized subjects; such a recommendation therefore may have been applicable to a considerable proportion of them once the bleeding risk phase was left behind.
The formalization and implementation of a specific protocol in each Unit, based both on the general recommendations of the clinical practice guides and on the specific characteristics of the patients admitted to the Unit (risk stratification), could improve the prevention of VTED in the critical patient.6,7,31,32 Our study showed only one-half of the participating Units to have a prophylaxis protocol–this proportion falling well short of the recommended quality index of 100% in the critical patient proposed by the SEMICYUC.7 Furthermore, only one of the Units included VTED risk stratification in their protocol, with a view to optimizing prophylaxis.31,33 This situation means that our clinical practice is not in line with that recommended by the guides which propose routine assessment of VTED risk in the critical patient (grade 1A recommendation).1
Lastly, mention should be made of the growing use of clinical ultrasound by intensivists in the critical care setting.34,35 The fact that VTED, and more specifically DVT, is often asymptomatic and silent in our patients, suggests that deep venous system ultrasound using the simplified compression technique (a simple and rapid procedure) may be very useful in screening for VTED in the ICU. In our case, only four of the participating Units routinely used ultrasound screening for VTED. In line with the recommendation of the current guides not to routinely screen for DVT (grade 2C recommendation),8,10,11 we consider that the technique should be reserved for those patients with an increased risk of suffering VTED, or in which adequate prophylaxis is not possible for some reason.
As commented above, the main limitation of our study was the impossibility of conducting a thorough and detailed analysis of the suitability of the prophylactic measures used. The initial aim of the study was fundamentally to assess the prevalence of prophylaxis and its different types; however, on posteriorly analyzing the results, we would have liked to have more information in order to adequately assess the suitability of the prophylactic measures adopted. Despite the large number of participating Units and the acceptable number of patients recruited, which could offer an impression of the routine results obtained in the ICUs of the Community of Madrid, the fact of having collected the data on a single day means that generalizations are not possible. Furthermore, performing the survey by e-mail implied possible errors both in forming and interpreting the questions, and even in answering them.
It seems obvious that a larger study is needed to correctly establish the situation of VTED prophylaxis in Spain. A prevalence cut at national level, with a more exhaustive and rigorous survey, could offer a more reliable idea of the current situation. The creation of a specific VTED working group probably could lead to studies designed to clarify the doubts on different aspects referred to the prevention of VTED: the true incidence of VTED in our Units, the most appropriate LMWH and its optimum dose, monitoring and dose adjustments based on anti-factor Xa levels, the efficacy of combined prophylaxis in high-risk critical patients, or the role of clinical ultrasound in prophylaxis, among other issues.
ConclusionsIn our study, pharmacological prophylaxis with LMWH was the most widely used VTED prevention measure. Mechanical prophylaxis was little used in patients with contraindications to pharmacological prophylaxis, and the use of combined prophylaxis was merely anecdotal. Many of the Units lacked specific prophylaxis protocols.
Financial supportThis study has received no financial support from public or private institutions.
Conflicts of interestPablo Garcia-Olivares, Jose Eugenio Guerrero-Sanz and Ana Maria Hernangomez have participated in different symposia on venous thromboembolic disease in the critical patient organized by the company Covidien Spain, S.L.
The authors wish to express their most sincere gratitude to the physicians of the different Intensive Care Units for their collaboration in this study.
Intensive Care Units of: Hospital La Paz, Hospital Clínico San Carlos, Hospital Puerta de Hierro, Hospital Infanta Sofía, Hospital Infanta Elena, Hospital Infanta Cristina, Hospital de Henares, Hospital de Getafe, Hospital de La Princesa, Hospital de Torrejón de Ardoz, Hospital del Sureste, Hospital Infanta Leonor, Hospital del Tajo, Hospital Doce de Octubre, Hospital Severo Ochoa, Hospital Madrid Norte San Chinarro.
Please cite this article as: García-Olivares P, Guerrero JE, Tomey MJ, Hernangómez AM, Stanescu DO. Profilaxis de la enfermedad tromboembólica venosa en el paciente crítico: aproximación a la práctica clínica en la Comunidad de Madrid. Med Intensiva. 2014;38:347–355.