This study evaluates the potential prognostic value of serial measurements of different biomarkers (procalcitonin [PCT], C-reactive protein and leukocytes [CRP]) in septic shock patients.
DesignProspective observational study.
SettingIntensive care unit of a third-level University Hospital.
PatientsThe study comprised a total of 88 septic shock patients defined using the 2001 Consensus Conference SCCM/ESICM/ACCP/ATS/SIS criteria. The PCT, CRP and leukocytes were recorded on admission to the ICU and again 72h after admission.
InterventionsNone.
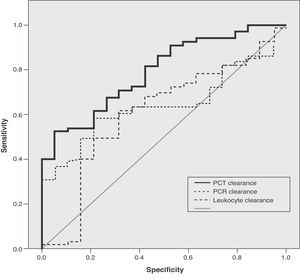
ResultsThose patients with increasing procalcitonin levels showed higher hospital mortality than those with a decreasing levels (58.8% vs 15.4%, p<0.01). No such effect was observed in relation to C-reactive protein or leukocytes. The best area under the curve for prognosis was for procalcitonin clearance (0.79). A procalcitonin clearance of 70% or higher offered a sensitivity and specificity of 94.7% and 53%, respectively.
ConclusionsSerial procalcitonin measurements are more predictive of the prognosis of septic shock patients than single measurements of this parameter. The prognostic reliability of the latter is also better than in the case of C-reactive protein and leukocytes. The application of serial procalcitonin measurements may allow the identification of those septic patients at increased mortality risk, and help improve their treatment.
El presente estudio evalúa la utilidad pronóstica que la determinación seriada de diferentes biomarcadores (procalcitonina [PCT], proteína C reactiva [PCR] y leucocitos) podría tener en los enfermos en shock séptico.
DiseñoEstudio prospectivo observacional.
ÁmbitoLa unidad de cuidados intensivos (UCI) de nuestro centro, un Hospital Universitario de tercer nivel.
PacientesOchenta y ocho pacientes en shock séptico según criterios de la Conferencia de Consenso SCCM/ESICM/ACCP/ATS/SIS de 2001. Se llevó a cabo la determinación de PCT, PCR y leucocitos al ingreso en la UCI y a las 72 horas del mismo.
IntervencionesNinguna.
ResultadosLos pacientes con incremento en los valores de PCT presentaron una mayor mortalidad hospitalaria en comparación con los que presentaron un descenso de los mismos (58,8 frente al 15,4%, p<0,01). Este efecto no se observó en las determinaciones de PCR ni los leucocitos. El mejor área bajo la curva ROC para el pronóstico correspondió al aclaramiento de PCT (0,79). El aclaramiento del 70% en los valores de PCT permitió discriminar la supervivencia hospitalaria con una sensibilidad del 94,7% y una especificidad del 53%.
ConclusionesLa determinación seriada de los valores de PCT predice mejor que la determinación única el pronóstico de los pacientes en shock séptico. Su fiabilidad pronóstica es superior a la de la PCR y los leucocitos. El uso de las determinaciones seriadas de PCT podría ayudar a identificar a aquellos pacientes sépticos con mayor riesgo de muerte permitiendo optimizar su tratamiento.
Sepsis and its consequences are one of the leading causes of death in Intensive Care units (ICUs).1,2 The growing prevalence of sepsis and its high mortality rate define it as a very important sociosanitary problem. In Spain, Esteban et al., in a study conducted in three hospitals in Madrid, recorded an incidence of 333 cases of sepsis per 100,000 inhabitants-year, with an in-hospital mortality rate of 13% for sepsis, 28% for severe sepsis, and 46% for septic shock.3 These figures place severe sepsis as the fifth ranking cause of mortality in Spain, after heart disease, cerebrovascular disease, cancer and chronic lung disease—without counting the contribution of sepsis to mortality associated with all of the aforementioned disorders.
Different initiatives have attempted to improve the survival of septic patients based on strategies designed to ensure the early diagnosis and treatment of these subjects.4–12 However, the complexity of these patients, who often develop multiorgan failure (MOF) and polymicrobial infections or infections produced by multiresistant pathogens, complicates the provision of optimum treatment. The early detection of those patients showing an initial unfavorable course or with an increased mortality risk is essential in order to prevent the progression of organ dysfunction, which would increase the frequency of complications and patient mortality.13,14 Although the use of complementary tests facilitates the detection of such patients, their usefulness is often limited,15 while other techniques such as cultures typically require long time intervals for obtaining results.3
Procalcitonin (PCT) is a peptide precursor of the hormone calcitonin found in very low (<0.05ng/ml) or even undetectable concentrations in healthy individuals. In situations of infection, however, different body tissues (kidney, adipose tissue, lung and liver) secrete PCT into the bloodstream—concentrations of over 0.5ng/ml being regarded as pathological.16 Different studies have found PCT to behave as a diagnostic marker of bacterial infection that is more reliable than other markers commonly used in clinical practice, such as C-reactive protein (CRP) or leukocyte count, and even more reliable than experimental markers such as interleukin-6 (IL-6) or IL-8.17 PCT also has prognostic value—its levels being related to the severity and mortality of infection.18,19 However, different factors, including the duration of sepsis or the presence of renal failure, can lead to a loss of reliability when PCT determination is carried out on an isolated basis.16,20 Serial measurements over time could remedy this limitation, though the existing experience in patients with septic shock is limited.
The present study examines the usefulness of the serial determination of plasma PCT in relation to the prognosis of patients with septic shock, comparing it with other markers used in routine clinical practice.
Materials and methodsPopulationA prospective, observational cohort study was carried out of all patients over 14years of age admitted to the ICU of Marqués de Valdecilla University Hospital in Santander (Spain) with septic shock according to the definitions proposed by the SCCM/ESICM/ACCP/ATS/SIS consensus conference,1 i.e., the presence of severe sepsis with arterial hypotension and/or persistent signs of tissue hypoperfusion refractory to the intravenous administration of fluids (20ml/kg), and requiring the infusion of vasoactive drugs. The study period extended from 1 January 2009 to 31 December 2009.
Infection was suspected from the presence of an infectious focus documented by compatible radiological or laboratory test findings, a clinical syndrome associated with a high probability of infection, or distributive shock not explainable by other causes.
We excluded patients aged ≤14years; patients with recent cardiac arrest or with instructions referred to the limitation of therapeutic effort; patients without severe sepsis or septic shock at the time of admission to the ICU but who developed such problems during admission to the Unit; and those patients who died or were discharged from the ICU in under 72h.
Recording of variablesThe demographic characteristics of the patients were documented, together with the laboratory test findings (basic biochemistry, lactate, PCT, CRP, complete blood count, coagulation and venous blood gases), microbiological culture results, number of days with antibiotics, the main final diagnosis, patient condition at hospital discharge and cause of death (related or unrelated to sepsis). Organ dysfunctions in turn were assessed based on the definitions proposed by the SCCM/ESICM/ACCP/ATS/SIS consensus conference.1
Determination of markersPlasma PCT and CRP were determined, together with the leukocyte count upon admission to the ICU and after 72h. We also calculated the clearance of each marker as the percentage variation in the value obtained in the last determination versus the first, based on the following formula: [(initial value−final value/initial value)×100]. CRP was assayed using an immunoturbidimetric test (CRPLX, 0–439) with the COBAS INTEGRA 400 analyzer (Roche, Germany). The plasma PCT concentrations were determined using a test based on TRACE (Time Resolved Amplified Cryptate Emission) technology with the Kryptor PCT analyzer (Brahms, Germany). The leukocyte counts were established by automatic laser mediated counting.
Statistical analysisThe continuous variables were expressed as the mean±SD, or as the median and interquartile range (IQR) in the case of a non-normal distribution. The qualitative variables were expressed as absolute variables and percentages.
Comparison of the means of continuous variables for two groups was based on analysis of variance or the nonparametric Kruskal–Wallis test, where indicated. The comparison of proportions was made using the chi-squared test, with Yates correction where applicable. An alpha risk with p<0.05 was assumed for considering a relationship to be statistically significant. A univariate analysis was performed to determine the association of the different variables to mortality.
For the diagnostic and prognostic evaluation of the biological markers we determined the corresponding diagnostic reliability indices: sensitivity, specificity, positive predictive value and negative predictive value for each cutoff point of each biological marker. Comparison of the diagnostic accuracy of the markers was made using ROC (receiving operator characteristic) curve analysis, calculating the area under the curve (AUC) and the standard error using the nonparametric method described by Hanley and McNeil.
Logistic regression analysis was carried out to estimate the mortality predicting capacity of the different biological markers. All those clinical and laboratory test parameters found by the univariate analysis to be correlated to the end prognosis (in-hospital mortality) were entered in the model (p<0.15). Adjustment was made for the following covariables: age, gender, immune depression, APACHE II score, SOFA score, and other identified possible confounding factors.
The study was approved by the Ethics Committee of the center. Given the observational nature of the study and the fact that the diagnostic tests involved were the same as those commonly used in clinical practice, no informed consent was considered necessary.
ResultsBetween 1 January 2009 and 31 December 2009, a total of 122 patients with septic shock were admitted to the ICU (Table 1). Limitation of therapeutic effort was applied to 5 of these patients; serial determination of the markers was not made in 26 subjects; and three patients died in under 72h after admission to the Unit. All these individuals were excluded from the study, leaving a final sample of 88 patients, of which 54 (61%) were males. The mean age was 64.8±18.7years, with an APACHE II score of 20.4±6.2 and a SOFA score of 9.1±3.1. Most of the patients (41%) came from the Emergency Department, 29 (32.9%) were admitted from surgical areas, 10 (11.3%) from medical wards, and 8 patients (0.09%) came from other hospitals. The most frequent location of infection was intraabdominal (36.6%), followed by the lungs (31.6%) and urinary tract (15.9%). The values corresponding to PCT, CRP and leukocyte count upon admission to the ICU were 13.2 (2.6–33.8)ng/ml, 21.0 (10.9–27.9)mg/dl and 15.1±10.2×103/mm3, respectively. The in-hospital mortality rate was 21.6%, while the mortality rate in the ICU was 17%. The patients who died had more frequent associated comorbidities and immune depression than the survivors, higher severity scores, and more often required mechanical ventilation and renal replacement techniques (Table 2).
Characteristics of the study population and comparison of the patients showing PCT clearance versus those without clearance.
| TotalNo.=88 | PCT clearanceNo.=71 | No PCT clearanceNo.=17 | p | |
| Characteristics | ||||
| Age (years) | 64.8±18.7 | 65.38±18.2 | 62.9±21.3 | 0.65 |
| Male gender n (%) | 56 (63.6) | 44 (62.0) | 12 (70.6) | 0.58 |
| SOFA admission | 9.0±3.0 | 8.9±3.0 | 9.3±3.2 | 0.71 |
| SOFA 72h | 6.1±3.6 | 5.5±3.0 | 8.6±4.7 | 0.01 |
| APACHE II score | 20.4±6.0 | 19.9±5.7 | 22.8±6.9 | 0.14 |
| Comorbidities n (%) | 74 (84.1) | 60 (84.5) | 14 (82.4) | 1.00 |
| Immune suppression n (%) | 12 (13.6) | 9 (12.7) | 3 (17.6) | 0.69 |
| Mechanical ventilation n (%) | 43 (53.8) | 30 (46.2) | 13 (86.7) | <0.01 |
| CVVHF n (%) | 10 (13.5) | 5 (8.2) | 5 (38.5) | 0.01 |
| SvcO2 (%) | 69.4±11.1 | 70.6±9.2 | 64.4±15.9 | 0.11 |
| Lactate (mg/l) | 31.7±28.7 | 31.3±28.8 | 33.7±32.3 | 0.90 |
| Infection site n (%) | 0.27 | |||
| Intraabdominal | 34 (38.6) | 27 (38.0) | 7 (41.2) | |
| Pneumonia | 32 (36.4) | 26 (36.6) | 6 (35.3) | |
| Urinary tract | 15 (17.0) | 14 (19.7) | 1 (5.9) | |
| Skin/soft tissues | 4 (4.5) | 2 (2.8) | 2 (11.8) | |
| Catheter bacteremia | 1 (1.1) | 1 (1.4) | 0 (0) | |
| Other infections | 1 (1.1) | 0 (0) | 1 (1.4) | |
| Unknown | 3 (1.1) | 1 (1.4) | 0 | |
| Outcome | ||||
| Hospital mortality n (%) | 21 (23.9) | 11 (15.5) | 10 (58.8) | <0.01 |
| ICU mortality n (%) | 15 (17.0) | 7 (9.9) | 8 (47.1) | <0.01 |
| Hospital stay (days) | 32.4±13.3 | 30.8±21.0 | 40.8±65.2 | 0.02 |
| ICU stay (days) | 11.5±13.5 | 11.4±14.3 | 11.7±8.2 | 0.19 |
APACHE II, Acute Physiology and Chronic Health Evaluation score; CVVHF, continuous venous–venous hemodiafiltration; PCT, procalcitonin; SOFA, Sepsis-related Organ Failure Assessment score; SvcO2, central venous oxygen saturation; ICU, Intensive Care Unit.
Infection marker values in the study population and comparison between survivors and deceased patients (in-hospital mortality).
| Markers | TotalNo.=88 | SurvivorsNo.=69 | DeceasedNo.=19 | p |
| Admission | ||||
| PCT | 13.2 (2.6–33.8) | 12.9 (3.0–41.0) | 13.5 (1.9–31.5) | 0.60 |
| CRP | 21.0 (10.9–27.9) | 21.6 (10.9–27.9) | 17.9 (10.6–27.9) | 0.85 |
| Leukocytes | 15.1±10.2 | 15.8±10.0 | 12.8±11.1 | 0.24 |
| 72h | ||||
| PCT | 4.4 (1.1–11.5) | 2.2 (1.0–9.6) | 20.0 (2.3–32.1) | <0.01 |
| CRP | 16.6 (10.8–25.1) | 14.8 (9.6–21.1) | 21.1 (12.1–27.0) | 0.09 |
| Leukocytes | 14.4±7.9 | 14.0±6.7 | 15.6±10.9 | 0.42 |
| Clearance | ||||
| PCT | 61.6 (24.0–83.8) | 73.9 (34.4–88.0) | 14.3 (−130.3–59.6) | <0.01 |
| CRP | 14.3 (−21.6–43.2) | 21.9 (−12.4–56.1) | 5.4 (−62.1–14.3) | 0.80 |
| Leukocytes | 9.9 (−70.8–40.0) | 18.5 (−58.9–40.8) | −40.3 (−107.1–26.0) | 0.75 |
CRP, C-reactive protein; PCT, procalcitonin.
Values of PCT in ng/ml, CRP in mg/dl, and leukocyte count in 103/mm3. Values of PCT, CRP and clearance expressed as median and interquartile range (IQR). Leukocyte counts expressed as mean±standard deviation (SD).
None of the infection markers (PCT, CRP or leukocyte count) proved predictive of mortality based on the single determination made upon admission to the ICU. However, after 72h, the survivors presented significantly lower PCT values than the deceased patients (Table 2). Accordingly, the patients with a decrease in PCT levels showed significantly lesser mortality compared with those who presented an increase in this marker both at hospital level (15.4% versus 58.8%; p<0.01) and in the ICU (9.8% versus 47%, p<0.001). Regarding marker clearance, the patients who died showed significantly lower PCT clearance values than the survivors. None of these effects were observed in the case of CRP or leukocyte count. The patients with renal dysfunction presented significantly higher PCT values upon admission to the ICU and after 72h than the patients without renal failure. However, no significant differences were observed between the two populations in relation to PCT clearance (Table 3).
Procalcitonin values according to the presence of renal dysfunction.
| No renal dysfunctionNo.=37 | Renal dysfunctionNo.=51 | p | |
| PCT values, ng/mla | |||
| Admission | 9.2 (1.6–21.5) | 17.2 (4.9–43.0) | 0.01 |
| 72h | 1.8 (0.4–9.5) | 6.6 (1.6–25.1) | 0.04 |
| Clearance | 53.7 (25.3–82.9) | 65.9 (19.8–84.7) | 0.89 |
PCT, procalcitonin.
Data expressed as median and interquartile range (IQR).
In the ROC curve analysis, the best area under the curve corresponded to PCT clearance (0.79), which exceeded that corresponding to CRP (0.64) and leukocyte count (0.60) (Fig. 1). The best cutoff point in discriminating hospital survival corresponded to a PCT clearance value of 70, with a sensitivity of 94.7% and a specificity of 53%.
In the logistic regression analysis, a descending trend in PCT was identified as an independent survival marker (OR: 0.10; 95%CI: 0.02–0.59; p<0.01), after adjusting for age, gender, immune depression, APACHE II, SOFA, the need for mechanical ventilation and renal replacement therapy.
DiscussionProcalcitonin clearance 72h after admission to the ICU was found to be a better prognostic indicator in patients with septic shock than a single PCT determination at the time of admission. A descending trend in PCT was identified as an independent marker of hospital survival. In contrast, variations in CRP and leukocyte count were not related to the end prognosis in patients of this kind.
The diagnostic and prognostic reliability of PCT as a single measurement can be affected by different factors. Transient elevations in PCT have been described in surgical patients,21 polytraumatized patients22 and in patients with acute respiratory distress syndrome (ARDS),23 among others—without such an elevation necessarily being associated to an infectious process. The fact that PCT is eliminated at least in part through the kidneys means that the presence of renal failure can also affect the results.20 Likewise, despite the early start of PCT release in response to an infectious stimulus (within 2–3h), determination of the parameter in very early stages could give rise to false negative readings. A number of studies have shown that the use of serial determinations increases the diagnostic reliability of PCT and can even guide us regarding the suitability of the treatment provided.24–27 From the prognostic perspective, increases in PCT have been associated to an increased mortality risk in patients with community-acquired pneumonia,28 pneumonia associated to mechanical ventilation,29 and in polytraumatized patients.30 Nevertheless, knowledge of how serial determinations of PCT could optimize the prognostic performance of this marker is limited, particularly as refers to patients with severe sepsis and septic shock. The study published by Claeys et al. is the only article exclusively centered on patients of this kind. It found that while single PCT determinations during the first 5days of treatment were unable to discriminate between survivors and deceased patients, a drop in PCT concentration in the first 48h after admission to the ICU was associated to a twofold greater probability of survival.31 More recently, Charles et al. and Karlsson et al. obtained similar results in patients with severe sepsis and septic shock.25,32
These findings are consistent with those of our own study, where neither PCT upon admission nor PCT after 72h yielded information on the survival of the patients. However, a decrease in PCT values was associated to a practically fourfold greater probability of survival. Our data suggest that those patients with PCT values that do not decrease by 72h after admission to the ICU should be re-evaluated in order to discard the existence of some undiagnosed infectious process or the use of an incorrect antibiotic treatment regimen, among other factors. Earlier studies that have found a good correlation between PCT increase and bacterial burden,33 and between variations in PCT values and the suitability of antibiotic treatment, would support this idea.25
Regarding the effect of renal failure upon the PCT values, in our study the patients with renal failure (according to the criteria of the 2001 consensus conference1) showed higher PCT levels upon admission and again after 72h than those who did not develop renal failure. However, on evaluating the clearance of the marker, no significant differences were observed between the two groups. PCT is a low molecular weight protein and therefore would be susceptible to elimination through the kidneys. This would explain the higher PCT levels found in the subgroups of patients with renal failure in different studies.20,32 However, the existence of renal dysfunction does not affect the half-life of PCT, and the kinetics of the latter therefore can be used for both diagnostic and prognostic purposes34—this consequently speaking in favor of the use of serial determinations rather than single determinations.
Exceptions aside,35,36 PCT is an earlier and more specific marker of infection than CRP or leukocyte count.37,38 The PCT values rise faster than those of CRP in response to infection, and likewise decrease faster as infection subsides. In the present study, neither isolated PCT measurements (both at baseline and after 72h) nor the tendencies of the marker were predictors of survival.
Mention must be made of the limitations of our study, including its single-center nature and relatively small sample size. This fact, and the variability of the biomarkers used, can affect the reliability and validity of the results obtained. For this reason we limited the study to a population of patients with septic shock.
In conclusion, during the first 72h of septic shock, a decrease in PCT was more often recorded among the survivors than in those who died. This suggests that the serial determination of PCT could offer a better prediction of patient outcome than a single quantification of the marker. The monitorization of PCT in clinical practice could help identify those patients at increased risk of death, contributing to improve their treatment and perhaps also their survival. Further studies are evidently needed to confirm the findings of our work and the validity of the postulated superior performance of serial PCT measurements.
Conflicts of interestDr. Suberviola has served as a consultant and collaborated in events sponsored by BRAHMS Iberia; the rest of the signing authors declare no conflicts of interest.