To evaluate the frequency of severe thrombocytopenia (STCP) (≤50,000/μl) in the first 24h in patients with multiple organ dysfunction syndrome, and the factors that influence its occurrence.
DesignA retrospective, observational study.
AreaMedical–surgical intensive care unit (ICU). Tertiary hospital.
PatientsThose with failure of at least two organs, according to SOFA criteria, with the exclusion of neurological and traumatologic critical cases.
VariablesMedical history, regular medication, baseline functional status, demographic variables, severity scores in ICU, multiple-organ failure data, course in ICU and main hospital data.
ResultsA total of 587 patients were included; 6.3% (37 patients) presented with STCP during the first day of admission; 64.6% were men; SOFA 8 (5–10); APACHE II 18 (13–24); APACHE IV 59 (46–73); 32.5% were surgical patients. A total of 79.9% subsequently needed mechanical ventilation, and 71.4% required vasoactive drugs. Overall stay in ICU: 4 (2–10) days, main hospital stay 18 (9–35) days. A total of 29.2% died in the ICU; 11.7% developed STCP during admission to the ICU. Multivariate analysis found the main determining factors in the occurrence of thrombocytopenia on admission to be: history of hospitalization in the last year, albumin and bilirubin levels, and sepsis.
ConclusionThe prevalence of STCP among critical patients was 6.3%. Its occurrence was associated with albumin and bilirubin levels, sepsis, and with patient admittance in the last year.
Evaluar la frecuencia de la trombocitopenia grave (TCPG) (≤50.000/μl) en las primeras 24 horas en pacientes con síndrome de disfunción multiorgánica (SDMO) y los factores asociados a su aparición.
DiseñoEstudio retrospectivo, con diseño observacional.
ÁmbitoUnidad de cuidados intensivos (UCI) médico-quirúrgica de un hospital de nivel III.
PacientesAquellos con disfunción de al menos dos órganos, según criterios SOFA; se excluyen neurocríticos y politraumatizados.
Variables de interésAntecedentes personales, medicación habitual, situación funcional basal, datos de filiación, puntuaciones de gravedad en UCI, datos de la disfunción multiorgánica, evolución UCI y datos hospitalarios.
ResultadosSe incluyeron 587 pacientes. El 6,3% (37 pacientes) presentaban TCPG durante el primer día de ingreso. El 64,6% eran hombres; la mediana de edad fue 69 (56-77) años; al ingreso, SOFA 8 (5-10); APACHE II 18 (13-24); APACHE IV 59 (46-73); 32,5% son quirúrgicos. Durante su evolución 79,9% necesitaron ventilación mecánica y el 71,4% requirió fármacos vasoactivos. Estancia en UCI 4 (2-10) días; estancia hospitalaria 18 (9-35) días. El 29,2% fallecieron en UCI. El 11,7% desarrollaron durante su ingreso en UCI TCPG. En el análisis multivariable los principales determinantes de la aparición de la trombocitopenia al ingreso fueron los antecedentes de ingreso hospitalario en el último año, el peor valor de bilirrubina y albúmina sanguínea y la sepsis.
ConclusiónLa prevalencia de TCPG en pacientes críticos con SDMO durante el primer día de estancia en UCI es del 6,3%. Los factores asociados son: la presencia o no de ingresos hospitalarios en el último año, los niveles de albúmina y bilirrubina y la sepsis.
Thrombocytopenia is the most common coagulation disorder seen in the Intensive Care Unit (ICU), and is a well established complication in critical patients.1,2 However, in addition to their participation in coagulation and thrombosis, platelets play an increasingly recognized physiopathological role in the mediation of inflammation and infection.3
The frequency of thrombocytopenia varies according to the definition used, the type of critical care population involved, and the time of determination.1 Thrombocytopenia is usually defined as a platelet count of <150,000/μl, while a count of ≤50,000/μl is taken to represent severe thrombocytopenia.4
In the context of the critical patient, thrombocytopenia presents a multifaceted pathogenic mechanism comprising hemodilution, increased platelet consumption (as in disseminated intravascular coagulation [DIC]), increased platelet destruction (immune mechanisms, etc.), decreased platelet production, increased platelet sequestration, and laboratory artifacts (pseudothrombocytopenia).5
Despite our knowledge of the condition, thrombocytopenia in the ICU continues to present many controversial aspects, including its possible impact upon the prognosis (particularly as regards an increase in mortality rate), and its probable role as a morbidity marker in serious disease. In the context of severe thrombocytopenia (STCP), it is difficult to clearly distinguish between the causes and consequences. Likewise, in relation to hemostasis among patients in the ICU, controversy remains regarding the thresholds for platelet transfusions and when to use them in the context of a decrease in count.1
The main objectives of the present study are to evaluate the frequency of STCP in the first 24h among patients with multiorgan dysfunction syndrome (MODS) in a clinical–surgical ICU, and the factors associated to the appearance of STCP.
Patients and methodsA retrospective observational study was carried out using a database compiled over a two-year period (2008 and 2009) and including all the patients admitted to the ICU of Virgen de la Salud Hospital (Toledo, Spain) with a diagnosis of MODS upon admission to intensive care. Our ICU is a polyvalent unit with 23 beds for critical patients and three beds for complicated postoperative cases. MODS is defined by the dysfunction of at least two organs, according to SOFA criteria.6 Neurocritical and polytraumatized patients were excluded.
The collected study data included the personal history, medication used before admission to hospital, baseline functional status, epidemiological data (gender, age, type of patient, previous disease, etc.), the ICU severity scores (APACHE II and IV), the SOFA score, clinical information (including global water balance as assessed by the nursing graphic registry, and clinical coagulopathy characterized by the presence of clinically visible bleeding) and biochemical parameters during the first day of admission. We also collected general data referred to patient stay and outcome (mortality or no mortality in the ICU), as well as the date and value of the lowest platelet count (nadir) during admission. STCP was defined by a platelet count of ≤50,000/μl.
Patient age, the severity scores, SOFA score, number of affected organs, vasoactive drug dosage, water balance and all the laboratory test parameters were defined as quantitative variables, while the rest of the data were of a categorical nature. The qualitative parameters were reported as absolute and relative frequencies (percentages), while the quantitative variables were reported as the median (interquartile range [P25–P75]). Categorical variables were compared using the chi-squared test, while the nonparametric Mann–Whitney U-test was applied for the comparison of quantitative variables. Logistic regression analysis in turn was used to associate the data to STCP; the dependent variable was the presence or absence of thrombocytopenia on the first day of admission to ICU, while the independent variables were those found to be significant in the bivariate model, age and gender. The parameters related to transfusion (administration of blood products) and the severity and SOFA scores were excluded from the model, in order to avoid collinearity problems. Forward stepwise regression was used. The variables included in the multivariate analysis were entered stratified by type (categorical or quantitative), as defined above. Statistical significance was considered for p<0.05.
ResultsThe study included a total of 587 patients. On the first day of admission, 352 patients (60%) had platelet counts of >150,000/μl; 114 (19.4%) had counts of 101,000–150,000/μl; 84 (14.3%) had counts of 51,000–100,000/μl; 30 (5.1%) had counts of 21,000–50,000/μl; and 7 patients (1.2%) had counts of <21,000platelets/μl. Thus, 6.3% of the patients presented STCP (≤50,000 platelets/μl) during the first day of admission. Males predominated (64.6%), and the median age was 69 years (range 56–77). On the first day of admission, the patients presented the following score distribution: SOFA 8 (5–10); APACHE II 18 (13–24); APACHE IV 59 (46–73). The number of affected organs was 3 (2–4).
The stay in the ICU was 4 days (2–10), with a hospital stay of 18 days (9–35). A total of 32.5% of the cases (191 patients) were surgical; 33% (194 patients) came from the emergency service, 36.6% (215 patients) from the hospitalization ward, 24.9% (146 patients) from the operating room, and 5.5% (32 patients) from another hospital center. During their clinical course, 79.9% of the subjects (414 patients) required mechanical ventilation, and 71.4% (390 patients) required vasoactive drugs. In turn, 29.2% of the patients (171 subjects) died in the ICU.
Excluding the patients already admitted with STCP in the first 24h, a total of 11.7% developed STCP during their stay in the ICU.
Referred to the global patients, the platelet count on the first day was 179,000/μl (110,500–256,000), with a minimum value (nadir) of 134,000/μl (76,000–200,000). The platelet nadir was recorded on day 2 (1–4 days), with a recovery (in those cases where recovery took place) to over 100,000/μl on day 6 (4–9 days).
A comparison was made of the characteristics between the patients with and without STCP in relation to the personal history and data on the first day of admission to the ICU (Tables 1 and 2). Regarding the evolutive data, there were no significant differences between the patients without versus the patients with STCP in terms of the duration of mechanical ventilation (4 [1–10.5] vs 1 [0–2.5] days), the duration of vasoactive drug administration (2 [1–5] vs 1 [0–5] days) and stay in the ICU (4 [2–10] vs 3 [1–6] days). In contrast, significant differences were recorded in terms of mortality in the ICU (27.7% vs 51.4%; p<0.01) and hospital stay (18 [10–36] vs 10 [3–21] days; p<0.05).
Personal history, regular medication, and baseline conditions in the patients with and without severe thrombocytopenia.
| Variable | Patients without severe thrombocytopenia (No.=550) | Patients with severe thrombocytopenia (No.=37) | Significance |
| Personal history | |||
| Arterial hypertension | 284 (51.8%) | 17 (45.9%) | NS |
| Smoker | 196 (35.6%) | 8 (21.6%) | NS |
| Regular alcohol consumption | 57 (10.4%) | 5 (13.5%) | NS |
| Hypercholesterolemia/hyperlipidemia | 146 (26.5%) | 3 (8.1%) | p<0.001 |
| Diabetes mellitus | 164 (29.8%) | 11 (29.7%) | NS |
| Chronic renal failure | 76 (13.8%) | 2 (5.4%) | NS |
| Ischemic heart disease | 75 (13.5%) | 6 (16.2%) | NS |
| COPD | 92 (16.8%) | 4 (10.8%) | NS |
| Peripheral vascular disease | 35 (6.4%) | 2 (5.4%) | NS |
| Acute stroke | 54 (9.8%) | 1 (2.7%) | NS |
| Clinical heart failure | 47 (8.5%) | 3 (8.1%) | NS |
| Liver cirrhosis | 21 (3.8%) | 3 (8.1%) | NS |
| Leukopenia (<500/μl) | 1 (0.2%) | 1 (2.7%) | NS |
| Drug abuse | 14 (2.5%) | 0 (0%) | NS |
| Neoplasm | 107 (19.5%) | 13 (35.1%) | p<0.05 |
| Chemotherapy/radiotherapy ≤6 months | 17 (3.1%) | 5 (13.5%) | p<0.01 |
| Obesity | 122 (22.2%) | 6 (16.2%) | NS |
| Admission to ICU <1 year | 24 (4.4%) | 0 (0%) | NS |
| Hospital admission <1 year | 187 (34%) | 21 (56.8%) | p<0.01 |
| Regular medication | |||
| Corticosteroids | 62 (11.3%) | 6 (16.2%) | NS |
| Insulin | 72 (13.1%) | 2 (5.4%) | NS |
| NSAIDs | 42 (7.6%) | 0 (0%) | NS |
| Antiarrhythmic drugs | 53 (9.6%) | 0 (0%) | p<0.05 |
| Antiseizure drugs | 11 (2%) | 0 (0%) | NS |
| Antidepressants | 63 (11.5%) | 3 (8.1%) | NS |
| Baseline condition | |||
| Institutionalized | 26 (4.7%) | 0 (0%) | NS |
| Asymptomatic at baseline | 397 (72.2%) | 29 (78.4%) | NS |
NSAIDs, nonsteroidal antiinflammatory drugs; NS, nonsignificant; COPD, chronic obstructive pulmonary disease. The categorical data are represented as absolute frequency (percentage).
Variables referred to data upon admission to the ICU (first 24h) in the patients with and without severe thrombocytopenia.
| Variable | Patients without severe thrombocytopenia (No.=550) | Patients with severe thrombocytopenia (No.=37) | Significance |
| Male gender | 354 (64.4%) | 25 (67.6%) | NS |
| Age (years) | 69 (56–77) | 70 (51–76) | NS |
| Readmission ICU | 46 (8.4%) | 1 (2.7%) | NS |
| Diagnostic group | |||
| Clinical | 372 (67.6%) | 24 (64.9%) | |
| Surgical (emergency) | 135 (24.5%) | 12 (32.4%) | |
| Surgical (elective) | 43 (7.8%) | 1 (2.7%) | NS |
| Principal disease/worsening | |||
| Abdominal | 153 (27.9%) | 12 (32.4%) | |
| Respiratory | 149 (27.1%) | 7 (18.9%) | |
| Cardiological | 122 (22.2%) | 3 (8.1%) | |
| Nephro-urological | 38 (6.9%) | 6 (16.2%) | |
| Vascular | 28 (5.1%) | 5 (13.5%) | |
| Soft tissues | 21 (3.8%) | 1 (2.7%) | |
| Unknown | 11 (2%) | 3 (8.1%) | |
| Neurological | 10 (1.8%) | 0 (0%) | |
| Intoxication | 9 (1.6%) | 0 (0%) | |
| Metabolic | 8 (1.5%) | 0 (0%) | p<0.05 |
| Sepsis | 220 (40%) | 23 (62.2%) | p<0.001 |
| APACHE II | 17 (13–23) | 23 (17.5–32.5) | p<0.001 |
| APACHE IV | 58 (45–72) | 82.5 (58.5–109.5) | p<0.001 |
| SOFA, global | 8 (5–10) | 13 (10–15) | p<0.001 |
| Number of affected organs | 3 (2–4) | 4 (4–5) | p<0.001 |
| Orotracheal intubation | 355 (64.9%) | 27 (73%) | NS |
| Noradrenaline (μg/kg/min) | 0.16 (0–0.45) | 0.3 (0–1) | p<0.01 |
| Dopamine (μg/kg/min) | 0 (0–2) | 0.5 (0–5.6) | p<0.05 |
| Water balance | 995 (−400 a 2610) | 1285 (0–3500) | NS |
| Continuous hemofiltration | 45 (8.4%) | 6 (1.6%) | NS |
| Clinical coagulopathy | 15 (5.5%) | 5 (25%) | p<0.01 |
| Red cell transfusion | 47 (9.6%) | 16 (51.6%) | p<0.001 |
| Plasma transfusion | 48 (9.6%) | 11 (35.5%) | p<0.001 |
| Platelet transfusion | 20 (4%) | 11 (36.7%) | p<0.001 |
| Prothrombin complexes | 19 (7%) | 6 (31.6%) | p<0.01 |
| Leukocytes (/μl) | 13,300 (9100–19,150) | 10,100 (3900–14,800) | p<0.01 |
| Hematocrit (%) | 33.8 (29.3–38.7) | 26.1 (20.5–31.5) | p<0.001 |
| Hemoglobin (g/dl) | 11.3 (9.8–13) | 8.5 (7.1–10.8) | p<0.001 |
| Platelets (/μl) | 188,000 (124,000–263,000) | 39,000 (28,000–44,000) | p<0.001 |
| Prothrombin activity (%) | 71.3 (56.8–84.7) | 56.7 (39.5–67.5) | p<0.001 |
| Glucose | 138 (110–190) | 116.5 (73–170) | NS |
| Creatinine | 1.2 (0.8–1.9) | 1.5 (1–2) | NS |
| Urea | 57 (38–93) | 71 (43.5–117.5) | NS |
| pH | 7.35 (7.28–7.42) | 7.31 (7.15–7.40) | p<0.01 |
| Bicarbonate (mmol/l) | 21.5 (17.8–25.1) | 17.6 (14.9–20.6) | p<0.001 |
| Lactic acid (mg/dl) | 17 (11–29) | 29 (16–83) | p<0.001 |
| Bilirubin (mg/dl) | 0.9 (0.5–1.4) | 1.8 (0.9–3.9) | p<0.001 |
| Proteins (g/dl) | 5.3 (4.4–5.9) | 4.2 (3.5–4.9) | p<0.01 |
| Albumin (g/dl) | 2.6 (2–3.1) | 1.9 (1.6–2.4) | p<0.001 |
Clinical coagulopathy: presence of clinically visible bleeding; NS, nonsignificant.
The categorical data are represented as absolute frequency (percentage) and the quantitative data as median (interquartile range).
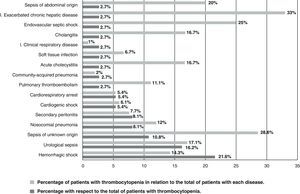
Fig. 1 describes the causal diseases among the patients admitted due to STCP, showing the percentage of the total patients with STCP and the percentage of patients with each disease involving STCP in relation to the total of patients with the mentioned disease.
The data of the logistic regression analysis relating the variables to the appearance of thrombocytopenia in the ICU during the first 24h are reported in Table 3.
Variables with statistical significance in the logistic regression model.
| Variable | OR | 95% CI OR | Significance |
| Constant | 0.23 | p<0.05 | |
| Admission to hospital in the ultimo year | 2.7 | 1.1–6.6 | p<0.05 |
| Septic patient | 2.8 | 1.1–7.6 | p<0.05 |
| Albumin first day | 0.3 | 0.2–0.7 | p<0.01 |
| Bilirubin first day | 1.1 | 1.1–1.3 | p<0.05 |
Dependent variable: existence of thrombocytopenia.
Coagulation disorders are a very frequent component of disease in the ICU, and among these disorders, thrombocytopenia as an expression of altered platelet homeostasis is the most common problem. Severe sepsis and other critical conditions are invariably associated to the activation of primary (platelet response) and secondary hemostasis (soluble coagulation factors). Primary hemostasis is characterized by platelet activation, adhesion and aggregation, and is clinically associated with thrombocytopenia.7
In our series we included all patients with dysfunction of at least two body organs, and in which disseminated intravascular coagulation (DIC) forms a part of the development of tissue ischemia.8 The prevalence of STCP on the first day of admission (6.3%) and over the subsequent clinical course (11.7%) coincides with the data found in the literature, with figures of 2.3–5% upon admission and 5–27% over the subsequent course in the ICU.9–16 At present, most studies establish a threshold of 100,000 or 150,000platelets/μl, and very few use the more strict cutoff point of ≤50,000 platelets/μl.4,17–19 Clearly, when the threshold used to define thrombocytopenia is raised (often referring it to ≤150,000platelets/μl), the frequency upon admission can reach 8.3–67.6%, with a subsequent incidence of 13–44%.4,7,11,20
Thrombocytopenia is not only conditioned by the definition used but also by the setting in which it is described. Thus, in most instances, including our own series, thrombocytopenia has been defined in the context of a clinical–surgical ICU, with higher incidences of STCP among surgical patients—though we observed no such predominance in our study.2,5
Thrombocytopenia is closely related to the diagnosis and characteristics underlying admission to the ICU, since a full 60% of all cases of thrombocytopenia are registered in the first two days after admission. In fact, such cases differ in many aspects from what is seen over the subsequent stay in the ICU.2 In our study we analyzed which diseases are most frequently associated with thrombocytopenia during the first hours of admission to the ICU (Fig. 1). Thrombocytopenia has been associated to a series of risk factors, of which sepsis is the most important.9,14 This is supported by the important role played by the platelets in host defense against infection and inflammation (the platelets can induce chemotaxis and release a number of inflammatory molecules). In effect, the platelets are known to be an important element of both innate and adaptive immunity.21–23 A number of studies have examined the relationship between thrombocytopenia and infection, recording a decrease in pneumonia acquired in the ICU and in bacteremias—with a rise in associated mortality.15,24 This association between septic processes and thrombocytopenia is due to an increase in platelet consumption, platelet destruction through immune mechanisms, and dilution.9,15,17,18,25–28 Our own findings support the above, since the multivariate analysis showed sepsis to be correlated to the appearance of STCP.
In our series the most common diseases among the patients with STCP were hemorrhagic shock, urological sepsis, sepsis of unknown origin, nosocomial pneumonia, secondary peritonitis, cardiogenic shock and cardiocirculatory arrest. The most thrombocytopenic disorders (i.e., those disorders associated with the highest percentage presence of STCP) were exacerbated chronic liver failure, sepsis of unknown origin, endovascular septic shock (catheter-related bacteremia), sepsis of abdominal origin and urological sepsis. Septic patients clearly show an increased frequency of thrombocytopenia. The literature describes its appearance in patients with intraabdominal, nephro-urological and vascular disease (dissecting aortic aneurysm, etc.), and in cases where the underlying origin cannot be established (sepsis of unknown origin)—in coincidence with our own observations. In this context, it has been suggested that an abnormal platelet count could be a better evolutive predictor than an abnormal leukocyte count.22
It is important to distinguish between thrombocytopenia present upon admission to the ICU and thrombocytopenia that develops in the course of ICU stay (often conditioned by drug-related toxicity).5 We selected patients with multiorgan dysfunction and analyzed them according to whether thrombocytopenia was initially present or not, since our aim was to study thrombocytopenia as a part of multiorgan dysfunction and appearing in the context of the latter, with a view to exploring its differential characteristics—assuming platelet consumption as one of the predominant mechanisms in our series. In fact, although moderate thrombocytopenia can be caused by sepsis alone, when the platelet count drops to under 50,000/μl, DIC is often present.29 This in turn is supported by the observed close correlation to coagulation disorders.
Independently of bleeding complications, some authors consider that there is an association between thrombocytopenia and a poor clinical outcome—with thrombocytopenia being regarded as a prognostic marker.1,5 In effect, thrombocytopenia is often associated to prolonged stay in hospital and in the ICU, the severity of the disease, sepsis and organ dysfunction.4,9,12,14,26 In our series we recorded no increased stay in the ICU, though a prolongation of hospital admission was noted. A number of studies, using multivariate analyses, have defined thrombocytopenia as an independent mortality predictor, adjusting for confounding factors.2,10,11,13–16,27,30 Some investigators have placed greater emphasis on examination of the profile of platelet reduction, rather than on the absolute platelet counts as such. In our global series we observed a platelet reduction profile similar to that described in the literature, with a significant drop in the first days of admission, reaching a nadir or low point by the fourth day, and followed by gradual recovery with a return to the previous levels by around day 7 after admission.20,31 It has been reported that alterations in platelet dynamics imply an increase in mortality, though in our series no evaluation was made of the changes in platelet dynamics. However, the mortality rate among subjects with thrombocytopenia is higher in critical patients due to a number of reasons, especially the underlying or background disease and its severity. Thus, thrombocytopenia could more often represent a prognostic indicator than a disease condition in itself.2
The multifactorial origin of thrombocytopenia in the critical patient implies that its appearance may be used as a severity indicator.32,33 In fact, both in our series and in other studies, patients with thrombocytopenia showed higher scores (APACHE II, APACHE IV and SOFA), longer hospital stays and greater mortality.10,11 The stay in the ICU was shorter among patients with thrombocytopenia, though not significantly so. This in turn is complemented by the contribution of other prognostic markers (vasoactive drug dosage, leukocytosis, increased lactic acid concentrations, reductions in protein levels, etc.). Neither patient gender nor age is the conditioning factor of STCP. The risk factors associated to STCP in the first 24h after admission in our series were antecedents of hospital admission and the septic nature of the disease, while worse albumin and bilirubin values in the first 24h appeared as conditioning factors. The literature in turn describes a broad range of risk factors for the development of thrombocytopenia in the ICU, based on the findings of multivariate analyses: the female gender, frozen fresh plasma transfusions, sepsis, pulmonary artery catheterization, a diagnosis of gastrointestinal disease, nonsteroidal antiinflammatory medication, dialysis, central venous or arterial catheterization, chemotherapy, elevated serum creatinine, elevated serum bilirubin, a low preoperative platelet count, DIC, C-reactive protein levels, a high SOFA score upon admission, APACHE II >20, vasopressor drug dosage, the PaO2/FiO2 ratio, and older patient age.9,11,12,16,17,26,28,34 Few publications have related thrombocytopenia to hyperbilirubinemia.9 In our series we found no association between thrombocytopenia and either hypoalbuminemia or previous admission to hospital. However, according to the literature, both hypoalbuminemia and hyperbilirubinemia are usually directly correlated to the presence of more serious and intense disease, and to a greater number of complications, and as such represent indicators of a poorer patient prognosis.34–38 We consider that these correlations would be analogous to the association between thrombocytopenia and poorer prognostic scores, an increased need for vasoactive medication, and higher mortality, among other parameters. Such factors therefore would reflect increased seriousness, with thrombocytopenia representing one of the components. We have found no references to hospital admission, though the significance of this parameter could point in the same direction.
The main strongpoint of this study is that it exclusively focuses on patients with multiorgan dysfunction, as a result of which DIC is often part of the underlying pathogenesis—representing some of the most critically ill patients we can find in the ICU. This fact moreover affords a certain study homogeneity. The main limitation is the retrospective nature of the study, where no ad hoc search for the disease was made despite the fact that the series formed part of a large registry. On the other hand, we focused our attention on STCP, as a result of which the patient sample size decreased, in the same way as the statistical power of the study.
Thrombocytopenia in the ICU remains the subject of debate, and its relevance extends beyond that of a mere laboratory test parameter expressing a decrease in an isolated hematological parameter. Indeed, among other aspects that still remain to be clarified, thrombocytopenia has a number of implications referred to the patient prognosis, severity, and the assessment of the true impact referred to bleeding. Our study shows thrombocytopenia to be associated with the most seriously ill patients and with the rest of the signs and symptoms of poor prognosis, as determined by the presence or absence of hospital admissions in the previous year, worse albumin and bilirubin levels in the first 24h, and the septic nature of the disease.
Financial supportThis study has received no financial support.
The present study forms part of the Doctoral Thesis of Dr. Carlos Miguel Marco-Schulke.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Marco-Schulke CM, et al. Trombocitopenia grave al ingreso en una unidad de cuidados intensivos en pacientes con disfunción multiorgánica. Med Intensiva. 2012;36:185–92.