To propose and validate a prediction score for intracerebral hemorrhage (ICH) patients at risk of hematoma expansion (HE).
DesignA retrospective observational study was designed to propose and validate the score.
SettingSanxiang Road branch and Xuguan branch belonging to the Second Affiliated Hospital of Soochow University (China).
PatientsA total of 317 ICH patients in Sanxiang Road branch were registered as the development cohort, and 109 ICH patients in Xuguan branch were enrolled as the validation cohort.
ProcedureIndependent risk factors for HE were identified using multiple logistic regression analysis. A prediction score was then proposed based on β coefficients and preliminarily verified in the validation cohort.
Main variablesAll clinical data of the patients were compiled from the electronic medical records. Hematoma expansion was defined as an increase in hematoma volume >33% or absolute hematoma growth >6ml from the initial scan. Specific non-contrast CT(NCCT) signs were identified by two observers independently.
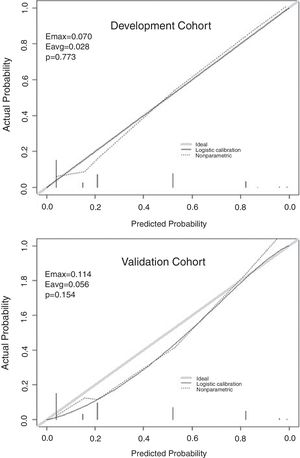
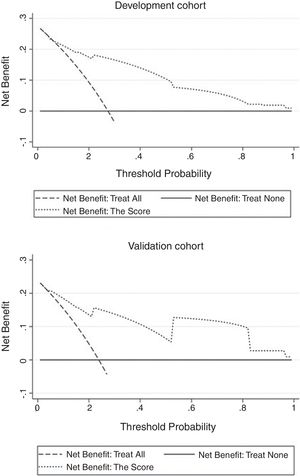
ResultsOur score demonstrated satisfactory discrimination ability for HE (area under the ROC curve 0.854 in the development cohort versus 0.893 in the validation cohort). Appropriate calibration was found in the development cohort, whereas calibration in the validation cohort was slightly lower but still within the accuracy range (maximum deviation, average deviation and P were 0.070, 0.028, 0.773, respectively, versus 0.114, 0.056, 0.156). Decision curve analysis of the score from two samples were both far from the curve of treat all and curve of treat none, which verified its security and reliability. Patients with a total score ≥4.5 were at greatest risk of HE.
ConclusionThe score may provide some reference and help in accurately identifying individuals at high risk of HE, allowing rapid guidance of clinical management and also serving as an aid in clinical trials.
Proponer y validar una puntuación de predicción de hemorragia cerebral (HC) en paciente con riesgo de expansión del hematoma (EH).
DiseñoSe diseñó un estudio observacional retrospectivo para proponer y validar la puntuación.
Ámbitoramas de Sanxiang Road y Xuguan pertenecientes al Segundo Hospital Afiliado de la Universidad de Soochow.
Pacientes317 pacientes con HE de la rama de Sanxiang Road fueron incluidos como la cohorte de desarrollo, y 109 pacientes con HC de la rama de Xuguan fueron incluidos como la cohorte de validación.
ProcedimientoSe obtuvieron los factores de riesgo independientes de EH de a partir de un análisis de regresión múltiple. A continuación, se propuso una puntuación de predicción basada en coeficientes β y se verificó de forma preliminar en la cohorte de validación.
Variables principalesTodos los datos clínicos de los pacientes se registraron consultando historias electrónicas. La EH se definió como un aumento del volumen del hematoma >33% o un crecimiento absoluto del hematoma >6ml respecto a la exploración inicial. Los signos específicos de la tomografía computerizada sin contraste (TCSC) fueron identificados de manera independiente por dos observadores.
ResultadosNuestra puntuación demostró de manera satisfactoria su capacidad de discriminación para la EH (el área bajo la curva ROC fue 0.854 en la cohorte de desarrollo frente a 0.893 en la cohorte de validación). Se observó un calibrado adecuado en la cohorte de desarrollo, mientras que el calibrado de la cohorte de validación fue ligeramente inferior, si bien se mantuvo dentro del intervalo de precisión (la desviación máxima, la desviación promedio y el valor P fueron respectivamente 0.070, 0.028 y 0.773, frente a 0.114, 0.056 y 0.156). Las curvas de análisis de la curva de decisión de la puntuación a partir de las dos muestras se situaron alejadas de la curva de tratar a todos y de la curva de no tratar a ninguno, lo cual verificó su seguridad y fiabilidad. Los pacientes con una puntuación total ≥4.5 corrían un mayor riesgo de EH.
ConclusiónEs posible que la puntuación sirva de referencia y ayuda para identificar con precisión a las personas con alto riesgo de EH, además de ofrecer una guía rápida sobre el tratamiento y de poder utilizarse en ensayos clínicos.
It is well known that intracerebral hemorrhage (ICH) is the second most common type of stroke, accounting for 10–15% of all stroke events.1 The fatality rate is relatively high, only 12–39% survivors could live independently.2 Early hematoma expansion (HE) occurs in 20–30% of ICH patients,3 accompanied by re-bleeding occasionally. Through the CT scanning and three-dimensional reconstruction analysis of the enlarged hematoma, Liotta4 found that HE was caused by the irregular expansion along the surface of the original hematoma, mainly within 24h after the onset. HE is an independent risk factor for disability and death in patients of ICH, so timely assessment and prevention of HE becomes the focus of current research. But treatments toward HE still exist some disputes and have not been standardized, including intensive blood pressure control, transamin,4 rectify the blood coagulation dysfunction, neurological intensive care unit and minimally invasive surgery. Saving lives, utmost retaining or even restoring neurological function are the fundamental purposes of treatments. Therefore, it is of great value to use simple predictors to screen out the high-risk patients who may have HE and make targeted treatments to curb the early deterioration of ICH. The objective of the study was exploring the risk factors of HE, proposing a prediction score and making a preliminary validation.
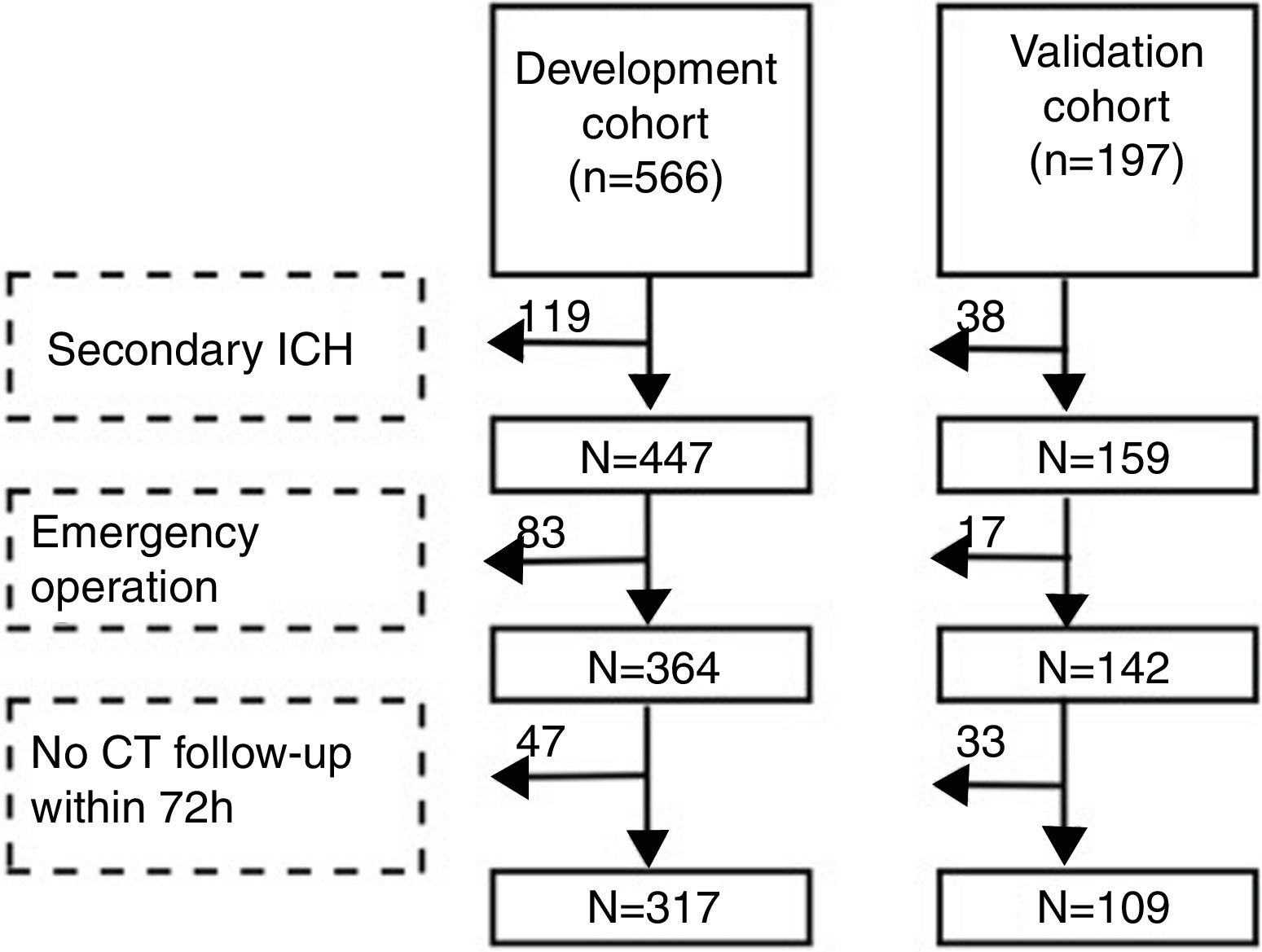
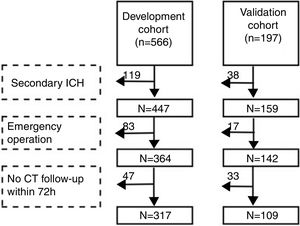
Patients and methodsStudy populationWe performed the research in two branches of the Second Affiliated Hospital of Soochow University. Patients admitted to the Sanxiang Road branch from January 2016 to May 2018 were consecutively registered as development cohort. Then we included patients in the Xuguan branch during the same time as validation cohort. The two branches are both academic medical center while the former is senior to the latter (number of beds: 1300 vs. 700). Inclusion criteria: 1. The first CT scan was acquired within 24h after the onset and the diagnosis was spontaneous ICH; 2. Age≥18. Exclusion criteria: 1. Secondary ICH (cerebral tumor, traumatic brain injury, arteriovenous malformation, cerebral aneurysm, hemorrhagic transformation of cerebral infarction); 2. Emergency surgery was performed before the second CT scan; 3. CT was not re-examined within 72h after the first scan. In brief, cohorts’ selection flowchart is shown in Fig. 1. The study was approved by the ethical committee of the Second Affiliated Hospital of Soochow University (JD-LK-2018-067-01). All study protocols and procedures were conducted in accordance with the declaration of Helsinki. Because this study was a retrospective observational study, patients’ information was anonymized and deidentified before analysis. Therefore, the need for patients’ consent was waived.
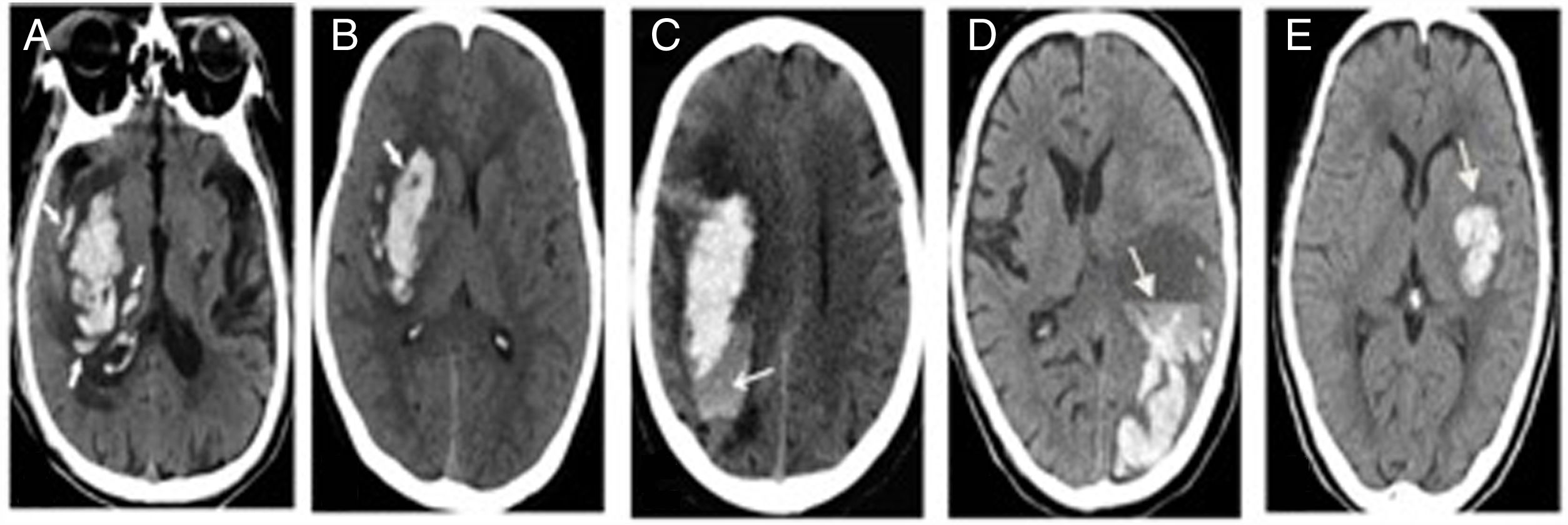
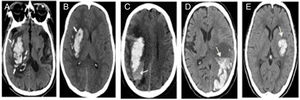
Research designRetrospective study was conducted to record the clinical data of patients by referring to electronic medical records and the subjects were divided into HE group and non-HE group. The score was proposed from development cohort and verified in the validation cohort afterwards. HE was defined as an increase of hematoma volume >33% or absolute hematoma growth >6ml from initial scan.2,4,5 Specific non-contrast CT(NCCT) signs (island sign, black hole sign, blend sign, edema, niveau formation) were identified and recorded referring to previous study.5 There were some photos of NCCT to illustrate the particular radiological signs in Fig. 2. The two observers (neuroradiologist and neurologist) were blind to the information and outcome of patients, independently evaluated the images. Disagreements were decided by consensus decision.
Statistical analysesStatistical analyses were performed using SPSS 25.0, Stata 15, and Rstudio. Medians and interquartile ranges (IQRs) or the mean±standard deviation (SD) was used to describe continuous variables, and percentage (%) was used to describe categorical variables. Statistical significance was assessed by χ2 test for categorical variables and Mann–Whitney U test for continuous variables. To propose the score, continuous variables had to convert into categorical variables by cut-off values, which were obtained by quartile, Youden index of receiver operating characteristic (ROC) curve and recursive partitioning as appropriate. To avoid collinearity, partial repeated variables were professionally removed. Categorical variables with P<0.05 between groups were included in the multivariate logistic regression. As a result, risk factors with P<0.05 in regression were added into score. The assigned scores for each item were derived by parameter estimates (β coefficients) from the regression and increased proportionately to the nearest integer by and large. Novel measures of score performance were utilized both in the development cohort and the validation cohort, including the area under ROC curve for discrimination, Hosmer–Lemeshow goodness-of-fit statistic and calibration plot for accuracy, and decision curve analysis (DCA) for clinical utility. A two-tailed P<0.05 was considered statistically significant.
ResultsBaseline characteristics of patientsBaseline characteristics of participants in the development cohort are shown in Table 1. HE was observed in 87 patients (27.4%) in the development cohort compared with 26 patients (23.9%) in the validation cohort. History of anticoagulants, D-Dimer≥0.65mg/l, potassium≤3.1mmol/l, hematoma growth (HG)≥2.7ml/h, Glasgow Coma Scale (GCS)≤8, and NCCT signs (island sign, black hole sign, blend sign, niveau formation) exist one or more, were risk factors of HE (P<0.05). Then the above factors were included in multivariate logistic regression and the detailed results were shown in Table 2. Finally, it was concluded that the history of anticoagulants, HG≥2.7ml/h, GCS≤8, and NCCT signs (island sign, black hole sign, blend sign, niveau formation) exist one or more, were independent risk factors of HE (P<0.05).
Baseline clinical characteristics of patients in the development cohort.
| Variables | Total (n=317) | HE (n=87) | Non-HE (n=230) | P |
|---|---|---|---|---|
| Age, years, median (p25–p75) | 64 (52–74) | 66 (52–75) | 64 (52–73) | 0.497 |
| Sex, male, n (%) | 200 (63.1) | 53 (60.9) | 147 (63.9) | 0.622 |
| Hypertension, n (%) | 239 (75.4) | 66 (75.9) | 173 (75.2) | 0.905 |
| Diabetes mellitus, n (%) | 48 (15.1) | 11 (12.6) | 37 (16.1) | 0.445 |
| Chronic kidney disease, n (%) | 13 (4.1) | 6 (6.9) | 7 (3.0) | 0.220 |
| Antiplatelets, n (%) | 20 (6.3) | 5 (5.7) | 15 (6.5) | 0.800 |
| Anticoagulants, n (%) | 9 (2.8) | 8 (9.2) | 1 (0.4) | <0.001 |
| Recurrent ICH, n (%) | 30 (9.5) | 8 (9.2) | 22 (9.6) | 0.920 |
| SBP, mmHg, median (p25–p75) | 163 (148–180) | 162 (148–182) | 165 (148–175) | 0.630 |
| Platelet, ×109l−1, median (p25–p75) | 196 (163–240.5) | 185 (149–229) | 204 (165–243.5) | 0.032 |
| Platelet≤135×109l−1, n (%) | 32 (10.1) | 13 (14.9) | 19 (8.3) | 0.078 |
| INR, median (p25–p75) | 1.03 (0.98–1.08) | 1.03 (0.98–1.08) | 1.02 (0.98–1.07) | 0.358 |
| Hb, g/l, mean (SD) | 140.65±17.96 | 141.06±20.22 | 140.50±17.06 | 0.804 |
| D-Dimer, mg/l, median (p25–p75) | 0.77 (0.41–1.15) | 0.98 (0.61–1.41) | 0.72 (0.39–1.08) | 0.001 |
| D-Dimer≥0.65mg/l, n (%) | 190 (59.9) | 65 (74.7) | 125 (54.3) | 0.001 |
| Potassium, mmol/l, median (p25–p75) | 3.57 (3.29–3.88) | 3.54 (3.16–3.76) | 3.59 (3.34–3.90) | 0.040 |
| Potassium≤3.1mmol/l, n (%) | 40 (12.6) | 19 (21.8) | 21 (9.1) | 0.002 |
| Calcium, mmol/l, median (p25–p75) | 2.24 (2.17–2.32) | 2.24 (2.16–2.31) | 2.25 (2.17–2.32) | 0.351 |
| Time to initial CT scan, h, median (p25–p75) | 3 (2–6) | 2.5 (2–4) | 3 (2–7.25) | 0.003 |
| Baseline ICH volume, ml, median (p25–p75) | 8 (4.5–18) | 23 (10–40) | 6 (3.5–13) | <0.001 |
| HG, ml/h, median (p25–p75) | 2.8 (1–7.5) | 8 (3.78–15.33) | 1.78 (0.7–4.58) | <0.001 |
| HG≥2.7ml/h, n (%) | 162 (51.1) | 76 (87.4) | 86 (37.4) | <0.001 |
| Supratentorial ICH, n (%) | 284 (89.6) | 82 (94.3) | 202 (87.8) | 0.095 |
| Intraventricular ICH, n (%) | 106 (33.4) | 35 (40.2) | 71 (30.9) | 0.115 |
| GCS, median (p25–p75) | 13 (11–14) | 11 (8–13) | 13 (12–15) | <0.001 |
| GCS≤8, n (%) | 43 (13.6) | 30 (34.5) | 13 (5.7) | <0.001 |
| Island sign, n (%) | 59 (18.6) | 51 (58.6) | 8 (3.5) | <0.001 |
| Black hole sign, n (%) | 74 (23.3) | 45 (51.7) | 29 (12.6) | <0.001 |
| Blend sign, n (%) | 28 (8.8) | 16 (18.4) | 12 (5.2) | <0.001 |
| Edema, n (%) | 37 (11.7) | 15 (17.2) | 22 (9.6) | 0.058 |
| Niveau formation, n (%) | 18 (5.7) | 16 (18.4) | 2 (0.9) | <0.001 |
| NCCT signs (island sign, black hole sign, blend sign, niveau formation) exist one or more, n (%) | 109 (34.4) | 63 (72.4) | 46 (20) | <0.001 |
GCS, Glasgow Coma Scale; HE, hematoma expansion; ICH, intracerebral hemorrhage; SBP, systolic blood pressure; Hb, hemoglobin; INR, international sensitivity index; NCCT, non-contrast CT; HG, hematoma growth; SD, standard deviation.
Multiple logistic regression of risk factors in the development cohort.
| Variables | β Coefficients | OR (95% CI) | P |
|---|---|---|---|
| Anticoagulants | 3.212 | 24.83 (1.95–315.48) | 0.013 |
| HG≥2.7ml/h | 1.688 | 5.41 (2.46–11.90) | <0.001 |
| GCS≤8 | 1.364 | 3.91 (1.63–9.40) | 0.002 |
| D-Dimer≥0.65mg/l | 0.633 | 1.88 (0.96–3.71) | 0.068 |
| Potassium≤3.1mmol/l | 0.705 | 2.02 (0.81–5.03) | 0.130 |
| NCCT signs (island sign, black hole sign, blend sign, niveau formation) exist one or more | 1.471 | 4.35 (2.24–8.46) | <0.001 |
GCS, Glasgow Coma Scale; ICH, intracerebral hemorrhage; NCCT, non-contrast CT; HG, hematoma growth; OR, odds ratio; CI, confidence interval.
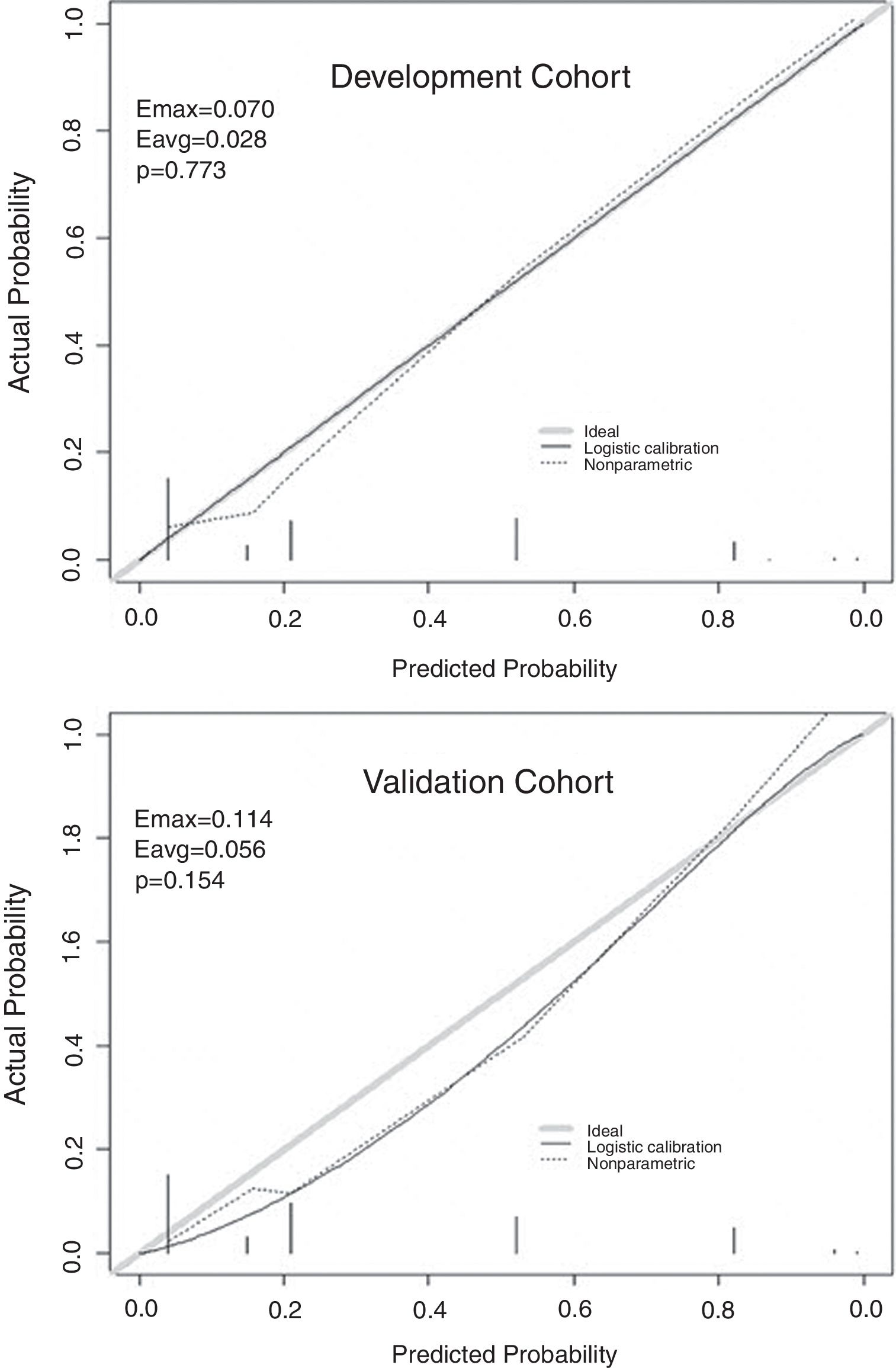
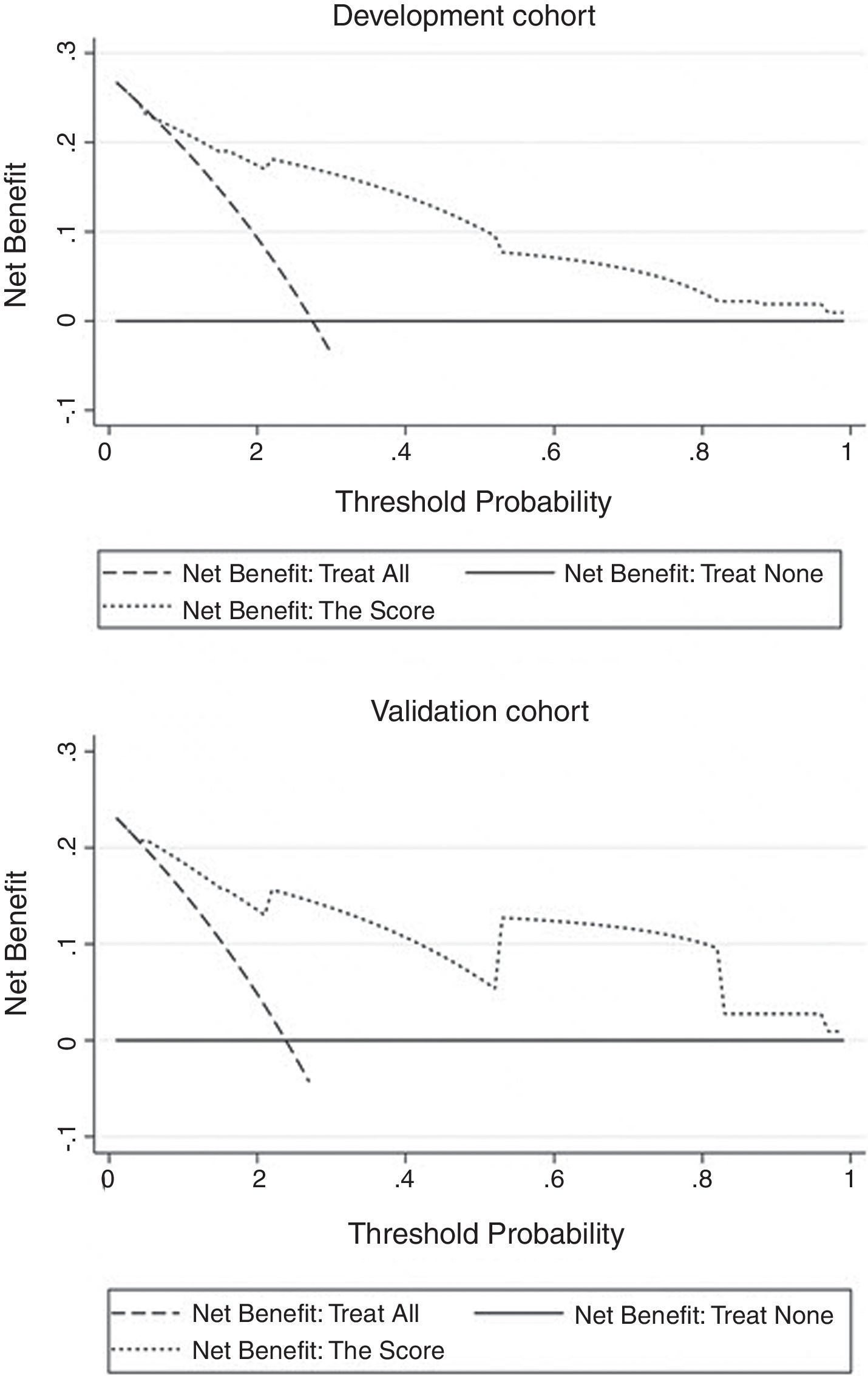
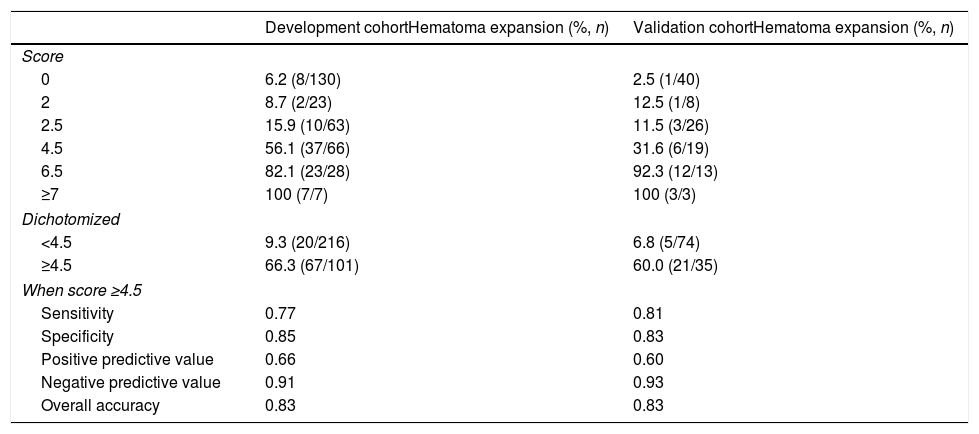
The independent risk factors were included in the model, and score was created based on the parameter estimates (β coefficients), as shown in Table 3. Further, the area under ROC curve was 0.854, 95% CI (0.803–0.904), P<0.001, in the development cohort compared with 0.893, 95% CI (0.816–0.970), P<0.001, in the validation cohort. Calibration plot is shown in Fig. 3, the maximum deviation (Emax), average deviation (Eavg) and P value of the score were 0.070, 0.028,0.773 in the development cohort, 0.114,0.056,0.156 in the validation cohort, respectively. Hosmer–Lemeshow goodness-of-fit test presented χ2=0.826, P=0.662 in the development cohort compared with χ2=6.106, P=0.107 in the validation cohort. In Fig. 4, DCA curves of score from two samples were both far from curve of treat all and curve of treat none, with a wide range of optional threshold probability and a high net benefit. In general, the incidence of HE increased with higher scores. When 4.5 was chosen as the cutoff value to dichotomize the score, the rate of HE in high risk group (score≥4.5) was 66.3% from development cohort compared with 60.0% from validation cohort, more details were demonstrated in Table 4.
Prediction score of HE in ICH.
| Variables | Points |
|---|---|
| GCS≤8 | 2 |
| HG≥2.7ml/h | 2.5 |
| NCCT signs (island sign, black hole sign, blend sign, niveau formation) exist one or more | 2 |
| Anticoagulants | 4.5 |
| Total | 0–11 |
GCS, Glasgow Coma Scale; HE, hematoma expansion; ICH, intracerebral hemorrhage; NCCT, non-contrast CT; HG, hematoma growth.
The proportion of patients experiencing HE by score.
| Development cohortHematoma expansion (%, n) | Validation cohortHematoma expansion (%, n) | |
|---|---|---|
| Score | ||
| 0 | 6.2 (8/130) | 2.5 (1/40) |
| 2 | 8.7 (2/23) | 12.5 (1/8) |
| 2.5 | 15.9 (10/63) | 11.5 (3/26) |
| 4.5 | 56.1 (37/66) | 31.6 (6/19) |
| 6.5 | 82.1 (23/28) | 92.3 (12/13) |
| ≥7 | 100 (7/7) | 100 (3/3) |
| Dichotomized | ||
| <4.5 | 9.3 (20/216) | 6.8 (5/74) |
| ≥4.5 | 66.3 (67/101) | 60.0 (21/35) |
| When score ≥4.5 | ||
| Sensitivity | 0.77 | 0.81 |
| Specificity | 0.85 | 0.83 |
| Positive predictive value | 0.66 | 0.60 |
| Negative predictive value | 0.91 | 0.93 |
| Overall accuracy | 0.83 | 0.83 |
We proposed and validated a novel HE score using two large spontaneous ICH cohorts. The score included 4 items: the history of anticoagulants, HG≥2.7ml/h, GCS≤8, and NCCT signs (island sign, black hole sign, blend sign, niveau formation) exist one or more, with a total score ranging from 0 to 11. In previous study,5–11 most of the evaluation methods were comparatively simple. Wielding DCA to assess the clinical utility were rare, which lead to the overlook of harm caused by false negative. In contrast, our study was the first to systematically evaluate the score in multiple dimensions (discrimination, accuracy, clinical utility). The score was divided into the high-risk group and the low-risk group by the cut-off value of 4.5, because the incidence of HE at this point was significantly higher than the average one. It is worth mentioning that no patients with a score of 4 were found, due to the fact that NCCT signs and low GCS scores were more likely to occur when hematomas were large. When the above two coexist, there was a high probability that HG≥2.7ml/h. The specificity of score were 0.85 (development cohort) and 0.83 (validation cohort), which may help the clinical trials focused on hemostatic drugs screen out the groups that benefit most from HE targeted intervention. In comparison with nomogram, the score is simple in form and content, quick in calculation, and combines imaging features with laboratory examination, allowing clinicians to make individualized treatments with limited information.
Veltkamp12 pointed out that the history of antiplatelets was negatively correlated with the prognosis of neurological function, but it's relationship with HE was highly controversial. In our case, the history of anticoagulants was an independent risk factor for HE while the history of antiplatelets was not. The differences may be attributed to the definition of hematoma enlargement, sample size, or demographic factors. Recently, computed tomography angiography (CTA) spot sign has been externally verified and considered as a potential risk factor for HE and poor prognosis, which has been recommended by the relevant American guidelines. However, in the ATACH-II clinical trial,13 >80% of the subjects were not examined by CTA and discrimination of CTA spot sign failed to meet expected theoretical level. In addition, emergency CTA is unavailable in some primary hospitals in Asia. Apart from the problems of allergy and renal insufficiency, spot sign should be distinguished from false positive, such as oligodendroglioma and moyamoya disease. Besides, a randomized clinical trial (SPOTLIGHT)14 using CTA spot sign for hemostasis was prematurely terminated due to low enrollment rates. In contrast, NCCT is the gold standard for the diagnosis of ICH, with strong universality and simple operation. It highlights the value in clinical trials by improving patient compliance and thus increases the enrollment. In our study, NCCT signs (island sign, black hole sign, blend sign, niveau formation) exist one or more was actually a novel quantification of the degree of hematoma heterogeneity, which overcame subjectivity to a certain extent and improved the reliability of prediction. Miyahara5 thought these signs may have undergone similar pathophysiological processes: active bleeding caused by secondary vascular rupture in different phases (avalanche effect). Veltkamp12 reported that edema was an independent risk factor for HE while niveau formation was not, which contradicted our study. The reason may be that it was difficult to identify when different NCCT signs coexist and overlapped. Another possibility was the lack of standardized training for observers. Recently, Li15 found out low GCS score was one of the key predictors of HE (P<0.001). Sakuta9 suggested that compared with GCS, NIHSS may better reflect the degree of mild or moderate neurological impairment. It is indicated that GCS and NIHSS could be complementary according to patients’ conditions, though the deep connection between the above two needs to be further clarified.
Previous scores5–11 have been published, most of which contained NCCT signs, but all were single signs. Zhang16 concluded in meta-analysis that it was not recommended to use separate NCCT signs to assess the risk of HE in routine clinical practice. Considering the complexity of actual clinical work, our study optimized and improved the single sign to NCCT signs (island sign, black hole sign, blend sign, niveau formation) exist one or more, so as to maximize the clinical information reflected in the limited scoring items. The reasonable infer of the possible mechanism is that, if there are multiple NCCT signs, it implies that there may be multiple bleeding spots around the hematoma, so the risk of HE will also increase. Although some literatures9,12 believed that HE peaked within 6h from the onset, the shorter the time from the onset to the first CT scan, the more likely HE will be detected. Given that patients with mild symptoms tend to delay hospitalization, to increase the enrolled population and make the score more widely applicable, we set the enrollment standard as 24h. The BAT score7 set the time window as 6h from the onset to the first CT scan; the HEP score8 was 12h; the 9 score10 and the NAG score9 were both 24h, consistent with our study. Brouwers10 enrolled more patients with initial hematoma volume >30ml, which was similar to the development cohort of our score from senior medical center. Consistent with the validation cohort of our score, Sakuta9 reported that the majority of the study population had mild symptoms, suggesting that different models may be compatible according to different clinical characteristics. Although the NAG score9 was simpler and more portable, it did not include imaging data. Since CT is the gold standard for the diagnosis of cerebral hemorrhage, its reliability needs to be further verified. Moreover, emergency blood glucose test is not a routine project in several medical institutions, which also limits its clinical application. The HEAVN score5 reckoned as useful in prediction of HE and neural function prognosis. However, the score was slightly complicated and added more subjective items, which required more clinical data to support and brought about deviations to the objective reflection of the disease. In addition, BRAIN score11 derived from the 9-point score also had the same problems, which was not conducive to make decision of clinical strategies rapidly.
Of course, our study had some limitations. 1. The results obtained from the Chinese population were of limited generalizability to other ethnic groups and lack prospective validation. 2. Retrospective study inherently increased the risk of selection bias and the number of patients was smaller in the validation cohort than the development cohort. 3. The time from onset to initial CT scan was sometimes imprecise or even unknown (wake-up strokes). Confounding factors such as scanning machine types and scanning parameters were usually variable. 4. Patients who received initial CT scan later may have developed HE but failed to be detected, which will affect the proportion of HE occurrence. 5. The definition of HE was based on neuroimaging, so the clear relationship between score and functional prognosis was not clarified. 6. A small number of patients were excluded from the study due to early death, abandonment of treatment or emergency surgery. This group was considered to have the highest incidence of HE, excluding them contributed to underestimate its real performance. 7. Given the imparity of academic level of hospitals, patients suffering severe symptoms were more common in development cohort than in the validation cohort, which means the difference of patients’ composition might affect the performance of the score.
ConclusionVia preliminarily validated externally, our score may provide some references and help for accurately identifying high-risk individuals of HE, swift guiding clinical treatments and also serving clinical trials. But prospective validation is required before score could be applied to routine clinical work.
Authors’ contributionsXYK study concept and design, analysis and interpretation of data, drafting of the manuscript. WQ acquisition of data, analysis and interpretation of data, drafting of the manuscript. JD acquisition of data, analysis and interpretation of data and revision of the drafting of the manuscript. ZYQ obtaining funding, study concept and design, study supervision or coordination, revision of the drafting of the manuscript. All authors read and approved the final manuscript.
FundingThis work was supported by the Technology Development Project of Soochow (SYSD2018102) and Postgraduate Practice Innovation Program of Jiangsu Province (SJCX18_0855).
The fund body took no part in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Conflict of interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The authors thank the participants and all who were involved in our study.