A study was made of the changes in the serum levels of thrombin activatable fibrinolysis inhibitor (TAFI), proinflammatory cytokines and acute phase proteins in the acute stage of acute coronary syndrome (ACS), in order to explore the possibility of using TAFI as a biomarker for ACS risk assessment.
MethodsA total of 211 patients with ACS were enrolled, and healthy subjects were used as controls. Blood samples were taken within 24h after admission. Serum TAFI levels were determined by immunoturbidimetry. Serum levels of interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) were determined by enzyme linked immunosorbent assay (ELISA). Procalcitonin (PCT) and C-reactive protein (CRP) levels were measured by gold-immunochromatographic assay.
ResultsSerum TAFI levels in ACS patients were significantly decreased versus the controls. The IL-1β, IL-6, TNF-α, PCT and CRP levels were markedly higher in the ACS patients than in the controls. Correlation analysis revealed a strong negative correlation between TAFI concentration and the IL-1β, IL-6, TNF-а, PCT and CRP levels in ACS patients and in controls. Multivariate logistic regression analysis suggested decreased serum TAFI to be an independent risk factor for ACS (OR 9.459; 95% CI 2.306–38.793; P=0.002). The area under the receiver operating characteristic (ROC) curve for TAFI was 0.872 (95% CI 0.787–0.909; P<0.001). The optimum TAFI cutoff point for the prediction of ACS was 24μg/ml, with a sensitivity of 75.83% and a specificity of 72.57%.
ConclusionThese findings suggest that TAFI can be useful as a potential biomarker for ACS risk assessment.
Esta investigación se ha diseñado para analizar el cambio en las concentraciones séricas del inhibidor de la fibrinólisis activado por trombina (TAFI), las citocinas proinflamatorias y las proteínas de la fase aguda en pacientes con síndrome coronario agudo (SCA) con el fin de explorar la posibilidad de utilizar TAFI como biomarcador para evaluar el riesgo de SCA.
MétodosSe incluyó un total de 211 pacientes diagnosticados de SCA y se seleccionó a voluntarios sanos como controles. Se extrajeron muestras de sangre de 24h después de la admisión. La concentración sérica de TAFI se determinó mediante inmunoturbidimetría. Las concentraciones séricas de interleucina (IL)-1β, IL-6 y factor alfa de necrosis tumoral se determinaron mediante ensayo de inmunoabsorción enzimática. Las concentraciones de procalcitonina y de proteína C reactiva se evaluaron mediante ensayo inmunocromatográfico.
ResultadosLa concentración sérica de TAFI en los pacientes con SCA fue significativamente menor que en el grupo control. Las concentraciones de IL-1β, IL-6, factor alfa de necrosis tumoral, procalcitonina y proteína C reactiva fueron notablemente mayores en los pacientes con SCA que en el grupo control. Un análisis de correlación mostró que existía una sólida correlación negativa entre la concentración de TAFI y las concentraciones de IL-1β, IL-6, factor alfa de necrosis tumoral, procalcitonina y proteína C reactiva, tanto en los pacientes con SCA como en los del grupo control. El análisis de regresión logística multivariante evidenció que la disminución de la concentración sérica de TAFI constituía un factor de riesgo independiente de SCA (OR 9,459; IC 95% 2,306-38,793; p=0,002). El área bajo la curva de eficacia diagnóstica (curva ROC) del TAFI fue de 0,872 (IC 95% 0,787-0,909; p=0,001). El punto de corte óptimo del TAFI para la predicción del SCA fue de 24μg/ml, con una sensibilidad del 75,83% y una especificidad del 72,57%.
ConclusiónEstos hallazgos evidencian que el TAFI constituye un posible biomarcador del riesgo de SCA.
Coronary heart diseases are a leading cause of morbidity and mortality in the world. Acute coronary syndrome (ACS) is a term representing the main clinical manifestation of atherosclerotic progression in the coronary plaque.1 In the pathological process of ACS, thrombosis plays a critical role. Disruption of an atherosclerotic plaque exposes flowing blood to subendothelial collagen, tissue factor, and other procoagulant substances that trigger the activation of platelets and the formation of fibrin within the local vessel lumen. Endothelial damage, inflammation and coagulation are closely related to the patho-physiology of acute coronary syndrome and might be inter-related.2
Thrombin activatable fibrinolysis inhibitor (TAFI) is a zymogen that links coagulation and fibrinolysis.3 When activated, it potently inhibits fibrinolysis through the removal of the carboxy-terminal lysine and arginine residues from partially degraded fibrin polymers. In addition, TAFI has a suppressive effect on conversion of inactive plasminogen to plasmin.4 Since impaired fibrinolysis is a well established risk factor for cardiovascular events, detecting TAFI concentration in ACS patients may be helpful to the risk assessment of this life threatening disease. However, the results of current investigation are paradoxical. Some studies showed a trend for increased TAFI level in coronary artery disease, while others found decreased level of TAFI in these patients.5–7
Inflammation plays an important role in the onset and development of atherosclerosis which is the underlying cause of ACS.8 Coagulation and inflammation are closely inter-related processes. Pro-inflammatory cytokines promote blood coagulation.9 Inflammation leads to activation of coagulation, and activated coagulation considerably affects inflammatory activities. Pro-inflammatory cytokines and other mediators are capable of activating coagulation system and downregulating important physiological anticoagulant pathways.10
Since TAFI is a connector of blood coagulation and inflammation, it seems reasonable to hypothesize that TAFI might be an indicator which comprehensively reflexes coagulation and inflammation status, and maybe it is possible to use TAFI as a risk factor to ACS patients. Thus, the present study was designed to test and verify this hypothesis.
Subjects and methodsStudy designWe performed a case–control study with prospective inclusion of the participants. Cases were consecutively recruited 211 patients with acute coronary syndrome, admitted to the Cardiovascular Department of Jinzhou Medical University between September 2014 and August 2015. No patient died during hospitalization, and all patients finished the study. Control subjects were normal volunteer subjects, recommended by Medical Examination Center of the hospital, and had no previous history of thromboembolic disease and were matched to the patients in age and sex. All subjects in this study were given informed consent according to a protocol approved by the local ethics committee.
Inclusion criteriaPatients with ACS enrolled in this experiment included acute myocardial infarction (AMI, total of 153 patients) and unstable angina (UA, total of 58 patients). AMI was defined according to the universal definitions of MI though characteristic symptoms and ECG changes, as well as cardiac marker elevation. The elevation of cardiac biomarker included that CK-MB fraction level increased at least twice the upper limit of normal or the level of troponin I or T increased above the cut-off level for MI. Unstable angina was diagnosed through symptoms and ECG changes complied with ACS, and cardiac marker levels in UA patients were lower than cut-off or normal levels. The standard for ST-elevation myocardial infarction (STEMI) diagnosis included evidence of AMI as above mentioned, and ST-segment elevation and/or new left bundle branch block on the initial ECG. The classification of Non-STEMI (NSTEMI) patients included those with ischemic symptoms, ST-segment depression or T-wave abnormalities in the absence of ST elevation on the initial ECG.11,12 All patients underwent coronary angiography and only those whose coronary artery occlusion area exceeded 50% were included.
Blood samples and proceduresAll venous blood samples were taken in about six o’clock in the morning after an overnight fast. The blood collection of patients was within twenty-four hours after admission. The blood samples were taken from the femoral vein into 0.1M trisodium citrate, and kept on ice and centrifuged within one hour at 2500g for 25min at 6°C. Plasma samples were stored at −80°C until analysis.
Laboratory assaysThe analysis was done blinded to the case identity. The samples were assayed by corresponding kits. TAFI, procalcitonin (PCT) and C-reactive protein (CRP) kits were from Liaoning MEDI Biotechnological Company (Benxi, China). TAFI level was assayed by immunoturbidimetry kit; PCT and CRP levels were determined by gold-immunochromatographic assay. Serum levels of interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) were measured by enzyme linked immunosorbent assay (ELISA) kits (Jingmei Biological Technology Company, Jiangsu, China). For immunoturbidimetry assay, automatic biochemical analyzer (Beckman Coulter, USA) was applied. For ELISA assay, microplate reader (Biotek ELx808, USA) was used. For gold-immunochromatographic assay, Colloidal Gold Immunoassay Analyzer was used (HY Triage, Taizhou, China). The coefficient of variations for all kits were less than 15%. All operations were carried out in accordance with manufacturers’ instructions.
Statistical analysesAll descriptive data were shown as mean±standard deviation. Statistical analyses were performed with the IBM SPSS Statistics 19. The data were analyzed by an ANOVA. We used the t-test for independent samples to assess the differences between two groups. Pearson's correlation was used to calculate correlations between TAFI and plasma cytokine levels. Multivariate logistic regression model was employed to identify the independent risk factors associated with ACS. The receiver operating characteristic (ROC) analysis was performed to evaluate the predictive value of TAFI for ACS and determine the best cut-off value of TAFI. P values <0.05 were considered statistically significant.
ResultsBasic clinical characteristics of patientsThe study population consisted of 211 ACS patients and 211 controls. Basic clinical characters of patients and controls were showed in Table 1. Patients with ACS were subdivided into STEMI, NSTEMI and UA. There was no significant difference in age, gender, history of hypertension, diabetes or tobacco use between ACS patients and healthy controls. Further analysis of the clinical classification of ACS patients showed no remarkable difference in lipid, lipoprotein fractions and fasting glucose among patients with STEMI, NSTEMI, UA and healthy controls.
Clinical characteristics of patients.
| Characteristics | STEMI (n=130) | P | NSTEMI (n=23) | P | UA (n=58) | P | Control (n=211) |
|---|---|---|---|---|---|---|---|
| Age | 62.5±8.7 | 0.82 | 63.1±7.6 | 0.80 | 61.5±7.9 | 0.29 | 62.7±6.9 |
| Sex (male/female) | 78/52 | 12/11 | 28/30 | 120/91 | |||
| Hypertension, n (%) | 69 (53.1) | 13 (56.5) | 31 (53.4) | 112 (53.1) | |||
| Diabetes, n (%) | 35 (26.9) | 5 (21.7) | 12 (20.7) | 45 (21.3) | |||
| Tobacco, n (%) | 37 (28.5) | 6 (26.1) | 16 (27.6) | 51 (24.2) | |||
| TC (mmol/L) | 3.82±0.95 | 0.84 | 3.68±0.93 | 0.44 | 3.91±0.94 | 0.63 | 3.84±0.96 |
| TG (mmol/L) | 1.66±0.79 | 0.72 | 1.72±0.84 | 0.86 | 1.85±0.85 | 0.19 | 1.69±0.76 |
| LDL-C (mmol/L) | 2.52±0.96 | 0.77 | 2.46±0.86 | 0.81 | 2.73±0.87 | 0.07 | 2.49±0.91 |
| HDL-C (mmol/L) | 1.15±0.26 | 0.28 | 1.23±0.29 | 0.43 | 1.21±0.21 | 0.35 | 1.18±0.24 |
| GLU (mmol/L) | 5.14±0.81 | 0.74 | 5.23±0.88 | 0.75 | 5.21±0.86 | 0.74 | 5.17±0.83 |
Data are presented as mean±SD. Data in STEMI, NSTEMI and UA are compared with controls respectively. TC, total cholesterol; TG, total triglycerides; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; GLU, fasting glucose.
As an initial attempt to explore whether TAFI could be used as a possible risk factor to ACS, we detected the variety of TAFI and inflammation status in ACS patients. The results were shown in Table 2. TAFI level in ACS patients was significantly lower than in controls. Pro-inflammatory cytokine levels of IL-1β, IL-6 and TNF-α were significantly increased when compared with that in controls. PCT and CRP levels in ACS patients were also notably higher than in controls. Collectively, these data suggest that the serum level of TAFI changes in opposite direction against pro-inflammatory cytokines and acute phase proteins in ACS patients.
Plasma levels of TAFI, pro-inflammatory cytokines and acute phase proteins in ACS patients and controls.
| STEMI (n=130) | P | NSTEMI (n=23) | P | UA (n=58) | P | Control (n=211) | |
|---|---|---|---|---|---|---|---|
| TAFI (μg/mL) | 20.79±3.64 ** | 0.0026 | 21.45±3.63** | 0.0011 | 22.12±3.42** | 0.0012 | 26.84±3.62 |
| IL-1β (pg/mL) | 8.69±2.94** | 0.0031 | 8.65±2.73** | 0.0034 | 7.93±2.53** | 0.0036 | 4.45±0.90 |
| IL-6 (pg/mL) | 104.72±12.75** | 0.0010 | 99.32±10.34** | 0.0034 | 101.43±11.43** | 0.0010 | 82.14±8.01 |
| TNF-α (pg/mL) | 13.76±2.28** | 0.0010 | 12.94±2.13** | 0.0021 | 12.89±2.24** | 0.0016 | 6.89±1.59 |
| PCT (ng/mL) | 0.78±0.15 ** | 0.0011 | 0.79±0.14** | 0.0010 | 0.82±0.16** | 0.0011 | 0.44±0.09 |
| CRP (μg/mL) | 15.98±3.21** | 0.0010 | 16.87±3.87** | 0.0011 | 17.23±3.93** | 0.0010 | 5.81±1.28 |
Data are presented as mean±SD.
P<0.01. Plasma levels of TAFI, pro-inflammatory cytokines and acute phase proteins in ACS patients compared with that in controls.
ACS, acute coronary syndrome; TAFI, thrombin activatable fibrinolysis inhibitor; IL-1β, interleukin-1β; IL-6, interleukn-6; TNF-α, tumor necrosis factor-α; PCT, procalcitonin; CRP, C-reactive protein.
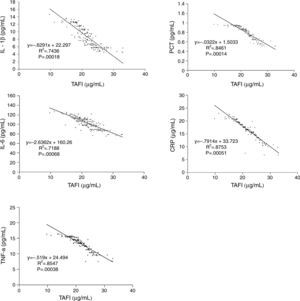
To explore the relationship of TAFI and inflammatory parameters in ACS, we evaluated the correlation between serum level of TAFI and pro-inflammatory cytokines and acute phase proteins in patients with ACS. A significant negative correlation was found between TAFI and IL-1β, IL-6, TNF-α, PCT and CRP in acute coronary patients (Fig. 1). This discovery strongly implies a negative relationship exists between TAFI and inflammation parameters.
Relationship between serum level of TAFI and pro-inflammatory cytokines and acute phase proteins in patients with acute coronary syndrome. A significant negative correlation existed in acute coronary syndrome patients between the serum level of TAFI and pro-inflammatory cytokines and acute phase proteins.
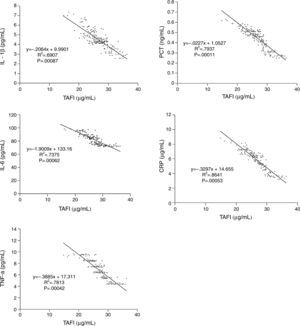
As a strong negative correlation existed between TAFI and inflammatory parameters in ACS patients, we further analyzed the relationship of TAFI and pro-inflammatory cytokines and acute phase proteins in healthy controls. Just as expected, we found that TAFI level in healthy controls was also negatively correlated with the serum levels of IL-1β, IL-6, TNF-α, PCT and CRP. Combined with the correlation analysis results in ACS patients, we speculated that maybe there exists an intrinsic regulation among serum TAFI concentration and IL-1β, IL-6, TNF-α, PCT and CRP levels (Fig. 2).
Serum TAFI level for ACS risk assessmentTo further investigate the association between serum TAFI and ACS, multivariate logistic regression analysis was conducted (Table 3). Only those parameters that were significantly differentiated between ACS and controls were considered as impacting factors. The results showed that after adjusting for IL-1β, IL-6, TNF-α, PCT and CRP, decreased TAFI was still significantly associated with an increased risk of ACS (OR, 9.459; 95% CI, 2.306–38.793; P=0.002). Additionally, increased IL-1β (OR, 67.918; 95% CI, 4.072–1132.738; P=0.003), TNF-α (OR, 46.437; 95% CI, 5.835–369.532; P=0.001) and CRP (OR, 5.038; 95% CI, 2.629–9.657; P=0.001) were also risk factors of ACS.
Multivariate logistic regression analysis of ACS risk factors.
| Variables | B | S.E | Wals | df | OR | 95% CI | P |
|---|---|---|---|---|---|---|---|
| TAFI | 2.247 | 0.720 | 9.738 | 1 | 9.459 | 2.306–38.793 | 0.002 |
| IL-1β | 4.218 | 1.436 | 8.632 | 1 | 67.918 | 4.072–1132.738 | 0.003 |
| IL-6 | 0.028 | 0.159 | 0.031 | 1 | 1.028 | 0.753–1.403 | 0.861 |
| TNF-α | 3.838 | 1.058 | 13.154 | 1 | 46.437 | 5.835–369.532 | 0.001 |
| PCT | 7.398 | 5.664 | 1.706 | 1 | 1631.991 | 0.025–1.080 | 0.191 |
| CRP | 1.617 | 0.332 | 23.739 | 1 | 5.039 | 2.629–9.657 | 0.001 |
TAFI, thrombin activatable fibrinolysis inhibitor; IL-1β, interleukin-1β; IL-6, interleukin-6; PCT, procalcitonin; CRP, C-reactive protein.
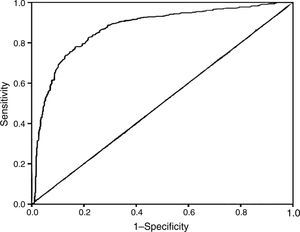
Since TAFI is the center among the parameters in this study, we put special emphasis on analyzing its role in ACS risk assessment. In order to calculate cut-off point of TAFI, ROC analysis was performed. The ROC curve analysis (Fig. 3) showed that the area under the curve of TAFI for predicting ACS was 0.872 (95% CI, 0.787–0.909; P<0.001). The optimum cut-off point of TAFI was under 24μg/mL, with sensitivity of 75.83% and specificity of 72.57%.
DiscussionIn present study, we demonstrated that plasma TAFI level decreases, while the plasma levels of pro-inflammatory cytokines and acute phase proteins increase in patients with ACS. There exists a strong negative correlation between TAFI level and IL-1β, IL-6, TNF-α, PCT and CRP concentration. After adjusting for pro-inflammatory cytokines, decreased TAFI is still associated with an increased risk of ACS. To our knowledge, this is the first investigation on the correlation between TAFI and pro-inflammatory cytokines, and exploring TAFI as a possible independent risk factor to ACS patients.
TAFI, a carboxypeptidase B (CPB)-like zymogen, activated by thrombin during coagulation, is synthesized by liver and released into circulating plasma.13 Thrombin cleaves TAFI into activation peptide and catalytic domain, the latter leads to decreased plasmin formation and enhanced stabilization of the clot.14 It seems that the elevated level of TAFI zymogen is associated with coronary heart disease. However, according to the course of TAFI activation, it is more likely that the active form of TAFI instead of TAFI zymogen, is responsible for clot stabilization effect. In fact, the reported serum levels of TAFI were ambiguous in coronary heart disease.5,6 The reasons for the difference of TAFI levels among researchers might can be explained as different cardiovascular disease stages15 or different assays or different antibody reactivity toward different TAFI isoforms.16
In present study, we found decreased serum TAFI level in ACS patients. Although definite evidence concerning the mechanisms of plasma TAFI decline is not available, it seems rational to partially explain the decrease as huge consumption of TAFI in the acute stage of ACS. This explanation gets support from a recent report, in which the continuously decreased serum TAFI concentration was shown to be closely associated with extensive TAFI activation, more severe initial stroke, and unfavorable stroke evolution in subacute phase and poor long-term stroke outcome.17
Genotyping analysis of TAFI promoter and 3′-untranslated regions showed polymorphisms, which might contribute to decreased serum TAFI level.7 The 2 main polymorphisms that closely associated with TAFI concentration are 1542C and Thr147 in the 3′-untranslated region. The carriers of these two alleles had higher serum TAFI levels.18 The GG genotype of the C+1542G polymorphism, the TT (Ile/Ile) genotype of the Thr325Ile polymorphism and the GG (Ala/Ala) genotype of the Ala147Thr polymorphism are associated with lower TAFI Ag and TAFI activity levels.19 We do not conduct TAFI polymorphism detection in present research, thus it is difficult for us to rule out the genetic impact on TAFI level. However, there is no report concerning significant difference of genotype distribution between case and control. Maybe it is justified to assume the influence of genetic factor on plasma TAFI level between case and control is ignorable in present research.
A recent research showed that TAFI expression can also be regulated by inflammatory mediators. Cultured HepG2 cells treated with pro-inflammatory cytokines of IL-6, TNFα and IL-1β, reduce TAFI production by half. In contrast, when treated with anti-inflammatory cytokine IL-10, HepG2 cells double TAFI expression. The mechanism for this modulation concerns binding of tristetraprolin to TAFI 3′-UTR, mediating TAFI mRNA destabilization.20 In present study, we found the increase of IL-1β, IL-6 and TNF-α in ACS patients. Maybe it is rational to partially attribute the decrease of plasma TAFI concentration to the inhibition effect of high level pro-inflammatory cytokines on TAFI expression in ACS patients.
Activated TAFI has a broad range of anti-inflammatory activities. It inactivates the activated inflammatory mediators, including complement elements of C5a and C3a, osteopontin and bradykinin.21 Inflammation in vascular wall is crucial to the development of atherosclerosis, and is responsible for several adverse vascular events, such as coronary artery disease and stroke. The expression of endothelial pro-inflammatory cytokines and adhesion molecules greatly accelerates atherosclerotic lesion formation in animal atherosclerosis model.22 Cytokines, such as IL-1β and TNF-a, act as mediators of innate and adaptive immunity in the formation and progression of atherosclerosis.23 In the progression of atherosclerosis plaque, macrophage cells accumulate in arterial intima and adventitia, releasing vasoactive factors and inflammatory cytokines.24 Thus it can be inferred that decreased plasma TAFI weakens its inhibition effect on vascular wall inflammation in ACS patients. The negative correlation between serum TAFI and IL-1β, IL-6, TNF-α, PCT and CRP levels strongly suggests the existence of an intrinsic regulation network. However, the precise mechanisms for this regulation are still unclear.
The main limitation for present study is the absence of follow-up. This limitation weakens the predictive capacity of TAFI in relation to cardiovascular risk. The second limitation is that this observation is based on a small number of patients and controls, so it is difficult to completely avoid the impact of individual difference of TAFI level between case and control.
In summary, this study found that plasma level of TAFI decreases, while the serum levels of IL-1β, IL-6, TNF-α, PCT and CRP increase in acute stage ACS patients. TAFI concentration is negatively correlated with IL-1β, IL-6, TNF-α, PCT and CRP. TAFI might be a prospective biomarker for the risk assessment of acute coronary syndrome.
Note: The first and corresponding author Hongbo Pang is now working in College of Chemistry and Life Science, Shenyang Normal University (No. 253 Huanghebei Street, Huanggu District, Shenyang City, China).
Ethical disclosuresAll subjects in this study were given informed consents according to a protocol approved by the local ethics committee. The contents of the consent included the aim of this research, the obligation and right of participants, the procedure of this research, and risk factors of this research.
Authors contributionHongbo Pang designed the study and wrote manuscript. Chuanhai Zhang performed collection of subjects and clinical work. Feng Liu performed the assay of all index. Xiaoli Gong conducted determination of TAFI and data analysis. Xuehua Jin conducted determination of cytokines. Chunyang Su performed cytokine assays.
Conflict of interestsThe authors declare that they have no conflict of interest.