To compare oxygen saturation index (rSO2) obtained simultaneously in two different brachial muscles.
DesignProspective and observational study.
SettingIntensive care unit.
PatientsCritically ill patients with community-acquired pneumonia.
InterventionsTwo probes of NIRS device (INVOS 5100) were simultaneously placed on the brachioradialis (BR) and deltoid (D) muscles.
VariablesrSO2 measurements were recorded at baseline (ICU admission) and at 24h. Demographic and clinical variables were registered. Pearson's correlation coefficient was used to assess the association between continuous variables. The consistency of the correlation was assessed using the intraclass correlation coefficient (ICC) and Bland•Altman plot. The predictive value of the rSO2 for mortality was calculated by ROC curve.
ResultsNineteen patients were included with an ICU mortality of 21.1%. The rSO2 values at baseline and at 24h were significantly higher in D than in BR muscle. Values obtained simultaneously in both limbs showed a strong correlation and adequate consistency: BR (r=0.95; p<0.001; ICC=0.94; 95% CI: 0.90•0.96; p<0.001), D (r=0.88; p=0.01; ICC=0.88; 95% CI: 0.80•0.90; p>0.001) but a wide limit of agreement. Non-survivors had rSO2 values significantly lower than survivors at all times of the study. No patient with rSO2 >60% in BR died, and only 17.6% died with an rSO2 value >60% in D. Both muscles showed consistent discriminatory power for mortality.
ConclusionBoth BR and D muscles were appropriate for measuring rSO2.
Comparar el índice de saturación tisular de oxígeno (rSO2) medido de forma simultánea en 2 diferentes músculos braquiales.
DiseñoEstudio prospectivo, observacional.
ÁmbitoServicio de Medicina Intensiva.
PacientesCríticos con neumonía comunitaria.
IntervencionesDos sensores con tecnología NIRS (INVOS⢢ 5100) fueron ubicados de forma simultánea en los músculos braquiorradial (BR) y deltoides (D).
VariablesLas mediciones del rSO2 se efectuaron al ingreso (basal) y a las 24h. Se registraron los datos demográficos y clínicos. La correlación de Pearson se utilizó para estudiar la asociación entre variables continuas. La concordancia de la correlación fue valorada mediante el coeficiente de correlación intraclase (ICC) y el análisis de Bland-Altman. El valor predictivo de rSO2 para mortalidad fue calculado mediante curva ROC.
ResultadosSe incluyeron 19 pacientes con una mortalidad de 21,1%. El valor basal y a las 24h de rSO2 fue significativamente mayor en D respecto del BR. Los valores obtenidos de forma simultánea en ambos miembros evidenciaron una buena correlación y una adecuada concordancia: BR (r=0,95; p<0,001. ICC=0,94; IC 95%: 0,90-0,96; p<0,001), D (r=0,88; p=0,01. ICC=0,88; IC 95%: 0,80-0,90; p<0,001), así como un amplio rango de concordancia. Los fallecidos presentaron valores de rSO2 significativamente menores que los supervivientes en todos los momentos del estudio. Ningún paciente con rSO2>60% en BR falleció, y solo el 17,6% fallecieron con un rSO2>60% en D. Ambos músculos evidenciaron un buen poder de discriminación para mortalidad.
ConclusionesTanto el músculo BR como el D fueron apropiados para la medición del rSO2.
Near-infrared spectrometry (NIRS) is a non-invasive technique that uses the differential absorption properties of oxygenated and deoxygenated haemoglobin to evaluate skeletal muscle oxygenation. This technique allows the assessment of the concentration of saturated haemoglobin and myoglobin and, indirectly, provides information about the state of the microcirculation. Two different primary variables can be obtained, depending on the device used: tissue oxygenation saturation (StO2)1,2 and regional oxygen saturation index (rSO2).3,4
Skeletal rSO2 muscle is equivalent to StO2. However, StO2 is defined as a quantification of the OxyHb/HbT ratio in microcirculation of skeletal muscle as an absolute number. On the other hand, skeletal rSO2 is an index of OxyHb present within a volume of tissue; this index is expressed as the percentage of oxygenated haemoglobin relative to total haemoglobin (HbO2/Hb sum). The INVOS 510 devices employ reflectance mode probes that have one 1.5mm optical fibre to illuminate the tissue and two optical fibres (30mm and 40mm) to detect the backscattered light from the tissue. The 40mm separated fibre measures a greater and deeper tissue volume than the 30mm separated fibre. The difference between the spectral absorbance measured with these two probing depths is used to calculate rSO2.
Nevertheless, this technique has some difficulties, mainly due to the lack of signal processing and acquisition procedures standardization.5,6 The first problem arises from the difficulty of establishing a relationship between the two variables and comparing data obtained with different devices.7 Moreover, there is no standard application site for measurements. Therefore, using different muscles may derive different values of the variables. These differences might be related, to the local perfusion characteristics (including its response to different stimulus), the different metabolic functions and states, and the morphological characteristics of each muscle, among others. In this regard, Bezemer et al.7 reported that forearm StO2 is a more sensitive parameter to haemodynamic changes than thenar StO2 and that the depth at which StO2 is measured is of minor influence. On the other hand, Ikossi et al.2 found no association between StO2 and tissue oxygen pressure (measured directly with electrodes) in deltoid muscle. Recently, our group reported that a brachioradialis rSO2 greater than 60% at ICU admission was associated with better outcome in septic patients.3
The skeletal rSO2 seems to be an adequate variable for evaluating the microvascular status in patients with severe sepsis.3 However, the site of application is important because there may be differences in the sensitivity of underlying muscle groups and other anatomical structures, to cardiovascular challenges. Unfortunately, there are no studies that have determined rSO2 in different application sites simultaneously using INVOS 5100 spectroscopy technology. We believe that contributing to clarifying these differences may be helpful in the management of critically ill patients, particularly in those where it is crucial to try to maintain the balance between oxygen supply and demand. The aim of this study was to investigate rSO2 behaviour in two different brachial muscles in septic patients, a proximal one (deltoid muscle, D) and a distal one (brachioradialis muscle, BR). The measurements were obtained initially at ICU admission and again 24h after starting the treatment. The specific objectives of the study were: (1) To compare rSO2 values obtained during the evolution of the patient and simultaneously in both muscles and (2) to determine the association between those values and ICU mortality.
Materials and methodsA prospective and observational study was conducted in a 30-bed medical•surgical ICU in a tertiary university hospital. The investigation was conducted according to the principles outlined in the Declaration of Helsinki. The study protocol was approved by the Joan XXIII University Hospital Ethics Committee (MICRO2 20/2010) and informed consent was given by each patient or their next of kin. Data obtained from rSO2 were not known by the care team and did not influence any decision about treatment or management.
PatientsSince patients with sepsis are heterogeneous, this study included only those with severe sepsis due to community-acquired pneumonia (CAP). Inclusion criteria were: (1) severe CAP; (2) age >18 years; (3) less than 6h between ICU admission and the first measurement; and (4) informed consent obtained from patients or relatives. Patients with morbid obesity (Corporal Mass Index >30kg/m2) were excluded.
Severe CAP was defined as an acute lower tract infection characterized by: (1) an acute pulmonary infiltrate evident on chest radiographs and compatible with pneumonia, (2) confirmatory findings of clinical examination, (3) acquisition of the infection outside a hospital, long-term care facility or nursing home8, and (4) ICU admission.9
ProceduresOnce the patient has been enrolled, demographic variables were registered and severity of illness was assessed using Acute Physiology and Chronic Health Evaluation II (APACHE II)10; Sequential Organ Failure Assessment score (SOFA)11 and Simplified Acute Physiology Score 3 (SAPS 3).12
The rSO2 measurements were taken by NIRS using system INVOS 5100 (Somanetics Corporation, Troy, MI, USA), by the technique previously described.3 Briefly, NIRS is a non-invasive technique that uses the differential absorption properties of oxygenated and deoxygenated haemoglobin to evaluate tissue oxygenation. The near-infrared light easily crosses biological tissues, which have a low absorption power, and is absorbed only by haemoglobin, myoglobin and oxidized cytochrome, although the contribution of these latter two to the light attenuation signal is very small. The NIRS INVOS system signal is limited to vessels that have less than 1mm (arterioles, capillary and venules), since the elevated blood concentration of vessels with greater diameter (and greater light absorption) makes the reflection less probable. The NIRS INVOS technique uses reflectance mode probes that have one 1.5-mm optical fibre to illuminate the tissue to a depth of 4cm, and two optical fibres to detect the backscattered light from the tissue. The signal is then transformed to a digital absolute number (rSO2) and carried to a display unit where the values and trends are displayed on the screen. The system updates the rSO2 value at 10s intervals.
In all patients, two probes were placed on the brachioradialis muscle, located on the anterior outside of the forearm, 1•2cm below its insertion on the external tip of the radius in both limbs, according to the technique describe by Bezemer et al.7 At the same time, another two probes were placed on the deltoid muscle in both limbs, 1•2cm below the acromioclavicular joint.2 After a stabilization period of 30•40s, values for rSO2 were registered for each of the studied muscles. Measurements were recorded at baseline (ICU admission) and 24h after initiating treatment. No vascular occlusion test was carried out in this study (see ‘Discussion tm) section).
Statistical analysisDifferences between groups were assessed using chi-square or Fisher's exact test for discrete variables and the Wilcoxon test and analysis of variance (ANOVA). The precision of the measurements obtained in both muscle groups was studied through the determination of the coefficient of variation (CV). Pearson's correlation coefficient was used to assess the association between continuous variables. The consistency of the values obtained in different application sites was assessed using the intraclass correlation coefficient (ICC), based on the model of analysis of variance for repeated measures by the process reliability. The strength of the consistency was assessed according to the value of the ICC as, very good (>0.90), good (0.71•0.90), moderate (0.51•0.70), mediocre (0.31•0.50) and null (<0.30).13 In addition, Bland•Altman analysis was performed.
The predictive value of the variables for mortality was calculated using the Receiver Operator Characteristic (ROC) curve, and the Area Under the ROC (AUROC) curve was computed. The ROC graph for each variable was a plot of all the sensibility/specificity pairs resulting from different cut-off points of mortality prediction. AUROC close to 1 was considered as a “perfect” prediction model, whilst values close to 0.5 were considered poor prediction models. Difference between AUROC was obtained by Hanley and McNeil analysis.14 Statistical significance was defined as p<0.05.
ResultsNineteen patients with severe CAP were enrolled over an 18-month period. Baseline characteristics are summarized in Table 1. Patients were relatively young, with a moderate degree of severity of illness and an expected mortality of between 20 and 25%. The ICU crude mortality was 21.1%. No significantly differences were observed between survivors and non-survivors. Only mechanical ventilation (MV) and the presence of shock at ICU admission were variables associated with mortality.
Baseline characteristic of the 19 patients with respiratory sepsis included in the study.
| Variable | All patients (n=19) | Survivors (n=15) | Non-survivors (n=4) | p-Value |
|---|---|---|---|---|
| Age (yr), mean (SD) | 55.0 (16.3) | 52.9 (17.0) | 63.0 (14.0) | 0.89 |
| Men, n (%) | 14 (73.7) | 12 (80.0) | 2 (50.0) | 0.27 |
| APACHE II score at day-1, mean (SD) | 15.6 (6.3) | 14.2 (3.9) | 21.0 (10.9) | 0.30 |
| SAPS 3 score at day-1, mean (SD) | 52.2 (7.0) | 51.1 (6.6) | 56.0 (9.4) | 0.24 |
| SOFA at day-1, mean (SD) | 4.2 (1.9) | 3.9 (1.4) | 5.0 (3.4) | 0.57 |
| ICU length of stay (days), mean (SD) | 12.6 (13.6) | 10.6 (10.1) | 20.1 (23.4) | 0.23 |
| Mechanical ventilation at day-1, n (%) | 9 (47.4) | 5 (33.3) | 4 (100) | 0.03 |
| Shock at day-1, n (%) | 9 (47.4) | 5 (33.3) | 4 (100) | 0.03 |
| ICU mortality rate, n (%) | 4 (21.1) | NA | NA | NA |
SD: standard deviation; APACHE II: Acute Physiologic Physiology and Chronic Health Evaluation II; ICU: intensive care unit; SOFA: sequential organ failure assessment; SAPS 3: Simplified Acute Physiology Score 3; NA: not applicable.
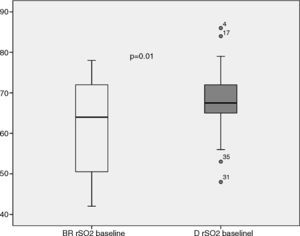
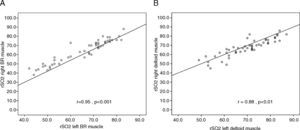
The rSO2 mean value at ICU admission was significantly higher in deltoid muscle than in brachioradialis muscle (Fig. 1). This trend was also found after 24h of treatment (65.8 [12.6] vs. 69.4 [10.5]; p=0.19). Measurements performed in both limbs allowed us to assess the variability due to this factor for each muscle. Thus, rSO2 values obtained simultaneously in left and right brachioradialis muscle showed a strong and significant direct correlation (r=0.95; p<0.001) (Fig. 2A), and an adequate consistency (ICC=0.94; 95% CI:0.90•0.96; p<0.001). Similar results were observed for deltoid muscle r=0.88; p=0.01 (Fig. 2B) and ICC of 0.88 (95% CI: 0.80•0.90; p<0.001). The precision of the measurements obtained simultaneously in both muscular groups was good, with a coefficient of variation of 3.68% and 4.38% for brachioradialis and deltoid muscles.
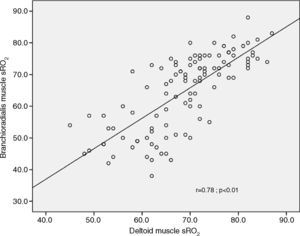
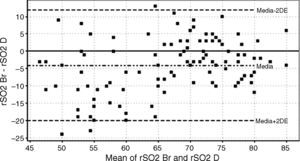
On the other hand, there was a good correlation between simultaneous grouped measurements of rSO2 between deltoid and brachioradialis muscle (r=0.78; p<0.01) (Fig. 3). The consistency of results was good (ICC=0.84; 95% CI: 0.77•0.89; p<0.001); however the Bland•Altman analysis shows a mean difference of ∧4.1 percentage points with a wide limit of agreement (Fig. 4).
Non-survivors (n=4) had values of rSO2 significantly lower than survivors, both at admission and at 24h (Fig. 5). Among non-survivor patients, rSO2 values in deltoid muscle were higher than brachioradialis muscle (Table 2). In contrast, there were no significant differences in rSO2 between the two muscle groups in survivors.
Skeletal regional saturation index (rSO2) in brachioradialis and deltoid muscle at ICU admission and at 24h in survivors and non-survivors.
| Survivors | Non-survivors | |||||
|---|---|---|---|---|---|---|
| rSO2 brchioradialis | rSO2 deltoid | p | rSO2 brachioradialis | rSO2 deltoid | p | |
| ICU admission | 66.9 (8.3) | 70.3 (6.7) | 0.09 | 45.7 (3.3) | 60.6 (6.9) | 0.001 |
| 24h | 71.3 (8.5) | 73.5 (8.0) | 0.32 | 48.4 (5.4) | 56.2 (5.8) | 0.01 |
rSO2 values are shown as mean and standard deviation.
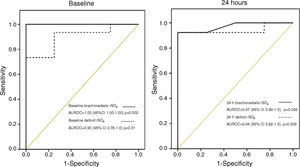
According to our previous findings3 a threshold of rSO2<60% in brachioradialis muscle on ICU admission was considered as “low” skeletal muscle oxygenation and associated with an unfavourable prognosis. No patients with brachioradialis rSO2≥60% died. In contrast, 3 of 17 (17.6%) patients with deltoid rSO2≥60% finally died. The presence of a low rSO2 (<60%) at ICU admission was associated with a mortality rate of 57% and 50% for brachioradialis and deltoid muscle respectively. Finally, even though both skeletal muscles showed consistent mortality discrimination, the AUROC curve was superior in brachioradialis values at admission and at 24h (Fig. 6). However, this difference does not reach statistical significance neither at ICU admission (z=0.81; p=0.41) nor at 24h (z=0.28; p=0.77).
DiscussionThis is the first study to compare rSO2 values obtained simultaneously in proximal and distal skeletal muscle areas of the limbs in patients with respiratory sepsis. Sepsis causes microcirculatory derangement, characterized by decreased vascular density, a large number of non-perfused and intermittently perfused vessels and heterogeneity of capillary transit time with an increase in the proportion of fast-flow to normal-flow times.1,15,16 Our results suggest that the distal muscular area (brachioradialis muscle) might better reflect changes in tissue oxygenation than the proximal muscular area (deltoid muscle) during the first 24h of treatment in the ICU, with no differences between the left and right sides of the body. In addition, our findings reinforce the concept that a major alteration in the muscle tissue oxygenation assessed by NIRS is associated with a worse prognosis.
Non-invasive determination of rSO2 in skeletal brachioradialis muscle has been proposed as a useful tool not only to quantify microvascular dysfunction, but also to predict the evolution of critically ill patients.1•7,17•20 Our group has reported that values of brachioradialis rSO2<60% at ICU admission are associated with a significant increase in mortality in septic patients. These findings are confirmed in the present study, focused on patients with respiratory sepsis. Nevertheless, sepsis physiopathology brings about alterations in tissue oxygenation, due to an imbalance between supply and demand, and can be heterogeneous in different sites. Even though some factors related to oxygen supply are relatively homogeneous (i.e. concentration and saturation of haemoglobin), others like perfusion may vary depending on local factors. Factors related to oxygen muscle utilization, such as tissue bioenergetics, muscle fibre composition, enzymatic aerobic activity and myoglobin concentration might be determinant of the heterogeneity of this metabolic imbalance.
Another important finding was that skeletal rSO2 values were higher in deltoid muscle than brachioradialis muscle over the entire study period, although there was a good correlation and concordance between them; the Bland•Altman plot evidences a wide limit of agreement. This suggest that rSO2 in deltoid muscle cannot be used in place of the rSO2 brachioradialis determination. These differences might be explained by the different metabolic activity of each muscle dependent not only on the composition of the muscle fibres (oxidative or glycolytic) but also the different capillary density.21,22 To our knowledge, no studies have been published on capillary density of each muscular group. However, we know the phenotype of muscle fibres in humans for brachioradialis muscle (40% type I fibres) and deltoid (48% type I fibres).23 We could speculate that this characteristic might result in a slightly smaller number of capillaries in brachioradialis muscle compared to deltoid.24,25 A different vascular reactivity resulting from sepsis could also be a differential element between the two muscle territories. Considering that this is one of the elements that can be inferred with NIRS technology, it is necessary to standardize measurement of the signal site. On the other hand, the prognostic value of the variables obtained depends on the sensitivity to changes they reflect. Our hypothesis was that these changes would be more marked in distal skeletal muscle territories, and our results support this idea. However, the discriminatory ability for mortality was similar for the rSO2 brachioradialis and deltoid muscle determinations. Given the difficulty of carrying out measurements in brachioradialis for anatomical or functional reasons (i.e. presence of catheters on the forearm), the deltoid might be a second option for patients with community-acquired respiratory sepsis.
Finally, another important finding of our study was the close association between early lower values of rSO2 and mortality in both muscles. These results are consistent with previous findings published by Ikossi et al.2 using another NIRS device (the InSpectra, Hutchinson Technology, Minneapolis, MN, USA). These authors analyzed 28 critically ill patients after a resuscitation period, and obtained mean values of tissue oxygen saturation (StO2) in the deltoid of 63%, similar to the one observed in our patients (68% at admission). In addition, StO2 values closely correlated with tissue oxygen pressure measured directly on the muscle. Both variables were associated with development of complications. Furthermore, animal models of haemorrhagic shock26 have shown an excellent correlation between liver and muscular (deltoid) tissue oxygenation.
The fact that we obtained discriminatory prognostic values without carrying out a vascular occlusion test (VOT) is an interesting finding of the present study that confirm what our group have previously reported.3 Some authors suggest that performing a VOT (interruption of the blood flow through the forearm with a pressure cuff for 3min) is a more appropriate way to assess the functionality of vascular response.27 It is not possible to realize this test in the deltoid muscle. In addition, the main objective of the present study was to compare rSO2 values obtained simultaneously in two muscular groups.
As a collateral finding, our results suggest that there are no significant differences between the rSO2 measured in both upper limbs. Physiologically, this would be due to the predictable symmetry of the factors that influence the state of tissue oxygenation. The clinical conclusion would be that, in the absence of local factors that contraindicate it, measurements are the same for both sides of the body. It also indicates that it is not necessary to obtain the measurements in the dominant limb.7,18
The present study has several limitations that should be addressed, even though the relevance of the findings is not challenged. First of all, there was no control group because our group had already validated rSO2 measurement techniques in healthy controls.3 This study only intended to compare the performance of the measurement of the rSO2 carried out in different skeletal muscle locations. A second potential limitation is the small sample size; therefore results need to be interpreted with caution especially those related to prognosis. However, results reach significance in a homogeneous group of patients (CAP patients) which confers internal validity. Finally, we were not able to carry out either morphological or metabolic tests in the muscles studied, which could have explained the differences observed in rSO2 values. The clinical condition of septic patients makes it difficult to conduct muscular biopsies. Even so, members of our group have recently published a paper about metabolic alterations that can be found in respiratory and lower limb muscles in septic patients.28
In conclusion, our results reinforce the potential usefulness of the rSO2 in the assessment of the severity and prognosis of patients with community respiratory sepsis. Brachioradialis and deltoid muscle were appropriate for measuring this variable. Furthermore, there were no significant differences between each side of the body.
Author contributionsRodriguez A, Marín J, Claverias L, Magret M, Bodi M, Cos E, Rosich S, Trefler S and Pascual S have made substantial contributions to conception and design, acquisition, analysis and interpretation of data.
Rodriguez A has drafted the submitted article. Marín L, Claverias L, Pascual S and Gea J have revised the manuscript critically for important intellectual content.
Rodriguez A and Gea J have provided final approval of the version to be published.
Conflicts of interestThe authors have no conflict of interest to disclose.
FundingThis study was partially supported by grants from the Fondo de Investigación Sanitaria (FIS PI10/01538 and PI13/02011) and Beca SEPAR264/2012.
The funding agency had no role in study design, in collection, analysis or interpretation of data; in writing of the manuscript; or in the decision to submit the paper for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Institute of Health Carlos III (ISC III) and SEPAR from Spain.
The authors wish to thank Francisco Avilèc)s MD, PhD for statistical support and Phil Hoddy for revision of the English version of the manuscript.