To evaluate the respiratory and hemodynamic changes during lung recruitment maneuvering (LRM) through stepwise increases and decreases in PEEP level.
Design and settingA retrospective study in a 17-bed ICU was carried out.
PatientsTwenty-one patients with acute respiratory failure and bilateral pulmonary infiltration.
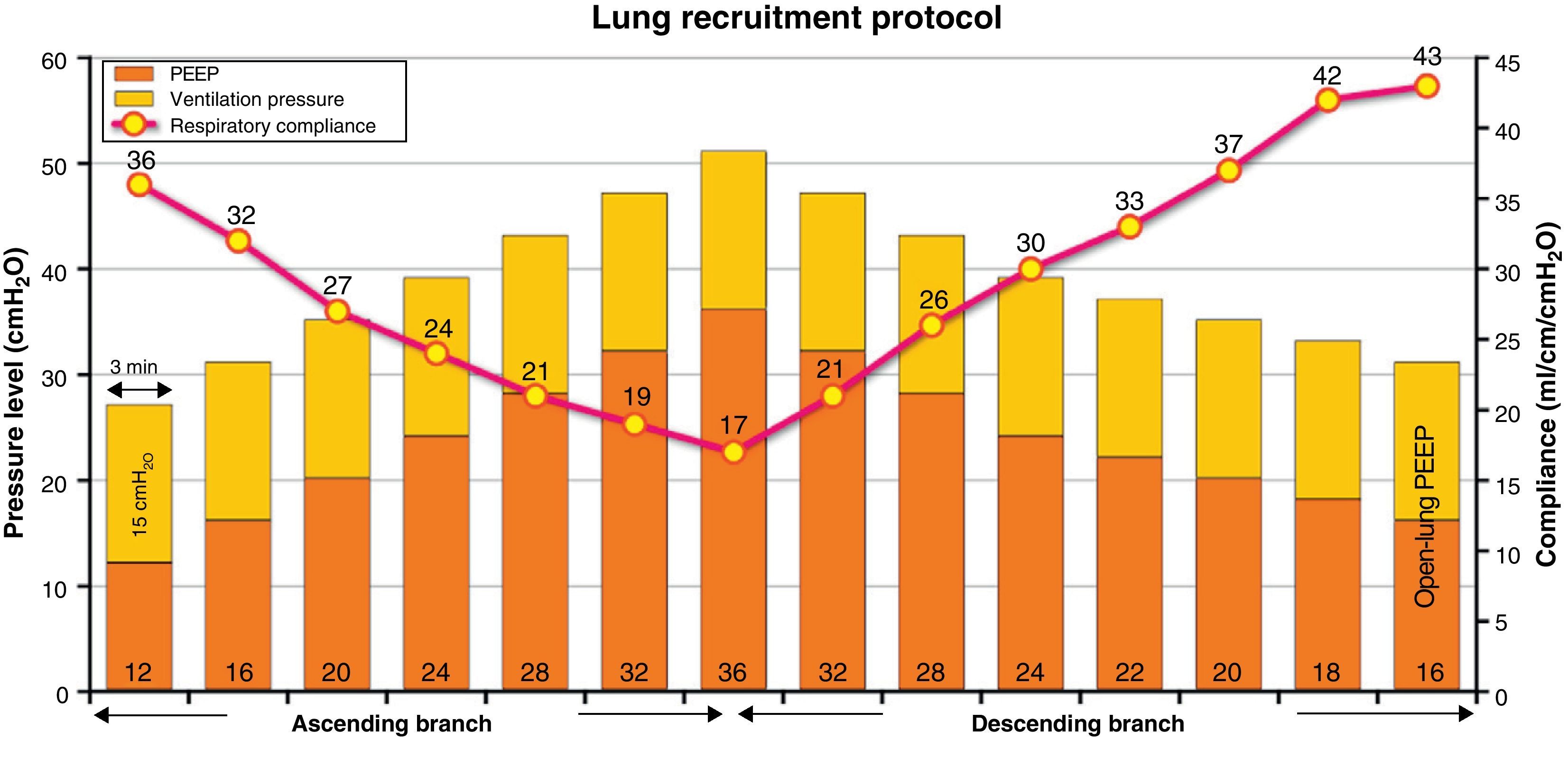
InterventionLRM was carried out, consisting of stepwise increases in PEEP (4cmH2O every 3min), with fixed ventilation pressure, until reaching a maximal value of 36cmH2O PEEP (ascending branch), followed by progressive decreases in PEEP (2cmH2O every 3min) until establishing the open-lung PEEP at the value associated to maximum respiratory compliance (Crs) (descending branch). Continuous hemodynamic monitoring was performed using an esophageal echodoppler probe.
ResultsCrs gradually decreased in the ascending branch of the LRM, and progressively increased surpassing the initial value after establishing the open-lung PEEP in the descending branch, reducing the ventilation pressure and increasing the SpO2/FiO2 ratio. Hemodynamic changes primarily consisted of a fall in cardiac output and left ventricular preload, together with an increased heart rate and cardiac contractility. At comparable levels of PEEP and mean airway pressure, these changes were more pronounced during the descending branch of the LRM.
Conclusions(1) LRM increased Crs, improving oxygenation and decreasing ventilation pressure; (2) the main hemodynamic consequence was the drop in cardiac output and left ventricular preload; and (3) the unequal hemodynamic derangement in both branches, at the same level of PEEP and mean airway pressure, showed that, along with intrathoracic pressure, other factors such as Crs and hypercapnia may have influenced the hemodynamic consequences of this type of LRM.
Estudiar los cambios respiratorios y hemodinámicos durante una maniobra de reclutamiento pulmonar (MRP) mediante incrementos y decrementos progresivos de PEEP.
Diseño y ámbitoEstudio retrospectivo en una UCI de 17 camas.
PacientesUn total de 21 pacientes con insuficiencia respiratoria aguda e infiltrados pulmonares bilaterales.
IntervenciónMRP consistente en incrementos progresivos de PEEP (4 cmH2O cada 3 minutos), con presión de ventilación fija, hasta alcanzar un valor máximo de 36 cmH2O de PEEP (rama ascendente), seguida de decrementos progresivos (2 cmH2O cada 3 minutos) hasta establecer la PEEP de apertura en el valor asociado a la máxima distensibilidad del sistema respiratorio (Dsr) (rama descendente). La monitorización hemodinámica se realizó de forma continua con una sonda ecodoppler esofágica.
ResultadosLa Dsr disminuyó gradualmente en la rama ascendente de la MRP y aumentó de forma progresiva superando el valor inicial al establecer la PEEP de apertura en la rama descendente, reduciéndose la presión de ventilación y aumentando la relación SpO2/FiO2. Los cambios hemodinámicos consistieron fundamentalmente en una disminución del gasto cardiaco y de la precarga del ventrículo izquierdo, junto con un aumento de la frecuencia y de la contractilidad cardiaca. A niveles equiparables de PEEP y presión media en vía aérea, estos cambios fueron más intensos durante la rama descendente.
Conclusiones(1) La realización de la MRP incrementó la Dsr mejorando la oxigenación y disminuyendo la presión de ventilación; (2) la principal consecuencia hemodinámica fue la disminución del gasto cardiaco y de la precarga ventricular izquierda; (3) la afectación hemodinámica desigual en ambas ramas, a niveles equiparables de PEEP y presión media en vía aérea, puso de manifiesto que, junto a la presión intratorácica, otros factores como la Dsr y la hipercapnia pudieron influir en las consecuencias hemodinámicas en este tipo de MRP.
“Open lung” mechanical ventilation is a protective ventilation modality that aims to minimize lung collapse, prevent cyclic alveolar aperture and closure, and reduce pulmonary overdistension.1–3 Its implementation is mainly based on three interventions: (1) the early application of lung recruitment maneuvering (LRM); (2) the use of a positive end-expiratory pressure (PEEP) level sufficient to stabilize and keep open the maximum possible number of alveolar units (the so-called “open-lung PEEP”); and (3) ventilation with as low a pulmonary distension pressure as possible.4,5 However, in order to reach these objectives it is necessary to at least temporarily use very high intrathoracic pressures, and for this reason application of the above ventilatory strategy remains the subject of debate.6,7
The advocators of open lung ventilation justify its use in terms of the harmful effects of lung collapse upon the evolution of the lesions associated to mechanical ventilation, favored by the use of small tidal volumes.8 In contrast, the critics of the technique point out that in addition to the absence of solid confirmation of its benefits in terms of lessened patient mortality, the application of high intrathoracic pressures is not risk-free, with non-negligible hemodynamic alterations and the appearance of barotrauma phenomena.6,9
In September 2003 we introduced an open lung mechanical ventilation protocol based on the use of LRM followed by the application of open-lung PEEP. The aim of this ventilatory strategy was to recruit the maximum possible number of alveoli, ventilating with maximum respiratory system compliance (Crs), and thus with the lowest lung ventilation pressure.2,10 The recent debate published in this journal on the use of this ventilation mode6,7 has prompted us to present some of the most relevant data which we have collected during these years of experience. Specifically, the present retrospective analysis has two aims: (1) to investigate the respiratory and hemodynamic effects of LRM in a group of patients subjected to careful monitoring; and (2) to establish the incidence and form of presentation of barotrauma, and describe the main characteristics and evolution of the patients who develop this complication. While the first of these two points constitutes the main objective of the present study, the second point, related to lung barotrauma, is dealt with in another part of this issue.11 Some of the results of the present study have been previously reported in abstract form.12
Patients and methodsPatientsDuring the period between September 2003 and January 2011, a total of 100 patients were ventilated with our open lung ventilation protocol due to severe hypoxemic acute respiratory failure, defined by the incapacity to maintain oxygen saturation measured by pulsioxymetry (SpO2)>90% with PEEP 10cmH2O and an inspiratory oxygen fraction (FiO2)≥0.6 (SpO2/FiO2≥150),13 in the presence of bilateral lung infiltrates on the chest X-rays. In 21 of these patients the hemodynamic and respiratory mechanical registries obtained during LRM were entered in an electronic database allowing posterior analysis; these patients constituted the present study population.
None of the patients were >80 or <16 years old, and none had suffered previous barotrauma, advanced chronic lung disease (chronic obstructive pulmonary disease [COPD]), uncontrollable progressive acidosis (pH<7.15), hemodynamic instability (defined as mean blood pressure [MBP]<65mmHg despite vasoactive medication), acute heart failure, signs of intracranial hypertension, or terminal stage disease.
This retrospective study was approved by the research bioethics subcommittee of Hospital SAS de Jerez (Spain). Informed consent was not deemed necessary, since the protocol formed part of the usual care of these patients.
General patient managementAll the patients were subjected to tracheal intubation and were ventilated in the supine position with the head of the bed raised 30°, using a Servo 300 (Siemens-Elema AB, Solna, Sweden) or Puritan Bennet 840 respirator (Tyco Healthcare, Gosport, UK), according to the institutional protective ventilation strategy protocol: ventilation in pressure control mode with ventilation pressure on PEEP to secure a tidal volume of 6–8ml/kg body weight, PEEP adjusted to maximize Crs14 and FiO2 to maintain SpO2≥90%. A necessary condition for LRM was taken to be stable basal hemodynamic conditions, defined by MBP≥65mmHg during the last hour, without modification of the vasoactive or inotropic medication doses in those patients receiving treatments of this kind.
The patients were sedated with a continuous perfusion of midazolam in combination with an opiate (morphine or fentanyl), plus bolus doses of midazolam to guarantee adequate sedation (Ramsay score 6). A muscle relaxant was administered (vecuronium bromide: 0.1mg/kg as a bolus dose) before starting LRM, to suppress any spontaneous respiratory effort. The administration of both fluids and vasoactive drugs remained without change throughout LRM. All the patients were subjected to continuous electrocardiographic, pulsioxymetric and blood pressure monitorization from the bedside screen (Datex CS/3, Datex-Ohmeda, Helsinki, Finland).
In all cases, at least one of the main investigators was present during LRM.
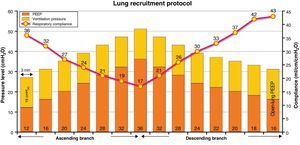
Lung recruitment maneuveringLRM was performed in pressure control mode with pressure over PEEP 15–20cmH2O, adjusted to secure a starting tidal volume of 6–8ml/kg ideal body weight, a respiratory frequency of 15–20rpm, FiO2 100%, and an inspiration/expiration ratio of 1:1 (duration of the inspiratory and expiratory times 1.5–2s, according to the respiratory frequency selected). LRM consisted of stepwise 4cmH2O increments in PEEP every 3min to a maximum of 32–36cmH2O (ascending branch). Posteriorly, PEEP was progressively decreased in progressive 4cmH2O steps every 3min to a value of 28–26cmH2O, followed by 2cmH2O decrements every 3min to the open-lung PEEP at the value associated to maximum Crs (descending branch). Fig. 1 graphically depicts the LRM protocol used.
Respiratory parametersRegistry of the respiratory parameters was carried out on a continuous basis every 30s using a spirometry module connected to the input of the tracheal tube and integrated with the bedside monitor of the patient (MCOVX, Datex-Ohmeda, Helsinki, Finland). The data were entered on a laptop computer with S/5 Collect software, version 4.0 (Datex-Ohmeda, Helsinki, Finland). Crs was calculated as the exhaled tidal volume/(peak pressure−PEEP), taking into account that the inspiratory time was sufficiently prolonged for the inspiratory flow to reach baseline before the end of inspiration (zero flow), so that the peak pressure equaled the plateau pressure.15 The exclusion of air trapping and auto-PEEP was ensured through careful observation of the expiratory flow tracing.
Hemodynamic monitorizationHemodynamic monitorization was carried out using an esophageal echodoppler system (Hemosonic 100, Arrow Intl., Everett., USA) inserted through the oral cavity until obtaining the best Doppler signal and adequate visualization of the anterior and posterior walls of the aorta.16,17 This device allows beat-by-beat evaluation of blood flow in the ascending aorta, based on the simultaneous measurement of aortic diameter and flow velocity by means of two transducers in the distal tip of the catheter (5MHz for Doppler and 10MHz for the M-mode). Cardiac output (CO) was calculated assuming that the arterial flow in the descending aorta represents 70% of the CO. The device also allows us to obtain other parameters related to preload (corrected left ventricle ejection time [LVETc])18 and cardiac contractility (maximum acceleration of aortic flow [Accel]).19 Cardiac power, a measure of the hydraulic efficiency of the heart, was calculated as MBP×CO/451.20 The hemodynamic data were registered and stored every 10s using specific software (Hemosoft, version 1.0. Arrow Intl., Everett., USA), with posterior analysis.
Blood gasesIn 15 of the 21 patients studied, central venous blood samples were collected for blood gas analysis at the start of the maneuver, after reaching maximum PEEP and after establishing open-lung PEEP.
Statistical analysisThe statistical analysis was carried out using MedCalc 11.1.7 (MedCalc Software, Mariakerke, Belgium). The results were expressed as the mean±standard deviation or the median (interquartile range). Normal distribution of the data was assessed using the D’Agostino–Pearson test. Use was made of the mean of each of the hemodynamic and respiratory parameters with each modification of PEEP for statistical purposes. Comparison of the hemodynamic, respiratory and blood gas data during the initial phase of the maneuver (preMRP phase), maximum PEEP (maxPEEP phase) and PEEP level at maximum respiratory compliance (bestPEEP phase) was made using analysis of variance (ANOVA) for repeated measures with Bonferroni correction. We selected four statistically comparable PEEP levels of the ascending and descending branches to evaluate the differences in respiratory and hemodynamic behavior between the two branches. Each pair of values was compared using the Student t-test for paired samples. Statistical significance was considered for p<0.05.
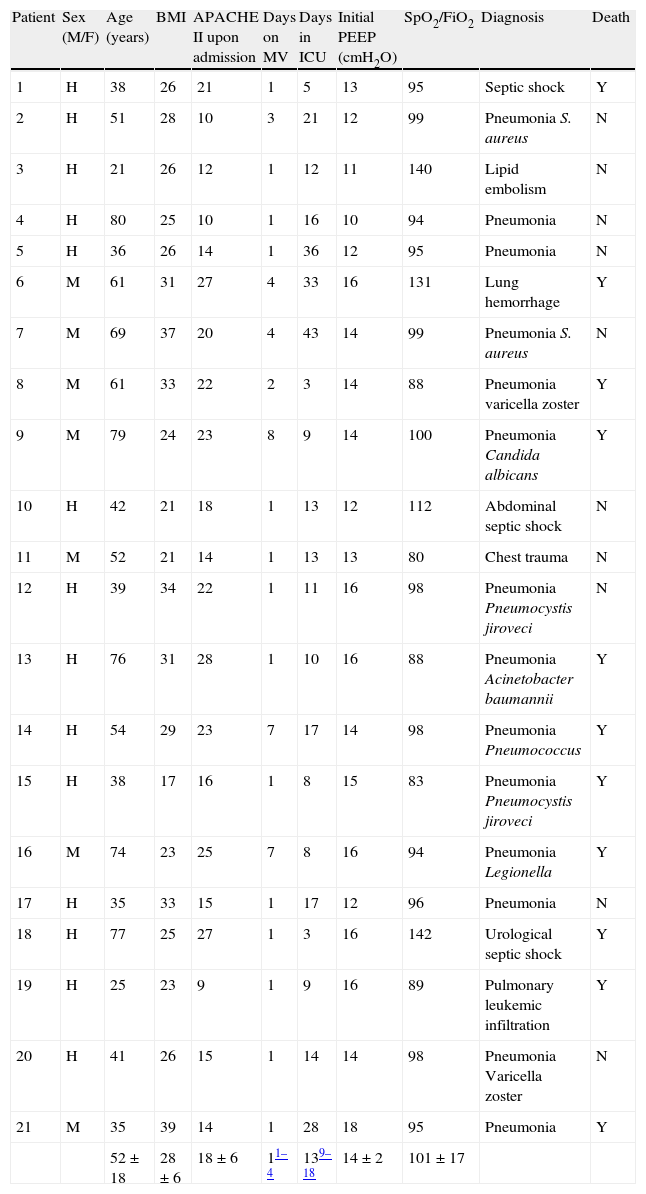
ResultsThe principal patients characteristics are summarized in Table 1. In most cases, the cause of respiratory failure was lung infection (67%). At the time of LRM, a total of 12 patients were receiving vasoactive or inotropic medication (noradrenalin in 9 cases, noradrenalin plus dobutamine in 2, and dobutamine alone in one patient). The mean noradrenalin and dobutamine dose was 0.24±0.09 and 6.91±0.43μgkg−1min−1, respectively. The respiratory frequency and tidal volume at the start of maneuvering was 16.6±2.2rpm and 6.9±1.1ml/kg body ideal weight, respectively.
Clinical characteristics of the patients.
| Patient | Sex (M/F) | Age (years) | BMI | APACHE II upon admission | Days on MV | Days in ICU | Initial PEEP (cmH2O) | SpO2/FiO2 | Diagnosis | Death |
| 1 | H | 38 | 26 | 21 | 1 | 5 | 13 | 95 | Septic shock | Y |
| 2 | H | 51 | 28 | 10 | 3 | 21 | 12 | 99 | Pneumonia S. aureus | N |
| 3 | H | 21 | 26 | 12 | 1 | 12 | 11 | 140 | Lipid embolism | N |
| 4 | H | 80 | 25 | 10 | 1 | 16 | 10 | 94 | Pneumonia | N |
| 5 | H | 36 | 26 | 14 | 1 | 36 | 12 | 95 | Pneumonia | N |
| 6 | M | 61 | 31 | 27 | 4 | 33 | 16 | 131 | Lung hemorrhage | Y |
| 7 | M | 69 | 37 | 20 | 4 | 43 | 14 | 99 | Pneumonia S. aureus | N |
| 8 | M | 61 | 33 | 22 | 2 | 3 | 14 | 88 | Pneumonia varicella zoster | Y |
| 9 | M | 79 | 24 | 23 | 8 | 9 | 14 | 100 | Pneumonia Candida albicans | Y |
| 10 | H | 42 | 21 | 18 | 1 | 13 | 12 | 112 | Abdominal septic shock | N |
| 11 | M | 52 | 21 | 14 | 1 | 13 | 13 | 80 | Chest trauma | N |
| 12 | H | 39 | 34 | 22 | 1 | 11 | 16 | 98 | Pneumonia Pneumocystis jiroveci | N |
| 13 | H | 76 | 31 | 28 | 1 | 10 | 16 | 88 | Pneumonia Acinetobacter baumannii | Y |
| 14 | H | 54 | 29 | 23 | 7 | 17 | 14 | 98 | Pneumonia Pneumococcus | Y |
| 15 | H | 38 | 17 | 16 | 1 | 8 | 15 | 83 | Pneumonia Pneumocystis jiroveci | Y |
| 16 | M | 74 | 23 | 25 | 7 | 8 | 16 | 94 | Pneumonia Legionella | Y |
| 17 | H | 35 | 33 | 15 | 1 | 17 | 12 | 96 | Pneumonia | N |
| 18 | H | 77 | 25 | 27 | 1 | 3 | 16 | 142 | Urological septic shock | Y |
| 19 | H | 25 | 23 | 9 | 1 | 9 | 16 | 89 | Pulmonary leukemic infiltration | Y |
| 20 | H | 41 | 26 | 15 | 1 | 14 | 14 | 98 | Pneumonia Varicella zoster | N |
| 21 | M | 35 | 39 | 14 | 1 | 28 | 18 | 95 | Pneumonia | Y |
| 52±18 | 28±6 | 18±6 | 11–4 | 139–18 | 14±2 | 101±17 |
Data expressed as the mean±standard deviation or median (percentiles 25–75).
APACHE: Acute Physiological and Chronic Health Evaluation; FiO2: inspiratory oxygen fraction; BMI: body mass index; SpO2: oxygen saturation measured by pulsioxymetry; MV: mechanical ventilation.
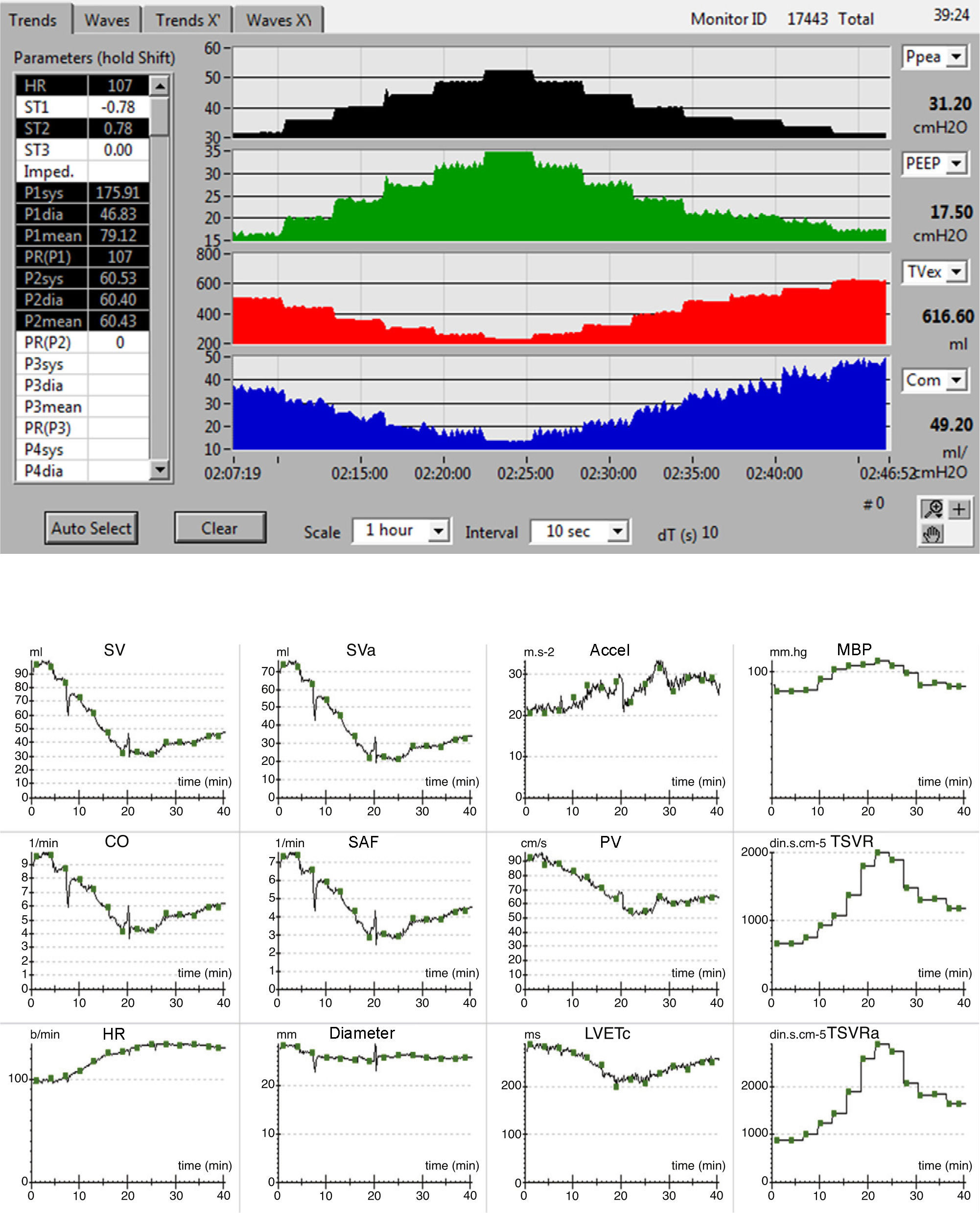
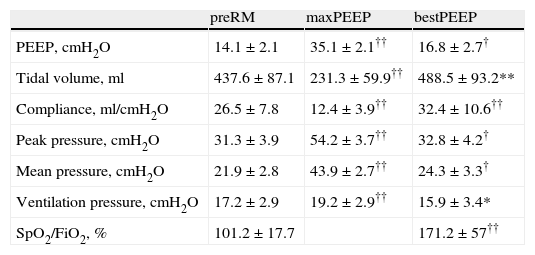
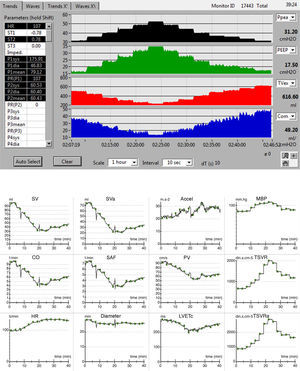
The evolution of the main respiratory parameters during LRM is reported in Table 2. Fig. 2A in turn shows the evolution of the respiratory parameters in one of the patients analyzed.
Respiratory changes during pulmonary recruitment maneuvering.
| preRM | maxPEEP | bestPEEP | |
| PEEP, cmH2O | 14.1±2.1 | 35.1±2.1†† | 16.8±2.7† |
| Tidal volume, ml | 437.6±87.1 | 231.3±59.9†† | 488.5±93.2** |
| Compliance, ml/cmH2O | 26.5±7.8 | 12.4±3.9†† | 32.4±10.6†† |
| Peak pressure, cmH2O | 31.3±3.9 | 54.2±3.7†† | 32.8±4.2† |
| Mean pressure, cmH2O | 21.9±2.8 | 43.9±2.7†† | 24.3±3.3† |
| Ventilation pressure, cmH2O | 17.2±2.9 | 19.2±2.9†† | 15.9±3.4* |
| SpO2/FiO2, % | 101.2±17.7 | 171.2±57†† |
Data expressed as the mean±standard deviation.
*P<0.05 vs preMR, **P<0.01 vs preMR, †P<0.001 vs preMR, ††P≤0.0001 vs preMR. bestPEEP: PEEP at maximum respiratory compliance; FiO2: inspiratory oxygen fraction; maxPEEP: maximum PEEP reached during recruitment maneuvering; PEEP: positive end-expiration pressure; PreRM: basal values, before start of recruitment maneuvering; SpO2: oxygen saturation measured by pulsioxymetry.
(A) Respiratory registry, from bottom to top: peak pressure, PEEP, tidal volume and respiratory compliance. (B) Hemodynamic registry: Accel: acceleration of aortic flow; CO: cardiac output; Diameter: aortic diameter; HR: heart rate; SAF: systolic aortic flow; LVETc: heart rate corrected left ventricle ejection time; MBP: mean blood pressure; SV: systolic volume; SVa: systolic volume, descending aorta; TSVR: total systemic vascular resistance; TSVRa: total vascular resistance of the descending aorta; PV: peak velocity.
The maximum PEEP level reached during maneuvering was 35.1±2.1cmH2O, with a maximum peak pressure of 54.2±3.7. The open-lung PEEP value was 16.8±2.7cmH2O, and was higher in obese patients (body mass index>30kg/m2): 19.6cmH2O (17.8–20.4) versus 15.7cmH2O (14.8–17.5; p<0.05). At maximum PEEP level, Crs decreased 52% with respect to the initial value (95%CI: 48.4–56.2%), while at the end of LRM it increased 23±15.2% (95%CI: 15.91–30.14%). In 16 patients (76%) this increase was ≥15% with respect to the basal value (15.3–56.3%), and in only 6 patients was it ≥30% (33.8–56.3%).
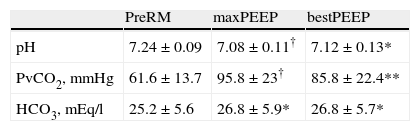
In the 15 patients subjected to central venous blood gas analysis, LRM induced or increased hypercapnia with respiratory acidosis during the maximum PEEP phase. After establishing the open-lung PEEP value, these alterations were maintained with respect to the initial values (Table 3), while the SpO2/FiO2 ratio increased significantly (Table 2).
Venous blood gas measurements during pulmonary recruitment maneuvering.
| PreRM | maxPEEP | bestPEEP | |
| pH | 7.24±0.09 | 7.08±0.11† | 7.12±0.13* |
| PvCO2, mmHg | 61.6±13.7 | 95.8±23† | 85.8±22.4** |
| HCO3, mEq/l | 25.2±5.6 | 26.8±5.9* | 26.8±5.7* |
n=15. Data expressed as the mean±standard deviation.
*P<0.01 vs preRM; **P<0.001 vs preRM; †P≤0.0001 vs preRM. bestPEEP: PEEP at maximum respiratory compliance; maxPEEP: maximum PEEP reached during recruitment maneuvering; PreRM: basal values, before start of recruitment maneuvering; PvCO2: venous CO2 pressure.
The hemodynamic effects during LRM are detailed in Table 4. The hemodynamic registry of one of the patients is shown in Fig. 2B.
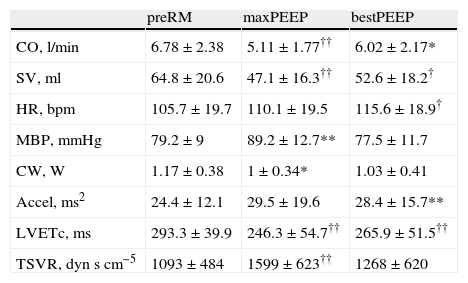
Hemodynamic changes during pulmonary recruitment maneuvering.
| preRM | maxPEEP | bestPEEP | |
| CO, l/min | 6.78±2.38 | 5.11±1.77†† | 6.02±2.17* |
| SV, ml | 64.8±20.6 | 47.1±16.3†† | 52.6±18.2† |
| HR, bpm | 105.7±19.7 | 110.1±19.5 | 115.6±18.9† |
| MBP, mmHg | 79.2±9 | 89.2±12.7** | 77.5±11.7 |
| CW, W | 1.17±0.38 | 1±0.34* | 1.03±0.41 |
| Accel, ms2 | 24.4±12.1 | 29.5±19.6 | 28.4±15.7** |
| LVETc, ms | 293.3±39.9 | 246.3±54.7†† | 265.9±51.5†† |
| TSVR, dynscm−5 | 1093±484 | 1599±623†† | 1268±620 |
Data expressed as the mean±standard deviation.
*P<0.05 vs preRM, **P<0.01 vs preRM, †P<0.001 vs preRM, ††P≤0.0001 vs preRM.
Accel: maximum acceleration of aortic flow; bestPEEP: PEEP at maximum respiratory compliance; CW: cardiac power; HR: heart rate; CO: cardiac output; LVETc: corrected left ventricle ejection time; maxPEEP: maximum PEEP reached during recruitment maneuvering; MBP: mean blood pressure; PreRM: basal values, before start of recruitment maneuvering; TSVR: total systemic vascular resistance; SV: systolic volume.
During the maximum PEEP phase, a decrease was recorded in CO, systolic volume (SV) and LVETc (23±18%, 26±18% and 16±13%, respectively) that persisted at the end of LRM despite the increase in heart rate and Accel. The MBP increased temporarily coinciding with the maximum PEEP phase, while cardiac power decreased during this LRM stage. Only one patient showed increased CO during the maneuver (patient 13).
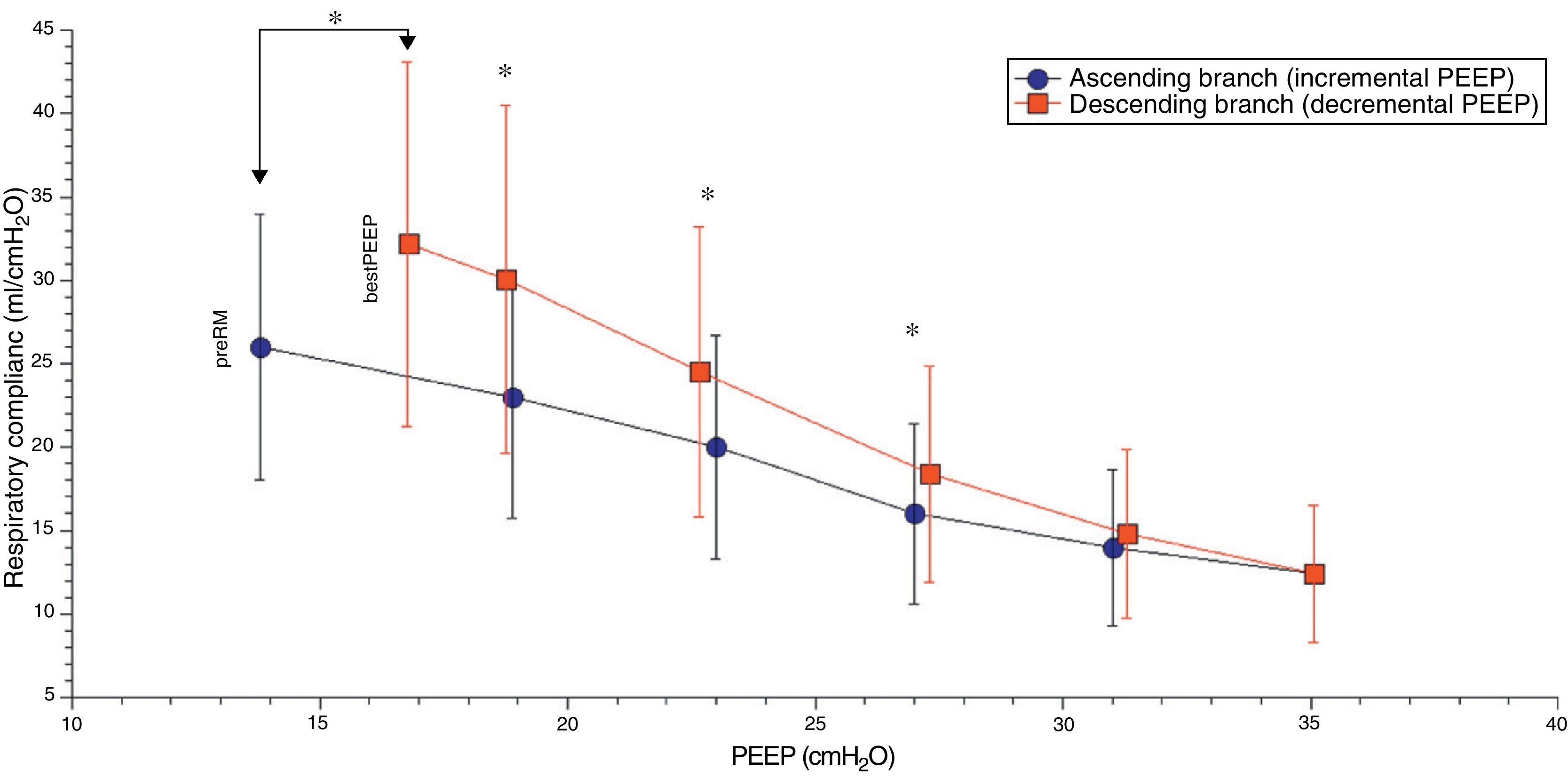
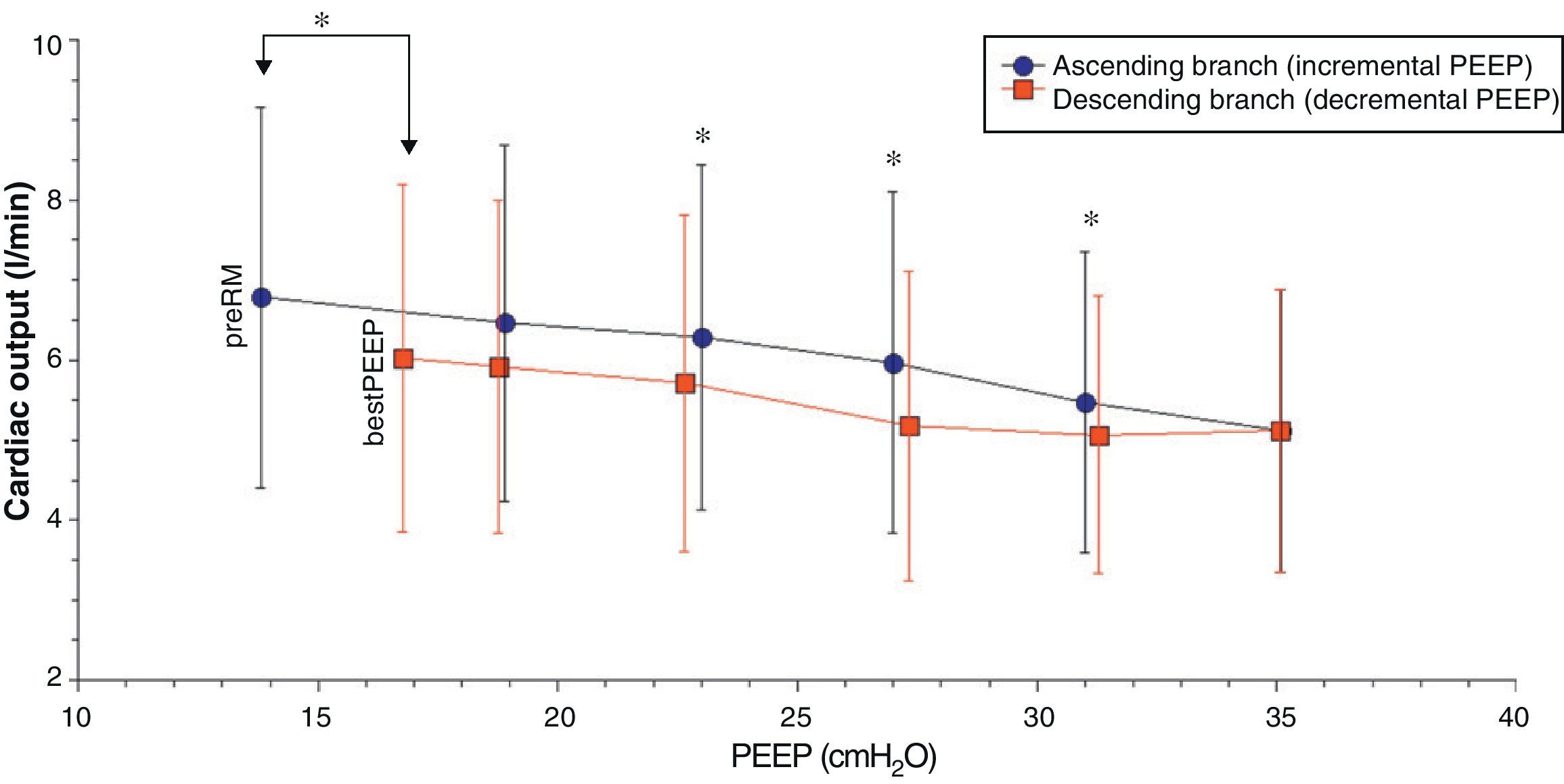
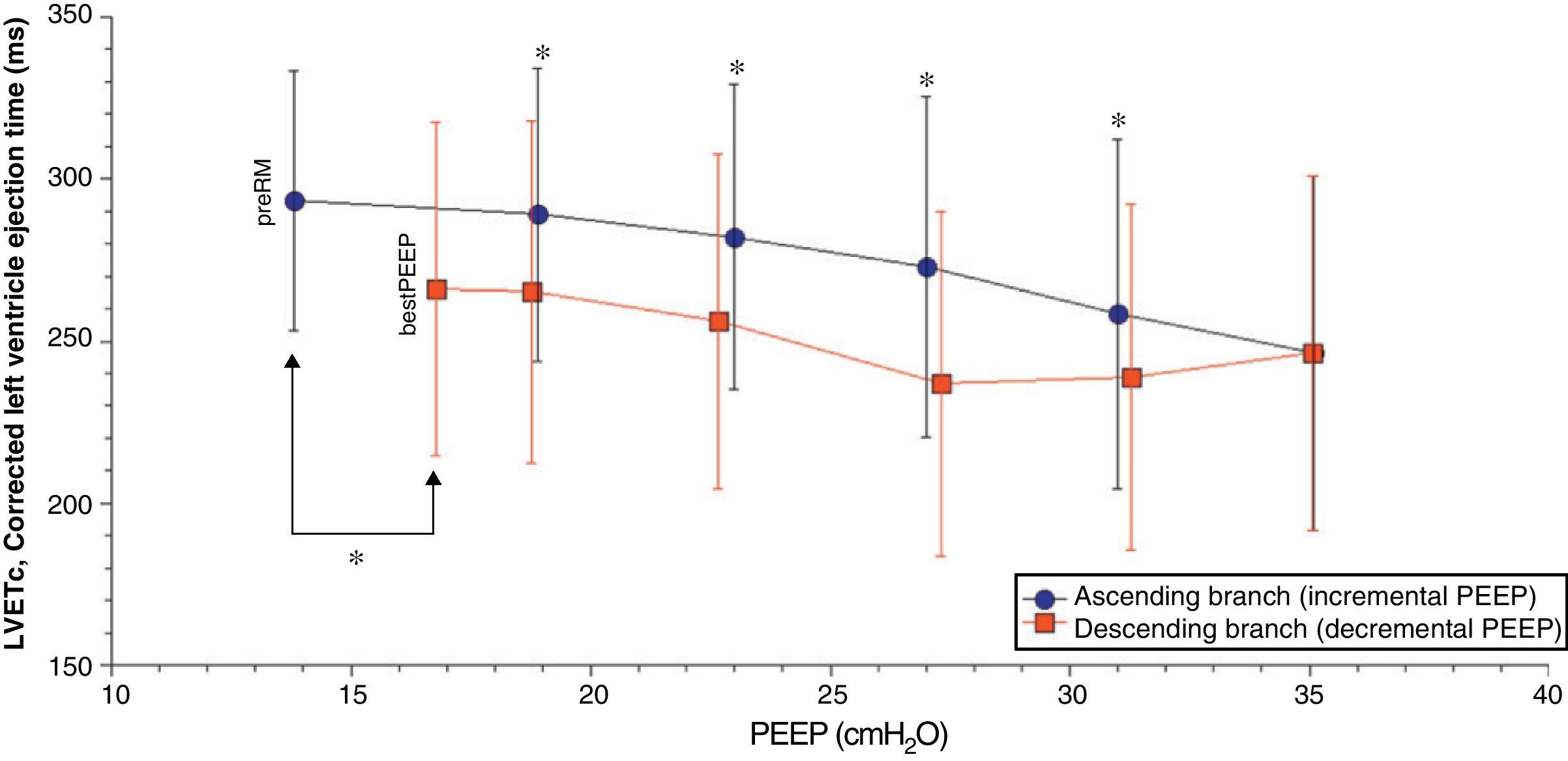
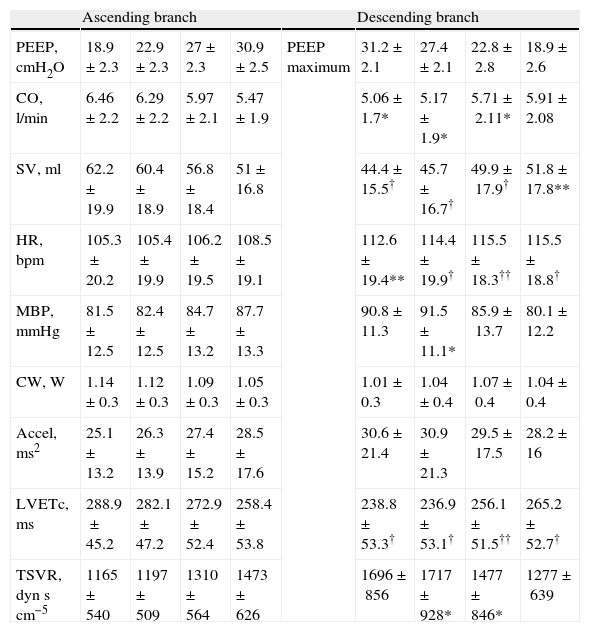
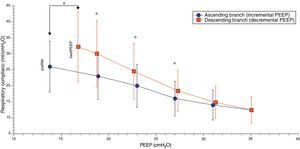
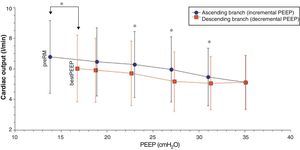
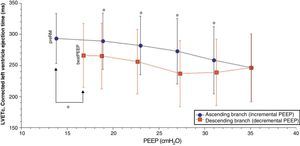
Comparison of the ascending and descending branches of recruitment maneuveringOn contrasting comparable PEEP levels in both LRM branches, with similar mean airway pressures, the Crs value was always greater over the descending branch (Fig. 3 and Table 5). From the hemodynamic perspective, the modification of LVETc, SV and CO was more pronounced over the descending branch, while heart rate was greater during this LRM stage (Figs. 4 and 5, and Table 6).
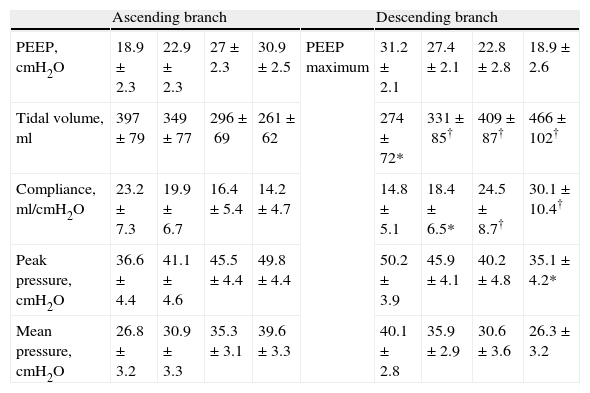
Respiratory values compared during the ascending and descending branches of recruitment maneuvering at comparable levels of PEEP.
| Ascending branch | Descending branch | ||||||||
| PEEP, cmH2O | 18.9±2.3 | 22.9±2.3 | 27±2.3 | 30.9±2.5 | PEEP maximum | 31.2±2.1 | 27.4±2.1 | 22.8±2.8 | 18.9±2.6 |
| Tidal volume, ml | 397±79 | 349±77 | 296±69 | 261±62 | 274±72* | 331±85† | 409±87† | 466±102† | |
| Compliance, ml/cmH2O | 23.2±7.3 | 19.9±6.7 | 16.4±5.4 | 14.2±4.7 | 14.8±5.1 | 18.4±6.5* | 24.5±8.7† | 30.1±10.4† | |
| Peak pressure, cmH2O | 36.6±4.4 | 41.1±4.6 | 45.5±4.4 | 49.8±4.4 | 50.2±3.9 | 45.9±4.1 | 40.2±4.8 | 35.1±4.2* | |
| Mean pressure, cmH2O | 26.8±3.2 | 30.9±3.3 | 35.3±3.1 | 39.6±3.3 | 40.1±2.8 | 35.9±2.9 | 30.6±3.6 | 26.3±3.2 | |
Data expressed as the mean±standard deviation.
*P<0.01 decremental vs incremental; **P<0.001 decremental vs incremental; †P≤0.0001 decremental vs incremental.
bestPEEP: PEEP at maximum respiratory compliance; maxPEEP: maximum PEEP reached during recruitment maneuvering; PEEP: positive end-expiratory pressure. Ventilation pressure=peak pressure−PEEP; PreRM: basal values, before start of recruitment maneuvering.
Hemodynamic values compared during the ascending and descending branches of recruitment maneuvering at comparable levels of PEEP.
| Ascending branch | Descending branch | ||||||||
| PEEP, cmH2O | 18.9±2.3 | 22.9±2.3 | 27±2.3 | 30.9±2.5 | PEEP maximum | 31.2±2.1 | 27.4±2.1 | 22.8±2.8 | 18.9±2.6 |
| CO, l/min | 6.46±2.2 | 6.29±2.2 | 5.97±2.1 | 5.47±1.9 | 5.06±1.7* | 5.17±1.9* | 5.71±2.11* | 5.91±2.08 | |
| SV, ml | 62.2±19.9 | 60.4±18.9 | 56.8±18.4 | 51±16.8 | 44.4±15.5† | 45.7±16.7† | 49.9±17.9† | 51.8±17.8** | |
| HR, bpm | 105.3±20.2 | 105.4±19.9 | 106.2±19.5 | 108.5±19.1 | 112.6±19.4** | 114.4±19.9† | 115.5±18.3†† | 115.5±18.8† | |
| MBP, mmHg | 81.5±12.5 | 82.4±12.5 | 84.7±13.2 | 87.7±13.3 | 90.8±11.3 | 91.5±11.1* | 85.9±13.7 | 80.1±12.2 | |
| CW, W | 1.14±0.3 | 1.12±0.3 | 1.09±0.3 | 1.05±0.3 | 1.01±0.3 | 1.04±0.4 | 1.07±0.4 | 1.04±0.4 | |
| Accel, ms2 | 25.1±13.2 | 26.3±13.9 | 27.4±15.2 | 28.5±17.6 | 30.6±21.4 | 30.9±21.3 | 29.5±17.5 | 28.2±16 | |
| LVETc, ms | 288.9±45.2 | 282.1±47.2 | 272.9±52.4 | 258.4±53.8 | 238.8±53.3† | 236.9±53.1† | 256.1±51.5†† | 265.2±52.7† | |
| TSVR, dynscm−5 | 1165±540 | 1197±509 | 1310±564 | 1473±626 | 1696±856 | 1717±928* | 1477±846* | 1277±639 | |
Data expressed as the mean±standard deviation.
*P<0.05 decremental vs incremental, **P<0.01 decremental vs incremental, †P≤0.001 decremental vs incremental, ††P≤0.0001 decremental vs incremental.
Accel: maximum acceleration of aortic flow; bestPEEP: PEEP at maximum respiratory compliance; CW: cardiac power; HR: heart rate; CO: cardiac output; LVETc: corrected left ventricle ejection time; maxPEEP: maximum PEEP reached during recruitment maneuvering; MBP: mean blood pressure; PEEP: positive end-expiratory pressure; PreRM: basal values, before start of recruitment maneuvering; TSVR: total systemic vascular resistance; SV: systolic volume.
In this study, lung recruitment maneuvering (LRM) comprising an ascending branch with progressive (stepwise) PEEP increments followed by a descending branch until reaching maximum respiratory system compliance (Crs) conditioned respiratory changes characterized by an important decrease in Crs at maximum PEEP level and an increase at the end of LRM with values higher than at the start, together with improvement of the SpO2/FiO2 ratio and a decrease in ventilation pressure. The hemodynamic changes fundamentally consisted of a decrease in CO, SV and LVETc, together with an increase in heart rate and contractility. At comparable PEEP levels and mean airway pressure, significant differences were observed between the two LRM branches, with more marked modification of left ventricle preload and CO over the descending branch.
The increase in Crs following LRM and its variations in the form of stepwise PEEP increments and decrements have been demonstrated in different clinical and experimental studies,21–24 and have been carefully detailed by Hickling in a theoretical mathematical model.25 According to the observations of this author, the changes in Crs during this kind of maneuver depend on: (a) the interaction between the number of alveoli that remain stable and inflated over the entire respiratory cycle; (b) the transalveolar pressure and degree of overdistension reached; and (c) the number of initially collapsed alveoli but which become inflated in the course of the inspiratory period (so-called “tidal recruitment”26). In our patients, the changes in Crs during LRM were similar to those predicted by the mathematical model of Hickling, with the exception that in our study the starting PEEP had been previously established according to the maximum Crs, though without prior opening maneuver. For this reason it is probable that in addition to the number of aerated stable alveoli and the transalveolar pressure reached, a non-negligible component of tidal recruitment could influence the Crs value before performing LRM. Likewise, the stepwise decrease in Crs with each PEEP increment after starting the maneuver could have been related to the gradual increase in transalveolar pressure and the successive decrease in tidal recruitment; accordingly, on reaching the upper transalveolar pressure limit, the lung overdistension value was maximum and tidal recruitment minimum or inexistent—this resulting in Crs at its lowest level. Lastly, during the descending phase, the stepwise decrease in transalveolar pressure gave rise to a gradual increase in Crs until reaching its maximum value, which in accordance to previous observations should coincide with the reinitiation of lung collapse.21,27 It is very likely that the explanation for the observed differences in Crs between both branches of the maneuver, at comparable PEEP levels, is the existence of a larger number of permanently aerated alveoli over the descending branch of the maneuver, which would support the efficacy of LRM.21,25
Together with an improvement in the SpO2/FiO2 ratio, another consequence of LRM was that the Crs increment allowed a reduction of ventilation pressure. The importance of this measure during open lung ventilation has already been underscored by Amato et al.,1,2 who established a value of <20cmH2O as one of the main objectives in their ventilation strategy. Conceptually, the relationship between plateau pressure, PEEP and tissue distension can be represented by a simple lever model in which PEEP represents the point of support or fulcrum, plateau pressure is the applied force, the degree of tissue stretch is the displacement reached, and ventilation pressure is the length of the lever torque arm.10 According to this model, on increasing the PEEP level without increasing plateau pressure—as occurred in our patients—LRM decreases the ventilation pressure and consequently also the length of the lever arm and tissue stretch, thereby exerting a favorable influence upon the course of lung damage.2,3
Although the effects and depth of the hemodynamic deterioration of LRM appear to be related to a series of factors, including the basal cardiovascular situation,28–30 the origin and time elapsed from lung injury,17 the type of maneuver employed31 and its duration,29 etc., the marked but generally transient drop in CO is the hemodynamic effect most often reported in different clinical17,32,33 and experimental studies.16,21,28,29,31 In contrast, however, some authors have observed no significant hemodynamic alterations.22,34 On the other hand, the cardiovascular consequences of LRM fundamentally have been attributed to a decrease in venous return and cardiac preloadm,28,32 as well as to an increase in right ventricle afterload22,28—the relative importance of each of these mechanisms being lesser or greater depending on the predominant effect of LRM upon transpulmonary or pleural pressure.35
The results of our study are consistent with the hemodynamic alterations described, with a decrease in CO (of up to 58% in some cases) and left ventricle preload, as assessed from the monitorization of LVETc. However, since right ventricle function was not evaluated, we cannot be sure whether these alterations were fundamentally due to changes in preload or in postload of the right ventricle. Nevertheless, the unequal behavior of Crs over both LRM branches at a comparable PEEP level and mean pressure suggests that transpulmonary pressure was also different in each stage of the maneuver, and therefore that the effect upon right ventricle afterload could be the most relevant factor over the ascending branch, while the decrease in venous return and right ventricle preload as a result of increased transmission to the pleural space could be the most important mechanism over the descending branch of LRM.
In accordance with the observations of previous studies, our results also confirm the scant usefulness of blood pressure as an exclusive parameter for monitoring the hemodynamic consequences of LRM.16,22,31,33,34 In effect, during the end stage of the ascending branch, MBP increased significantly, behaving in a way opposite to CO, and therefore without reflecting the global effect of LRM upon the cardiocirculatory system. Moreover, and curiously, despite the increase in MBP, cardiac power (a parameter assessing the efficiency of the heart as a hydraulic pump, combining simultaneous measurement of flow and pressure)36 decreased significantly over the ascending branch. However, since this parameter appears to be influenced not only by contractility but also by the changes in preload and heart rate,36 we do not know the precise significance of its evolution during LRM, since there were marked changes in these latter parameters during maneuvering. Nevertheless, since acceleration of aortic flow and heart rate increased during the maneuver, we suspect that the changes in cardiac power could be related mainly to worsened preload, and therefore that heart function of the patients was preserved.
On the other hand, although blood gas measurements were not obtained in each PEEP step or in all patients, it can be assumed that progressive hypoventilation with hypercapnia was an accompanying phenomenon during LRM that could have influenced the observed hemodynamic effects. Although there is some controversy regarding the principal hemodynamic effects of acute hypercapnia, they appear to comprise the generation of a hyperdynamic state (high CO, elevated heart rate and lessened systemic vascular resistances), as well as an increase in pulmonary artery pressure that could negatively affect right ventricle function.37–39 It is therefore very probable that all these mechanisms were implicated in the end hemodynamic effect of LRM, and that the combined result of these factors contributed in some way and degree to the observed cardiovascular consequences.
Before drawing conclusions, the present study has important limitations that must be commented. Firstly, this is a retrospective analysis with a limited number of patients—the main cause of respiratory failure being pulmonary infection. It is well known that lung consolidation predominates over collapse in pneumonia. As a result, the recruitment potential is lower than in other forms of respiratory failure, particularly cases of extrapulmonary origin.40 In addition, in the opinion of some investigators, the hemodynamic effects of LRM could be more serious in cases of lung injury induced by an infectious process.31 Lastly, although the hemodynamic monitorization used in this study, in contrast to the intravascular pressures, offers a certain advantage in that it does not interfere with the changes in intrathoracic pressure,19 the behavior of the right-side heart chambers (which are those most affected during LRM, and therefore the main causes of the hemodynamic alterations) could not be evaluated in this study. Despite these limitations, we consider that the study offers relevant information for clinicians wishing to use this form of LRM. Firstly, the maneuver was useful not only for improving oxygenation but also for facilitating selection of open-lung PEEP and lowering lung ventilation pressure. Secondly, since the hemodynamic consequences, and particularly the decrease in CO, were significant and in some cases notorious, we consider that it is not enough to monitor blood pressure during LRM. In this context, careful hemodynamic monitorization should be ensured, if possible using devices that do not generate artifacts with changes in intrathoracic pressure, and which moreover inform us of the course of right ventricle function.
In conclusion, LRM with stepwise increments and decrements in PEEP facilitated open-lung PEEP on the basis of maximum Crs, improving oxygenation and reducing lung ventilation pressure—the main hemodynamic consequence being a reduction in left ventricle preload and cardiac output. The unequal hemodynamic derangement in both LRM branches at comparable PEEP levels and mean airway pressure values in addition showed that apart from intrathoracic pressure, other factors such as Crs and probably hypoventilation with hypercapnia influenced the hemodynamic consequences of this type of maneuver. Lastly, since these alterations were intense and were not reflected in the mean blood pressure changes, it is necessary to emphasize the importance of adequate hemodynamic monitorization, beyond the simple monitoring of blood pressure.
Conflicts of interestM. Ignacio Monge-Garcia is a consultant to Edwards Lifesciences. The rest of the authors declare no conflicts of interest.
Please cite this article as: Monge García MI, et al. Cambios respiratorios y hemodinámicos durante una maniobra de reclutamiento pulmonar mediante incrementos y decrementos progresivos de PEEP. Med Intensiva. 2012:36:77–88.