To determine the risk factors for severe acute respiratory failure requiring invasive mechanical ventilation (SARF-MV) and its effect upon clinical outcomes in critically ill cancer patients.
DesignA retrospective cohort study was carried out.
SettingA 12-bed oncological intensive care unit (ICU) from January 2014 to December 2015.
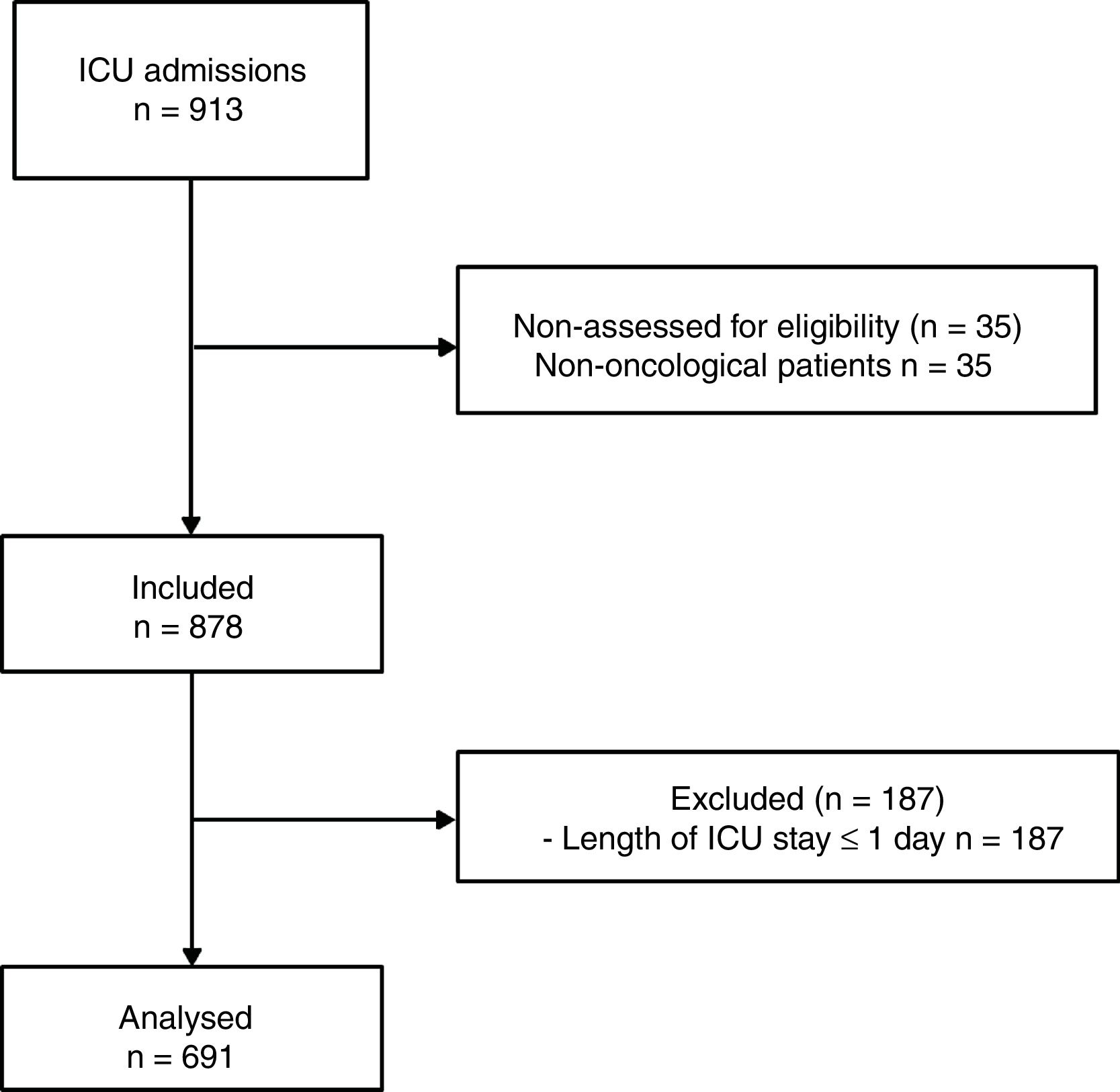
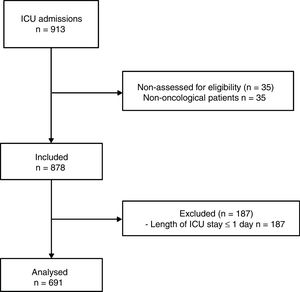
PatientsA total of 878 consecutive cancer patients were included. Patients with an ICU stay of ≤1 day were excluded. The final sample size was 691 patients.
InterventionsNone.
VariablesClinical variables at ICU admission were extracted from the medical records. The primary outcome was SARF-MV. We also measured ICU and hospital mortality, as well as length of stay.
ResultsThe SARF-MV rate was 15.8%. The multivariate analysis identified brain tumour (OR 14.54; 95%CI 3.86–54.77; p<0.0001), stage IV cancer (OR 3.47; 95%CI 1.26–9.54; p=0.016), sepsis upon admission (OR 2.28; 95%CI 1.14–4.56; p=0.020) and an APACHE II score≥20 points (OR 5.38; 95%CI 1.92–15.05; p=0.001) as being independently associated to SARF-MV. Compared with the patients without SARF-MV, those with SARF-MV had a prolonged length of ICU stay (p<0.0001), a lower ICU survival rate (p<0.0001) and a lower hospital survival rate (p<0.0001).
ConclusionsA number of clinical factors are related to SARF-MV. In this regard, SARF-MV is a powerful factor independently correlated to poor outcomes. Future studies should investigate means for preventing SARF-MV in critically ill cancer patients, which may have an impact upon outcomes.
Determinar los factores de riesgo para insuficiencia respiratoria grave que requiere ventilación mecánica (IRG-VM) y sus efectos sobre los resultados clínicos en pacientes críticos con cáncer.
DiseñoEstudio de cohorte retrospectivo.
ContextoDesde enero de 2014 a diciembre de 2015 en una unidad de cuidados intensivos (UCI) oncológicos de 12 camas.
PacientesSe incluyeron consecutivamente 878 pacientes. Se excluyeron aquellos con una estancia en UCI≤un día. Finalmente la muestra fue de 691.
IntervencionesNinguna.
VariablesDe los registros médicos se extrajeron las variables clínicas a la admisión en UCI. La variable de respuesta primaria fue la IRG-VM. También se analizó la mortalidad y estancia en UCI/hospitalaria.
ResultadosLa tasa de IRG-VM fue del 15,8%. En el análisis multivariado el tumor cerebral (OR 14,54; IC 95% 3,86-54,77; p<0,0001), la etapa IV del cáncer (OR 3,47; IC 95% 1,26-9,54; p=0,016), la sepsis (OR 2,28; IC 95% 1,14-4,56; p=0,020) y la escala APACHE II≥20 puntos (OR 5,38; IC 95% 1,92-15,05; p=0,001) fueron factores de riesgo independientes de IRG-VM. La IRG-VM se asoció con una mayor estancia en la UCI (p<0,0001), así como con una menor tasa de supervivencia en UCI (p<0,0001) y hospitalaria (p<0,0001).
ConclusionesAlgunos factores clínicos se relacionan con la IRG-VM. Este trastorno es un factor que se relaciona poderosamente con un peor pronóstico. Se requieren estudios futuros que investiguen las formas de prevención de la IRG-VM en los pacientes oncológicos críticos, lo cual podría tener un impacto en los resultados.
Acute respiratory failure (ARF) is a frequent disorder in cancer patients, commonly caused by several conditions including local effect of tumour, pulmonary infiltrates, pneumonia, acute respiratory distress syndrome and congestive heart failure. Supplemental oxygen and treatment of the underlying disorder is the mainstay of treatment for ARF, but severe cases require ventilatory support. Indeed, the need of this treatment modality is one of most frequent reasons for admission in the intensive care unit (ICU) for critically ill cancer patients.1–3
Mechanical ventilation (MV) is a primary method of organic function support in critically ill cancer patients treated in ICU, usually indicating a severe ARF (SARF). However, despite significant advances in ventilatory support and cancer management, MV remains associated with a high ICU, hospital and long-term mortality rate.4,5 In addition, the need of MV because of postoperative respiratory failure in operated cancer patients has been reported as high as 37%,6 which is associated with unfavourable outcomes as well.6,7
Although some previous studies have reported an association between SARF and VM with mortality in critically ill cancer patients, this relation is not clear because of methodological designs, participants’ characteristics and limitations of the studies. Conversely, the risk factors for SARF have not been consistently investigated in this type of patients. The study was aimed: 1) to identify the risk factors for severe acute respiratory failure requiring invasive mechanical ventilation (SARF-MV); and 2) to determine the effects of SARF-MV on clinical outcomes in cancer patients admitted in an oncological ICU.
Patients and methodsDesign and settingThis was a retrospective cohort study conducted in the oncological ICU of the Institute of Oncology and Radiobiology (IOR). This is a 220-bed, university-affiliated, tertiary care referral centre for cancer patients in Havana, Cuba. The ICU has 12 beds and provides care for about 500 medical and surgical cancer patients per year. Data source was the prospective database of the ICU and hospital records (January 2014–December 2015). The study was approved by the Scientific Council and the Ethics Committee for Scientific Research of the IOR. Informed consent was waivered because of the retrospective nature of the study. It was conducted in accordance with the Declaration of Helsinki.
ParticipantsOver the study period, a total of 878 consecutive cancer patients were included. Patients with a length of ICU stay ≤1 day were excluded (Fig. 1).
For those patients who were admitted more than once to the ICU during the same hospitalization, only the first data on ICU admission were analyzed. To be admitted, the oncologist and the ICU physician generally were agreed that patients had a potential chance of recovering from the acute problem. In contrast, patients without any further oncology specific treatment options were offered end-of-life care on the referring ward and not transferred to the ICU.
Data collection and outcomesThe following variables on ICU admission were extracted from the medical records: age, sex, Age-Adjusted Charlson Comorbidity Index score with the exclusion of malignancy,8 primary tumour location, clinical stage of cancer,9 patient origin (hospital ward or emergency department), malignancy-unrelated admission, type of admission (medical or surgical), emergency surgery, Acute Physiology and Chronic Health Evaluation (APACHE) II score, transfusion of blood products within 15 days prior to ICU admission, sepsis according to the Surviving Sepsis Campaign,10 and adverse event to chemotherapy as cause for admission in ICU.
The outcome of interest was the SARF-MV, defined as the need of invasive mechanical ventilation because of the presence of respiratory distress symptoms such as tachypnea (respiratory rate>30min–1), an arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ratio<300mmHg or hypercapnia with abnormal mental status. We also assessed the effect of SARF-MV on clinical outcomes such as length of ICU and hospital stay, as well as ICU and hospital mortality.
Statistical analysisCategorical variables are showed as count with percentage and numerical variables as median with 25th to 75th interquartile range (IQR). Difference between groups was performed using Pearson's chi-square test (χ2) or Fisher's exact test as appropriated for categorical variables; because of lack of normality, the Mann–Whitney U test was used for numerical variables.
Multivariate logistic regression model was used to identify risk factors associated with SARF-MV. Explanatory variables yielding p-values≤0.05 by univariate analysis and those clinically relevant were considered to enter in the initial model. Parsimony of the model was guaranteed. Because of lack of normality, numerical variables were stratified according to the analysis of the functional form and clinical significance. Development of model was based on backward stepwise (likelihood ratio) significance testing (p-value to enter: 0.10; p-value to remove: 0.15). The Hosmer–Lemeshow test was used to check the goodness of fit. Discrimination capability was evaluated by determination of the area under the receiver operating characteristic (ROC) curve. Results were summarized as odds ratio (OR) and respective 95% confidence interval (CI).
The survival was estimated by the Kaplan–Meier method. The follow-up time was marked by the date of ICU admission and by the date of hospital discharge. The survival rate was compared using the log-rank test.
Statistical test with a two tailed p-value≤0.05 was considered as significant. Data were analyzed using IBM® SPSS® Statistics 21.0 (IBM, Chicago, IL, USA).
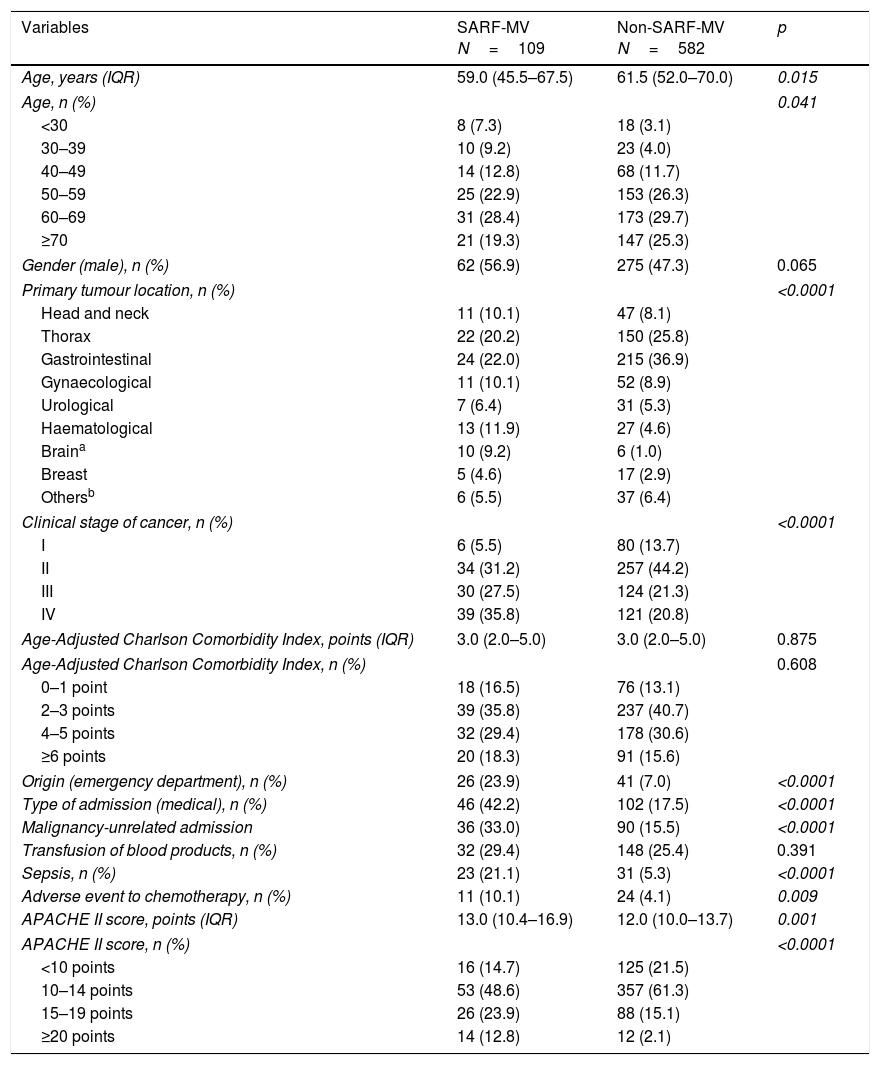
ResultsCharacteristics of study populationA total of 691 patients were analyzed (Fig. 1). The main characteristics of study population are depicted in Table 1. Patients with history of chronic disease were 468 (67.7%). The median in the Age-Adjusted Charlson Comorbidity Index score was 3.0 points (IQR 2.0–5.0 points); a score≥2 points was observed in 86.4% of subjects.
Univariate analysis of factors associated with severe acute respiratory failure requiring invasive mechanical ventilation.
| Variables | SARF-MV N=109 | Non-SARF-MV N=582 | p |
|---|---|---|---|
| Age, years (IQR) | 59.0 (45.5–67.5) | 61.5 (52.0–70.0) | 0.015 |
| Age, n (%) | 0.041 | ||
| <30 | 8 (7.3) | 18 (3.1) | |
| 30–39 | 10 (9.2) | 23 (4.0) | |
| 40–49 | 14 (12.8) | 68 (11.7) | |
| 50–59 | 25 (22.9) | 153 (26.3) | |
| 60–69 | 31 (28.4) | 173 (29.7) | |
| ≥70 | 21 (19.3) | 147 (25.3) | |
| Gender (male), n (%) | 62 (56.9) | 275 (47.3) | 0.065 |
| Primary tumour location, n (%) | <0.0001 | ||
| Head and neck | 11 (10.1) | 47 (8.1) | |
| Thorax | 22 (20.2) | 150 (25.8) | |
| Gastrointestinal | 24 (22.0) | 215 (36.9) | |
| Gynaecological | 11 (10.1) | 52 (8.9) | |
| Urological | 7 (6.4) | 31 (5.3) | |
| Haematological | 13 (11.9) | 27 (4.6) | |
| Braina | 10 (9.2) | 6 (1.0) | |
| Breast | 5 (4.6) | 17 (2.9) | |
| Othersb | 6 (5.5) | 37 (6.4) | |
| Clinical stage of cancer, n (%) | <0.0001 | ||
| I | 6 (5.5) | 80 (13.7) | |
| II | 34 (31.2) | 257 (44.2) | |
| III | 30 (27.5) | 124 (21.3) | |
| IV | 39 (35.8) | 121 (20.8) | |
| Age-Adjusted Charlson Comorbidity Index, points (IQR) | 3.0 (2.0–5.0) | 3.0 (2.0–5.0) | 0.875 |
| Age-Adjusted Charlson Comorbidity Index, n (%) | 0.608 | ||
| 0–1 point | 18 (16.5) | 76 (13.1) | |
| 2–3 points | 39 (35.8) | 237 (40.7) | |
| 4–5 points | 32 (29.4) | 178 (30.6) | |
| ≥6 points | 20 (18.3) | 91 (15.6) | |
| Origin (emergency department), n (%) | 26 (23.9) | 41 (7.0) | <0.0001 |
| Type of admission (medical), n (%) | 46 (42.2) | 102 (17.5) | <0.0001 |
| Malignancy-unrelated admission | 36 (33.0) | 90 (15.5) | <0.0001 |
| Transfusion of blood products, n (%) | 32 (29.4) | 148 (25.4) | 0.391 |
| Sepsis, n (%) | 23 (21.1) | 31 (5.3) | <0.0001 |
| Adverse event to chemotherapy, n (%) | 11 (10.1) | 24 (4.1) | 0.009 |
| APACHE II score, points (IQR) | 13.0 (10.4–16.9) | 12.0 (10.0–13.7) | 0.001 |
| APACHE II score, n (%) | <0.0001 | ||
| <10 points | 16 (14.7) | 125 (21.5) | |
| 10–14 points | 53 (48.6) | 357 (61.3) | |
| 15–19 points | 26 (23.9) | 88 (15.1) | |
| ≥20 points | 14 (12.8) | 12 (2.1) | |
APACHE: acute physiology and chronic health evaluation; IQR: 25th to 75th interquartile rank; SARF-MV: severe acute respiratory failure requiring invasive mechanical ventilation.
Postoperative care was the most common cause for admission in ICU (78.6%). Gastrointestinal tract (46.6%) and thorax (27.4%) were the most frequent sites for surgical interventions. The emergency surgery was carried out in 5.5% of the operated patients. The most common causes for admission in ICU in medical patients were acute respiratory failure (32.4%), infections (30.4%), cardiovascular disorders (19.6%), neurological disorders (9.5%), and others (8.1%).
Probability of death estimated by APACHE II was 11.7% (IQR 8.5–18.5%). Eighty-one patients (11.7%) required vasopressors during their stay in ICU. Thirty-four patients (4.9%) were re-admitted to ICU during the same hospitalization.
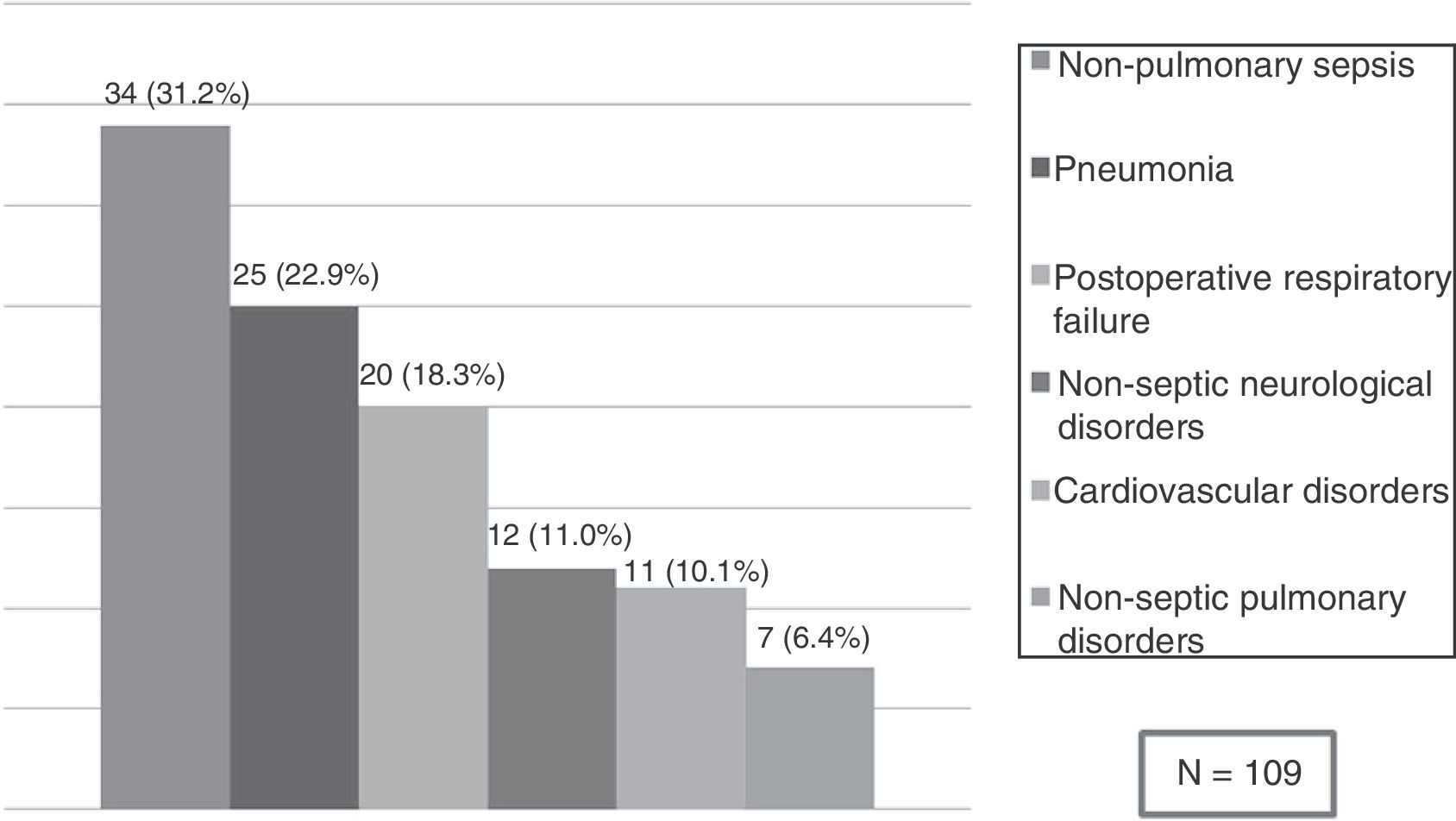
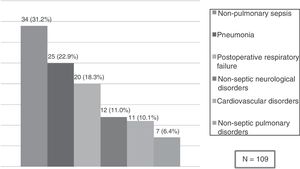
Risk factors for SARF-MVA total of 109 patients (15.8%) developed SARF-MV. The SARF-MV rate was 31.1% for medical patients and 11.6% for surgical patients. ARF secondary to non-pulmonary sepsis (31.2%) such as peritonitis (25 cases), urinary tract infection (two cases), soft tissue infection (six cases) and central nervous system infection (one case), as well as pneumonia (22.9%) were the main causes of SARF-MV. Other causes were postoperative respiratory failure (18.3%) because of difficult weaning (seven cases), circulatory instability (six), atelectasis (two cases) or volume overload (five cases); non-septic neurological disorders (11.0%) such as brain metastasis (four cases), ischaemic stroke (five cases) or brain haemorrhage (three cases); cardiovascular disorders (10.1%) such as acute pulmonary embolism (four cases), acute myocardial infarction (three cases), acute pulmonary oedema (two cases) or haemorrhage (two cases); and non-septic pulmonary disorders (6.4%) such as aspiration (two cases) or acute respiratory distress syndrome because of pancreatitis (one case) and drugs reaction (four cases) (Fig. 2).
In the univariate analysis, primary tumour location, clinical stage of cancer, admission from the emergency department, medical admission, malignancy-unrelated admission, sepsis, adverse event to chemotherapy, and APACHE II score were related with SARF-MV (Table 1).
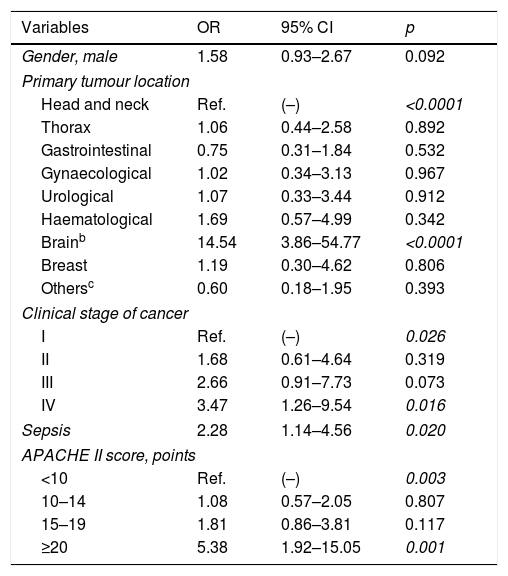
In multivariate logistic regression analysis, brain tumour, stage IV-cancer, sepsis at ICU admission, and APACHE II score≥20 points were independently associated with increased risk of SARF-MV (Table 2).
Results of multivariate logistic regression analysisa of factors associated with severe acute respiratory failure requiring invasive mechanical ventilation.
| Variables | OR | 95% CI | p |
|---|---|---|---|
| Gender, male | 1.58 | 0.93–2.67 | 0.092 |
| Primary tumour location | |||
| Head and neck | Ref. | (–) | <0.0001 |
| Thorax | 1.06 | 0.44–2.58 | 0.892 |
| Gastrointestinal | 0.75 | 0.31–1.84 | 0.532 |
| Gynaecological | 1.02 | 0.34–3.13 | 0.967 |
| Urological | 1.07 | 0.33–3.44 | 0.912 |
| Haematological | 1.69 | 0.57–4.99 | 0.342 |
| Brainb | 14.54 | 3.86–54.77 | <0.0001 |
| Breast | 1.19 | 0.30–4.62 | 0.806 |
| Othersc | 0.60 | 0.18–1.95 | 0.393 |
| Clinical stage of cancer | |||
| I | Ref. | (–) | 0.026 |
| II | 1.68 | 0.61–4.64 | 0.319 |
| III | 2.66 | 0.91–7.73 | 0.073 |
| IV | 3.47 | 1.26–9.54 | 0.016 |
| Sepsis | 2.28 | 1.14–4.56 | 0.020 |
| APACHE II score, points | |||
| <10 | Ref. | (–) | 0.003 |
| 10–14 | 1.08 | 0.57–2.05 | 0.807 |
| 15–19 | 1.81 | 0.86–3.81 | 0.117 |
| ≥20 | 5.38 | 1.92–15.05 | 0.001 |
APACHE: acute physiology and chronic health evaluation; CI: confidence interval; OR: odds ratio; Ref.: reference category.
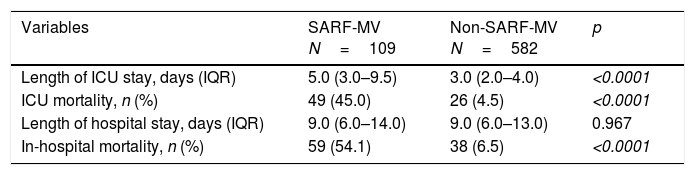
The overall ICU and hospital mortality rate was 10.9% and 14.0%, respectively. Median length of ICU and hospital stay was 3.0 days (IQR 2.0–4.0 days) and 9.0 days (IQR 6.0–13.0 days), respectively. Compared with patients without respiratory failure, those with SARF-MV had a prolonged length of ICU stay (Mann–Whitney U test, p<0.0001), an increased ICU mortality rate (χ2=155.53; p<0.0001), and an increased hospital mortality rate (χ2=172.37; p<0.0001) (Table 3).
Outcomes of patients with severe acute respiratory failure requiring invasive mechanical ventilation.
| Variables | SARF-MV N=109 | Non-SARF-MV N=582 | p |
|---|---|---|---|
| Length of ICU stay, days (IQR) | 5.0 (3.0–9.5) | 3.0 (2.0–4.0) | <0.0001 |
| ICU mortality, n (%) | 49 (45.0) | 26 (4.5) | <0.0001 |
| Length of hospital stay, days (IQR) | 9.0 (6.0–14.0) | 9.0 (6.0–13.0) | 0.967 |
| In-hospital mortality, n (%) | 59 (54.1) | 38 (6.5) | <0.0001 |
IQR: 25th to 75th interquartile rank; ICU: intensive care unit; SARF-MV: severe acute respiratory failure requiring invasive mechanical ventilation.
The median ICU and hospital survival time of the total cohort was 14.0 days (95% CI 11.4–16.6 days) and 39.0 days (95% CI 34.6–43.4 days), respectively. Of the 109 patients with SARF-MV, 49 died in ICU. Of the 49 patients, 27 died from septic shock, 10 from respiratory failure, six from neurological disorders, five from cardiogenic shock, and one from hypovolemic shock due to haemorrhage. Therefore, the ICU mortality rate for SARF-MV patients was 45.0%, with a median ICU survival time of 11.0 days (95% IC 8.6–13.4 days). Additionally, other 10 patients with SARF-MV died in hospital wards after being transferred from ICU; of them, eight patients died from septic shock and two from acute cardiovascular problems, so the hospital mortality rate for SARF-MV patients was 54.1%, with a median hospital survival time of 14.0 days (95% CI 11.2–16.8 days).
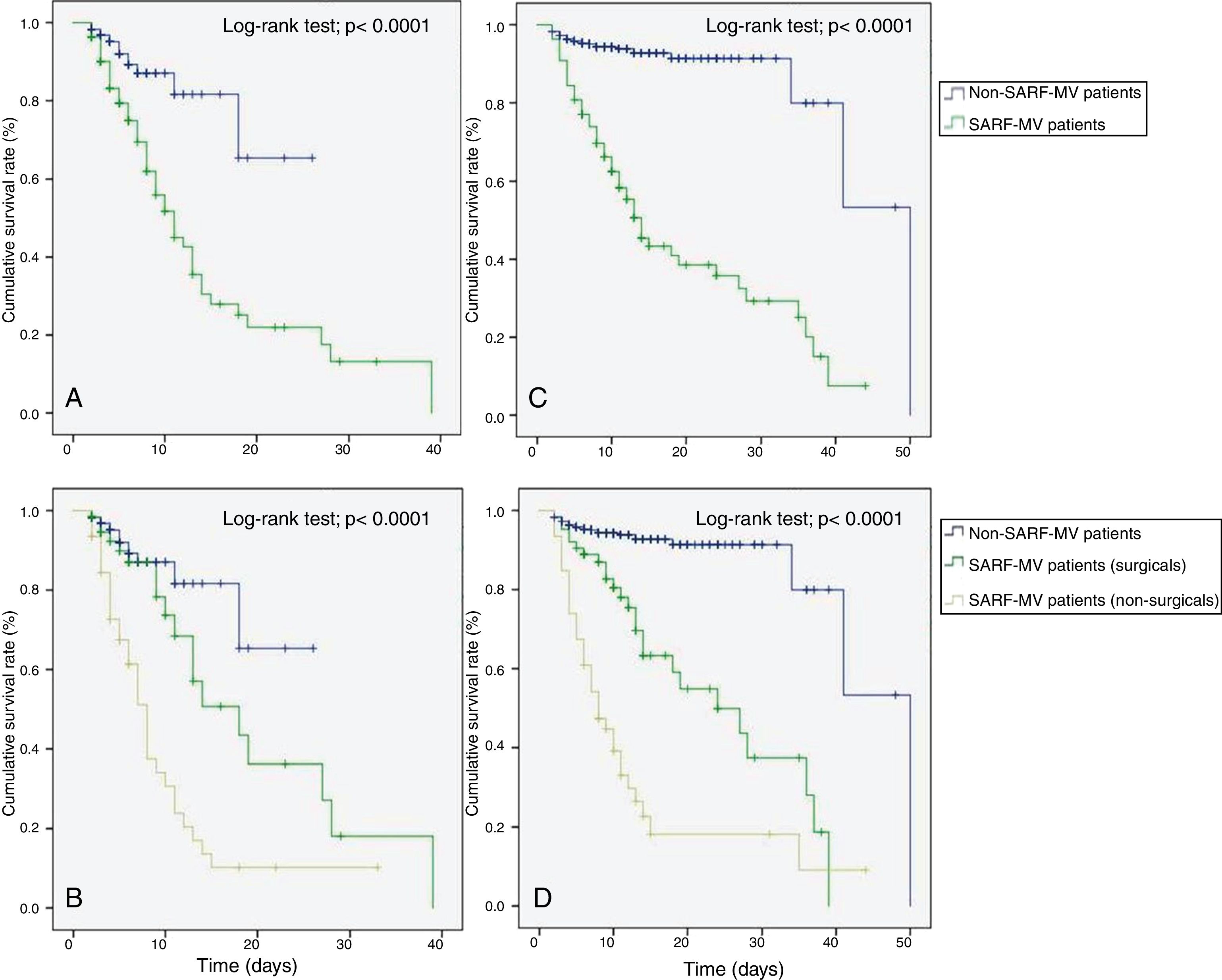
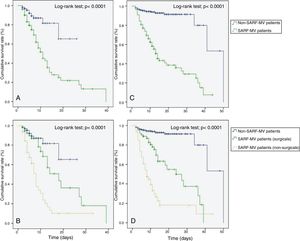
The Kaplan–Meier method showed that patients with SARF-MV had a decreased ICU (Log-rank test=21.75; p<0.0001) and hospital survival rate (Log-rank test=128.91; p<0.0001) compared with patients without SARF-MV (Fig. 3A and C). In a subgroup analysis, both surgical and non-surgical patients with SARF-MV had a decreased ICU (Log-rank test=43.19; p<0.0001) and hospital survival rate (Log-rank test=176.06; p<0.0001) compared with patients without SARF-MV (Fig. 3B and D).
Kaplan–Meier analysis depicting the impact of severe acute respiratory failure requiring invasive mechanical ventilation on intensive care unit survival – all patients (A), intensive care unit survival – subgroup patients (B), hospital survival – all patients (C), and hospital survival – subgroup patients (D). SARF-MV: severe acute respiratory failure requiring invasive mechanical ventilation.
This retrospective analysis showed that patients with cancer admitted to the ICU had a SARF-MV rate of 15.8%. The need of MV was higher in others studies, accounting for 35–50% of critically ill cancer patients.4,11,12 These differences with our results appear to derive from differences in the composition of the studied participants. In our study, most patients were admitted to the ICU for elective postoperative care. However, SARF-MV rate in medical patients was comparable to those reported in the international cohorts.13,14
We found that brain tumour, stage IV of cancer, sepsis at ICU admission and APACHE II score≥20 points were independently associated with SARF-MV. There are not studies in the literature evaluating risk factors for SARF-MV in critically ill cancer patients, so that this is the first study conducted under that purpose.
We observed an advanced cancer (tumour stage III or IV) rate of 63.3% for SARF-MV patients. Other authors found similar results. From 53 terminal cancer patients, Singh et al. found a MV rate of 77%.15 In patients with advanced solid cancer admitted to ICU, Xia and Wang observed that 57.4% of them required invasive MV.16 Thus, advanced oncological disease may be related with SARF-MV. This association is explained by several factors such as primary or metastatic pulmonary infiltrates, infectious complication secondary to immunosuppression, organ dysfunction, respiratory muscle weakness due to malnutrition, and drug-induced lung toxicity,4,17–19 all of which can predispose to SARF-MV.
Tumour site such as brain or lung can be a factor related with the need of MV in critically ill cancer patients, which is mainly explained by the local effect of the tumour. Patients with primary lung cancer or secondary pulmonary infiltrates are at high risk of MV as reported in previous studies.3,4 Pulmonary infiltrates predispose to infection, atelectasis, hemoptysis, pulmonary oedema, and pleural effusion,4,5 with gas exchange disturbances and a high respiratory muscles work increasing the risk of SARF-MV. On the other hand, we identified that brain tumour was associated with SARF-MV. The most frequent intracranial brain tumours are brain metastases, and all types of cancer can develop this complication. Primary o secondary brain tumours cause brain oedema, intracranial hypertension, alteration in consciousness level, and need of MV.20 In addition, some intervention such as neurosurgery or chemotherapy also would explain the higher rates of MV. Yutaka et al. observed that neurosurgery for brain tumour was associated with the need of MV.21 Flexman et al. reported similar results.22 Chemotherapy may have detrimental effects on central nervous system. Central nervous system neurotoxicity resulting from chemotherapy manifests as a wide range of clinical syndromes including acute, subacute, and chronic encephalopathies, posterior reversible encephalopathy, acute cerebellar dysfunction, chronic cognitive impairment, myelopathy, meningitis, and neurovascular syndromes.23 So that, MV would be required as supportive therapy for these clinical entities, which vary by causative agent, degree of severity, evolution, and timing of occurrence.
Sepsis affects the respiratory muscle because of muscular autophagy, dysfunction, decreased synthesis, and degradation of contractile proteins.24,25 More than fifteen years ago, Soares et al. identified that 63% of cancer patients requiring mechanical ventilatory support for more than 24h had sepsis.26 Recently, Yoo et al. also observed an infectious aetiology rate of 64% for cancer patients with diffuse pulmonary infiltrates causing acute respiratory failure admitted in ICU.4 We identified that sepsis was an independent factor associated with increased risk of SARF-MV, which suggests that sepsis acts as early prognostic factor in cancer patients admitted in ICU as observed in critically ill non-cancer patients.27
The APACHE II score was associated with SARF-MV. This model was developed for mortality prediction,28 but higher values indicate a greater severity of illness. Hence, the underlying pathophysiological disturbances may be associated with pulmonary damage and need of MV in critically ill cancer patients.
According to methodological design and included patients in previous studies, the ICU mortality rate and the hospital mortality rate in cancer patients range between 30% and 77%,1,15 and 30% and 56%,3,29 respectively. Over time, however, mortality rates have decreased.30
In ventilated cancer patients, some studies have shown an ICU mortality rate and a hospital mortality rate greater than 50%,11,26 and 65%,11,26,31 respectively. We observed a similar mortality rate for SARF-MV patients. SARF-MV is known as a risk factor for mortality in non-cancer patients32; however, their effect on outcomes has not been completely established for critically ill cancer subpopulation before.
MV is a temporary life-support method; however, despite the benefits of this therapy, many patients die after the initiation of mechanical ventilation, even though their arterial blood gases may have normalized. Mortality associated with SARF-MV can be associated with multiple factors including complications of ventilation such as barotrauma, oxygen toxicity, haemodynamic compromise, and ventilator-induced lung injury,33–35 as well as infectious complication such as ventilator-associated pneumonia.36 In cancer patients the local and systemic effects of tumour may play an additional role.
The length of hospital stay was similar between those patients with SARF-VM and those patients without SARF-VM. This is because higher hospital mortality rate among those subjects with SARF-VM, which in fact, died earlier. So that, the median length of hospital stay for ventilated patients was close to the median length of hospital stay for non-ventilated patients.
The present study has several shortcomings. First, our study was conducted at a single institution confined to oncological patients with a specialized ICU for critically ill cancer patients. Additionally, most patients were admitted to the ICU for postoperative care. Therefore, results of this study can only be applicable to other centres in which experienced intensivists are available for oncological and surgical critical care. Secondly, although data were extracted from a prospective database, given its retrospective nature, selection bias may have influenced our findings.
Results of this study demonstrated a significant association between some clinical factors with SARF-MV in cancer patients including primary tumour location, clinical stage of cancer, sepsis and APACHE II score. Our finding also highlights the powerful effect of SARF-MV on outcomes. However, this observation needs to be further evaluated by a multicentre, prospective study.
Author's contributionFDMB contributed in the Concepts, Design, Definition of intellectual content, Literature search, Clinical studies, Experimental studies, Data acquisition, Data analysis, Statistical analysis, Manuscript preparation, Manuscript editing, Manuscript review.
AGN contributed in the Design, Definition of intellectual content, Clinical studies, Data acquisition, Manuscript editing, Manuscript review.
MB contributed in the Literature search, Data analysis, Manuscript preparation, Manuscript editing, Manuscript review.
NAD contributed in the Literature search, Data analysis, Manuscript preparation, Manuscript editing, Manuscript review.
Conflict of interestsThere are no conflicts of interest.
The authors thank the medicine doctor's team of oncological intensive care unit of the Institute of Oncology and Radiobiology of Cuba for contribution with data collection.