To analyze the factors influencing in-hospital mortality among cancer patients admitted to an Intensive Care Unit (ICU).
DesignA retrospective observational study was carried out.
SettingThe ICU of a community hospital.
PatientsAdults diagnosed with solid or hematological malignancies admitted to the ICU, excluding those admitted after scheduled surgery and those with an ICU stay of under 24h.
InterventionsReview of clinical data.
Variables of interestReferring ward and length of stay prior to admission to the ICU, type of tumor, extent, Eastern Cooperative Oncology Group (ECOG) score, reason for ICU admission, severity (SOFA, APACHE-II, SAPS-II), type of therapy received in the ICU, and in-hospital mortality.
ResultsA total of 167 patients (mean age 71.1 years, 62.9% males; 79% solid tumors) were included, of which 61 (36%) died during their hospital stay (35 in the ICU). The factors associated to increased in-hospital mortality were ECOG scores 3–4 (OR 7.23, 95%CI: 1.95–26.87), metastatic disease (OR 3.77, 95%CI: 1.70–8.36), acute kidney injury (OR 3.66, 95%CI: 1.49–8.95) and SOFA score at ICU admission (OR 1.26, 95%CI: 1.10–1.43). A total of 60.3% of the survivors were independent at hospital discharge.
ConclusionsIn our series, only one-third of the critically ill cancer patients admitted to the ICU died during hospital admission, and more than 50% showed good performance status at hospital discharge. The clinical prognostic factors associated to in-hospital mortality were poor performance status, metastatic disease, SOFA score at ICU admission and acute kidney injury.
Analizar qué factores clínicos influyen en la mortalidad de pacientes con cáncer que ingresan en UCI.
DiseñoEstudio observacional retrospectivo.
ÁmbitoUCI de un hospital secundario.
PacientesAdultos ingresados en UCI con diagnóstico de cáncer (sólido o hematológico), excluyendo a aquellos ingresados en el postoperatorio de resección programada del tumor o con estancia inferior a 24 h en UCI.
IntervencionesRevisión de datos clínicos.
Variables de interésTipo de tumor, extensión, escala oncológica funcional Eastern Cooperative Oncology Group (ECOG), motivo de ingreso en UCI, gravedad (SOFA, APACHE-II, SAPS-II), terapia recibida y mortalidad hospitalaria.
ResultadosSe incluyó a 167 pacientes (edad media 71,1 años; 62,9% varones; el 79% con tumor sólido), de los cuales fallecieron 61 (36%) durante su estancia hospitalaria (35 en UCI). Los factores clínicos asociados a mayor riesgo de muerte hospitalaria fueron la puntuación 3-4 en la escala ECOG (OR 7,23; IC 95%: 1,95-26,87), extensión metastásica del tumor (OR 3,77; IC 95%: 1,70-8,36), insuficiencia renal (OR 3,66; IC 95%: 1,49-8,95) y puntuación SOFA al ingreso (OR 1,26; IC 95%: 1,10-1,43). El 60,3% de los supervivientes eran independientes al alta hospitalaria.
ConclusionesEn nuestra serie, solo un tercio de los pacientes con enfermedad oncológica grave que requieren ingreso en UCI fallecen durante el ingreso hospitalario y más de la mitad de los supervivientes presentan una situación de independencia al alta hospitalaria. Los factores clínicos asociados a la mortalidad hospitalaria fueron la mala situación funcional previa, el antecedente de tumor metastásico, la puntuación SOFA al ingreso en UCI y la presencia de insuficiencia renal aguda.
Cancer considered globally is the most frequent cause of death among men and the second most common cause of death in women, with an annual mortality rate of 204/100,000 inhabitants.1 Advances in the early diagnosis of the disease and the development of new treatments have improved the survival of cancer patients. As a result, an increasing number of such patients are admitted to the Intensive Care Unit (ICU) for the management of complications related to the treatment of cancer, side effects or disorders independent of cancer disease and which are considered to require admission to intensive care.2
A number of studies have reported a high mortality rate among oncological patients admitted to the ICU. The main factors associated to mortality over the short term are patient age, severity upon admission, organ failure, acute respiratory failure, the need for mechanical ventilation, late admission to the ICU, the presence of comorbidities, poor functional condition prior to admission, and advanced stage tumor disease, among others.3
Few studies in our setting have evaluated cancer patient mortality in the ICU, and the available data are moreover contradictory.4,5 While some authors question the true benefits of cancer patient admission to the ICU, in view of the high mortality and significant resource utilization involved,4 others consider that it is not possible to deny intensive care to all medical oncological patients.5
The admission of cancer patients to the ICU is a complex decision, since patient quality of life, the short and long term prognosis, and the tumor treatment options must be taken into consideration. The admission of such patients to the ICU is usually decided in order to treat a potentially reversible complication (whether related to the cancer or not), and the aim is to return the patient to a previous clinical condition allowing the continuation of active tumor therapy or discharge from hospital with acceptable quality of life. Such admission decisions are usually made in emergency situations, and the intensivist often does not have all the clinical information of the patient.6 Knowledge of the clinical parameters that could help predict the short term outcome of cancer patients suffering critical complications could be of help in the decision making process. The present study was designed to evaluate which clinical factors at the time of admission to the ICU have an impact upon in-hospital mortality, and to assess functional condition among the survivors at discharge from hospital.
Material and methodsStudy designA retrospective observational study was made, with the inclusion of patients admitted to the ICU of a second level hospital in the period between January 2011 and December 2016. The study protocol was approved by the local Ethics Committee.
PatientsThe study included patients aged 18 years or older and admitted to the ICU with solid or hematological tumor disease. Patients admitted in the immediate post-tumor resection period were excluded, as were those with an ICU stay of under 24h.
Data compilationThe data were compiled on a retrospective basis using the Critical Care Manager application, which records clinical severity scores, all management activities and techniques used in the ICU; and the Selene program, which records all information referred to patient admission in other areas apart from the ICU of the hospital.
Demographic data were recorded, along with body mass index (BMI), comorbidities, the Department or service of origin, the presence of neutropenia upon admission (absolute neutrophil count <1.5×109/l, considering the following neutropenia categories: mild: 1.0–1.5×109/l; moderate: 0.5–0.9×109/l; severe: <0.5×109/l), days of hospital stay prior to ICU admission, type of tumor (solid or hematological), cancer status (phase I: potentially curable; phase II: non-curable, prolongation of life; phase III: palliative care), disease condition in relation to treatment response (induction therapy, partial or complete remission, stable disease, disease progression), local or metastatic spread, and types of cancer treatments received. We also recorded the Eastern Cooperative Oncology Group (ECOG) score for assessing quality of life, where 0=asymptomatic (fully active, capable of all activities of daily living before the disease); 1=symptomatic but fully ambulatory (strenuous physical activity is restricted, but the patient is able to work); 2=symptomatic, spending less than half of the day in bed, ambulatory (capable of self-care but unable to work); 3=symptomatic, spending more than half of the day in bed, but not bedridden (with self-care limitations); 4=bedridden and completely disabled (self-care not possible, totally confined to bed or chair); and 5=death.7 In accordance with previous studies of patients in the ICU, the ECOG scale was recoded into three 3 categories: ECOG 0–1; ECOG 2 and ECOG 3–4.8 The reason for admission to the ICU was documented, considering the following categories: shock (septic, cardiogenic or hypovolemic); clinical suspicion of infection (possible infectious focus and presence of systemic inflammatory response syndrome [SIRS] criteria)9; respiratory failure defined by the presence of any of the following symptoms: resting dyspnea, tachypnea (respiratory frequency >24rpm), hypoxemia (PaO2<60mmHg), desaturation (SatO2 determined by pulsioximetry <90%); renal failure (defined according to the KDIGO 2012 criteria)10; and other reasons (including cancer treatment, metabolic disorders, neurological complications, pulmonary thromboembolism or cardiac arrest). In turn, we recorded severity upon admission to the ICU (SAPS II, APACHE-II and SOFA scores)11–13 and the type of therapy received in the unit: ventilatory support (including high-flow nasal oxygen, noninvasive mechanical ventilation [NIMV] and invasive mechanical ventilation [IMV)]), blood product transfusions, vasoactive drugs, and extrarenal replacement therapy indicated on the basis of the criteria usually employed in the ICU.14 Days of ICU stay and in-hospital mortality were also recorded. Furthermore, we documented the cases of limitation of therapeutic effort following the protocol of the ICU. Such limitation was decided on confirming that the patient condition was irreversible or terminal, with consensus among all the intensivists and with participation of the patient and family. Lastly, we recorded functional condition (ECOG score) among the survivors at hospital discharge.
Statistical analysisThe SPSS version 15.0 statistical package for MS Windows (SPSS Inc., Chicago, IL, USA) was used to analyze the data. Categorical variables were reported as percentages, and comparisons between groups were made using the chi-squared test or Fisher exact test for dichotomic variables. Continuous variables were reported as the mean (standard deviation [SD]) or median (interquartile range [IQR]), and comparisons were made using the Student t-test, Mann–Whitney U test or analysis of variance (ANOVA), as applicable. Statistical significance was considered for p<0.05. Forward stepwise multiple logistic regression models were used to identify the factors associated to in-hospital mortality, entering those clinical variables yielding p≤0.2 in the univariate analysis. Statistical significance was measured based on the likelihood ratio method.
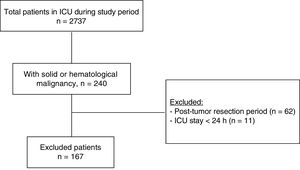
ResultsA total of 240 cancer patients were admitted to our ICU during the study period. Of these subjects we excluded 62 elective tumor surgery patients and 11 patients with an ICU stay of under 24h. The final study sample thus consisted of 167 patients. The study flowchart is shown in Fig. 1. The mean patient age was 71.1 years (SD 10.9); 62.9% of the patients were males; and the mean body mass index was 25.3kg/m2 (SD 4.9). Solid tumors predominated over hematological cancer (79% versus 21%, respectively). The most frequent solid tumors were gastrointestinal malignancies (n=64; 38.3%), followed by genitourinary cancer (n=34; 20.4%) and lung tumors (n=17; 10.2%). In turn, the most common hematological malignancies were lymphoma (n=15; 9%), followed by leukemia (n=13; 7.8%) and multiple myeloma (n=6; 3.6%). The patients came from the conventional hospital ward in 59.3% of the cases, the emergency service in 36.5%, and the postsurgery resuscitation room in 3% (due to complications of urgent and non-elective surgery). Only 10.3% of the patients presented prior dependency with an ECOG score of 3–4.
A total of 61 patients died during hospital admission, including 35 in the ICU (20 following limitation of therapeutic effort). Table 1 describes the demographic characteristics, comorbidities and condition (phase, status and spread) of the neoplastic disease upon admission to the ICU. The deceased patients more often presented metastatic spread upon admission (59% vs 30.8%; p=0.01), were receiving palliative care (37.7% vs 11.3%; p<0.001), and a larger proportion of them presented ECOG functional scores of 3–4 (21.3% vs 4.7%). Hospital stay before arrival in the ICU was significantly longer among the deceased patients (median 5 days versus 2 days; p=0.005). In turn, upon arrival in the ICU, the patients that finally died yielded comparatively higher severity scores (APACHE-II, SAPS-II and SOFA) (Table 2).
Basal characteristics of the patients.
| In-hospital mortality | p | ||
|---|---|---|---|
| No (n=106) | Yes (n=61) | ||
| Age in years, mean (SD) | 71.8 (11) | 71.44 (10) | 0.893 |
| Male gender, n (%) | 68 (64.2) | 37 (60.7) | 0.387 |
| ECOG upon admission | |||
| 0–1 | 61 (57.5) | 25 (41) | 0.003 |
| 2 | 40 (37.7) | 23 (37.7) | |
| 3–4 | 5 (4.7) | 13 (21.3) | |
| Body mass index, mean (SD) | 26.2 (4.9) | 24.0 (4.7) | 0.003 |
| Comorbidity, n (%) | |||
| Chronic obstructive pulmonary disease | 29 (27.4) | 23 (44.2) | 0.112 |
| Heart disease | 58 (54.7) | 33 (54.1) | 0.533 |
| Diabetes mellitus | 36 (34) | 20 (32.8) | 0.508 |
| Vascular disease | 31 (29.2) | 16 (26.2) | 0.408 |
| Type of tumor, n (%) | |||
| Solid | 85 (80.2) | 47 (77) | 0.385 |
| Hematological | 21 (19.8) | 14 (23) | |
| Tumor phase | |||
| I: Diagnostic or potentially curable: n (%) | 74 (69.8) | 27 (44.3) | <0.001 |
| II: Non-curable, prolongation of life: n (%) | 20 (18.9) | 11 (18) | |
| III: Palliative care: n (%) | 12 (11.3) | 23 (37.7) | |
| Tumor spread, n (%) | |||
| Locoregional | 71 (68.3) | 24 (39.3) | 0.001 |
| Metastasis | 32 (30.8) | 36 (59) | |
| Tumor treatment received before admission to ICU, n (%) | |||
| Chemotherapy | 49 (46.2) | 32 (52.5) | 0.269 |
| Radiotherapy | 25 (23.6) | 13 (21.3) | 0.446 |
| Hormone therapy | 9 (8.5) | 4 (6.6) | 0.450 |
| Disease state, n (%) | |||
| Stable | 27 (25.5) | 6 (9.8) | 0.084 |
| Induction | 26 (24.5) | 15 (24.6) | |
| Progression | 30 (28.3) | 21 (34.4) | |
| Remission | 23 (21.7) | 19 (31.1) | |
| Days of hospital stay until admission to ICU, median (interquartile range) | 2 (1;6) | 5 (1;12) | 0.005 |
| Neutropenia upon admission, n (%) | 12 (11.3) | 10 (16.4) | 0.241 |
SD: standard deviation; ECOG: Eastern Cooperative Oncology Group; ICU: Intensive Care Unit
Severity scores, reasons for admission and treatments received in the ICU.
| In-hospital mortality | p | ||
|---|---|---|---|
| No (n=106) | Yes (n=61) | ||
| Severity scores upon admission to ICU, median (interquartile range) | |||
| APACHE-II | 18 (14.75;22) | 21 (17;26) | 0.005 |
| SAPS-II | 68 (58;75) | 75 (56;75) | <0.001 |
| SOFA | 5 (3; 8) | 8 (6; 10) | <0.001 |
| Reason of admission to ICU, n 8%) | |||
| Shock | 63 (59.4) | 42 (68.9) | 0.148 |
| Suspected infection | 53 (50) | 41 (67.2) | 0.022 |
| Respiratory failure | 41 (38.7) | 36 (59) | 0.009 |
| Renal failure | 17 (16) | 24 (39.3) | 0.001 |
| Other reasonsa | 28 (26.4) | 17 (27.9) | 0.488 |
| Treatments in ICU, n (%) | |||
| Ventilatory support | 59 (55.7) | 50 (82) | <0.001 |
| Vasoactive drugs | 65 (61.3) | 47 (77) | 0.027 |
| Blood products | 42 (39.6) | 23 (37.7) | 0.469 |
| Extrarenal replacement therapy | 7 (6.6) | 10 (16.4) | 0.042 |
| Days of stay in ICU, median (interquartile range) | 3 (2;5.25) | 3 (2;11.5) | 0.845 |
SD: standard deviation; ICU: Intensive Care Unit.
The main reason for admission to the ICU was shock of one type or other, though without differences between groups (Table 2). However, the deceased patients more often presented suspected infection or renal or respiratory failure upon admission to the ICU (Table 2). Of the 93 patients with suspected infection upon admission, microbiological confirmation was obtained in 65 cases (69%). The most frequently affected site was the lungs among the patients that died (17.2%), and the abdominal cavity among the survivors (19.3%) – though without significant differences in the distribution of infectious sites between the two groups (p=0.349). The most frequently implicated pathogens were gram negative bacilli (n=28; 27.9%), followed by gram positive cocci (n=11; 11.8%). There were no significant differences between the deceased patients and the survivors (p=0.283), though the former tended to yield a slightly higher frequency of fungal isolates (12.5% vs 3.7%).
Upon admission to the ICU, 22 patients (13.2%) presented neutropenia, which proved severe in 12 cases (7.2%), moderate in 6 (3.6%) and mild in 4 (2.4%). There were no significant differences in the frequency of severe neutropenia between the patients that died in hospital and the survivors (9.8% vs 5.7%; p=0.707). Most of the patients with neutropenia (90.9%) were admitted to the ICU due to suspected infection, with no significant differences in the percentage of infection with respect to the intensity of neutropenia (mild 100%; moderate 83.3%; severe 91.7%; p=0.662).
Ventilatory support, vasopressor drug treatment and extrarenal replacement therapy were significantly more often needed among the patients that died (Table 2). The most frequently used ventilatory support mode was IMV (44.3% vs 18.9%; p<0.001), with a median duration of two days (interquartile range 0–7.5) and a median positive end-expiratory pressure (PEEP) of 8 cmH2O (interquartile range 6–10)—both values being significantly greater than among the survivors (p=0.008 and p=0.004, respectively). Fifty patients received NIMV (29.9% of the total), and high-flow nasal oxygen was administered to only 7.2% of the global patients, with no differences between groups. A total of 40 of the 50 patients that initially received NIMV finally required IMV. None of the cases initially managed with high-flow nasal oxygen required any other ventilatory support mode.
Sixty-five patients required the transfusion of blood products (38.9%): packed red cells (n=56; 33.5%), fresh plasma (n=19; 11.4%) and platelets (n=18; 10.8%), with no significant differences between the patients that died and the survivors.
Mortality risk factorsThe multivariate logistic regression analysis showed the most potent in-hospital mortality risk factor to be an ECOG score of 3–4, followed by metastatic tumor spread and the SOFA score upon admission. No influence was observed on the part of the tumor stage and phase, APACHE-II or SAPS-II scores, or the presence of respiratory failure or suspected infection upon admission to the ICU (Table 3).
Clinical factors upon admission to the ICU independently associated to in-hospital mortality.
| Adjusted OR | 95%CI | p | |
|---|---|---|---|
| ECOG upon admission 3–4 | 7.23 | 1.95–26.87 | 0.003 |
| ECOG upon admission 2 | 0.78 | 0.33–1.80 | 0.565 |
| ECOG upon admission 0–1 | Reference | Reference | Reference |
| Tumor spread: metastatic | 3.77 | 1.70–8.36 | 0.001 |
| Renal failure upon admission to ICU | 3.66 | 1.49–8.95 | 0.004 |
| SOFA upon admission | 1.26 | 1.10–1.43 | <0.001 |
ECOG: Eastern Cooperative Oncology Group; CI: confidence interval; OR: odds ratio.
Variables included in the model, but not significant in the final model: tumor phase, tumor state, APACHE-II, SAPS-II, respiratory failure upon admission to the ICU, suspected infection upon admission to the ICU.
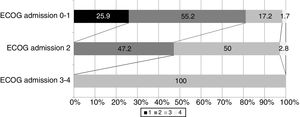
Among the 106 patients that survived, information referred to the ECOG score at discharge was available from 98 patients: 16 had scores of 0–1 (16.3%), 48 had a score of 2 (49%), and 34 had scores of 3–4 (indicative of strong dependency) (34.6%). Fig. 2 shows the distribution of the ECOG scores at discharge in relation to the scores upon admission. Over 80% of the survivors with ECOG scores of 0–1 upon admission had scores of ≤2 at discharge, and 47% of those presenting an ECOG score of 2 upon admission showed the same score at discharge.
DiscussionThe main finding of this study was that only one-third of all patients with severe cancer requiring admission to the ICU died during hospital admission, and only one out of every 5 patients died during ICU stay. The main factors associated to in-hospital mortality among such patients were the prior functional condition, followed by metastatic spread of the disease, the presence of renal failure, and the SOFA score upon admission. The tumor stage and phase, the APACHE-II and SAPS-II severity scores, and the presence of respiratory failure or suspected infection upon admission to the ICU exerted no significant influence.
The mortality observed in our series is consistent with the data found in other studies (24–75%).15–20 This broad difference in mortality between publications may be explained by the great heterogeneity of the study settings involved (medical, surgical or exclusively oncohematological) and the inclusion criteria used. Almost all of our patients (79%) had solid tumors, with medical complications associated to the malignancy. The incidence of oncohematological patients was very low because bone marrow transplantation is not available in our hospital – thus causing such patients to be sent to reference centers.
In our study, the most potent predictor of in-hospital mortality was the prior functional condition as determined upon admission by the ECOG score. This is concordant with the observations of other authors.5,8,19,21–24 However, none of these studies evaluated the ECOG score at hospital discharge. This is one of the contributions of our study, since we found over 80% of the survivors with ECOG scores of 0–1 upon admission to present scores of ≤2 at discharge, and 47% of those presenting an ECOG score of 2 upon admission showed the same score at discharge. Therefore, treatment in the ICU of these patients due to severe complications related or not related to the tumor disease does not necessarily imply a poorer functional prognosis over the short term.
The presence of renal failure upon admission was also significantly associated to the risk of in-hospital death. Renal failure is a common complication in cancer patients and tends to be of a multifactorial nature, involving mechanisms directly related to the tumor disease – such as acute tumor lysis syndrome, urinary tract obstruction, renal multiple myeloma or hypercalcemia – but also other indirect mechanisms and factors such as hypoperfusion/shock of any kind, the administration of nephrotoxic drugs, the use of radiological contrast media, chemotherapeutic agents (methotrexate, cisplatin) or other drugs (nonsteroidal antiinflammatory drugs, angiotensin converting enzyme inhibitors), and conditions derived from allogenic transplantation (sinusoidal obstruction syndrome, hemolytic-uremic syndrome).25 Renal failure has been reported to occur in 13–42% of all critical cancer patients, being more common in oncohematological cases, and with a mortality rate of 44–91%, depending on the literature source.23,25,26 Nevertheless, it may be regarded as a modifiable condition, since extrarenal replacement therapy is able to significantly lower the mortality rate, provided it is started early (on the first day of admission to the ICU).23
Different authors have reported that severe acute respiratory failure upon admission to the ICU and the need for ventilatory support are important predictors of mortality,17,18,21,22,27,28 and it has been suggested that high-flow nasal oxygen can be useful in patients of this kind. In our series, although the need for ventilatory support was not identified as an independent prognostic factor in the multivariate analysis, those patients who died required more frequent ventilatory support. Invasive mechanical ventilation was the most frequently used ventilation mode. High-flow nasal oxygen was very little used, though none of the patients that received such treatment required IMV. Nevertheless, very recent publications conclude that high-flow nasal oxygen has no impact upon the mortality figures.29,30
With regard to the severity scores in the ICU, only the SOFA score upon admission to the ICU was seen to be associated to increased in-hospital mortality risk. This is consistent with the observations of other studies in which the number of organ dysfunctions was identified as a main prognostic conditioning factor in critical cancer patients.2,20
Different admission and treatment modalities have been proposed for cancer patients in the ICU, including: (a) treatment without limitations in patients with recently diagnosed malignancy; (b) the so-called “ICU test”, involving initial treatment without limitations, with mandatory and repeated re-evaluations from day 3–5 of admission in those cases where clinical deterioration is potentially reversible or where there is uncertainty regarding the prognosis; (c) “limited ICU therapy” in patients requiring for example the administration of vasopressor drugs but who are not considered candidates for intubation or cardiopulmonary resuscitation; (d) palliative care in the ICU, where the symptoms are treated without adopting measures to prolong survival; and (e) the exclusion of admission to the ICU in patients who reject invasive treatment or present limited prior function (ECOG score >3) or recurring/progressing disease.31,32 Which of these modalities was considered in each case was not documented in our series.
Our study has a number of limitations. Firstly, its retrospective, descriptive observational design does not allow the exclusion of selection bias, since we only considered those patients who were admitted to the ICU. Secondly, the sample size was small, and this limits the reliability of the statistical findings, since the hospital where the study was made is a second level center. On the other hand, the types of patients seen in our hospital may not be representative of those seen in other centers – thereby precluding the generalization of our findings to other settings. Nevertheless, in order to strengthen our results, we developed logistic regression models to allow the identification of factors associated to mortality among the cancer patients admitted to the ICU – with findings similar to those reported in larger series.
In conclusion, only one-third of all patients with severe cancer requiring admission to the ICU of a second level center died during hospital admission, and over 50% of the survivors showed no dependency at hospital discharge. The tumor stage, APACHE-II and SAPS-II severity scores, and the presence of respiratory failure or suspected infection upon admission to the ICU had no significant impact upon in-hospital mortality among these patients. In this regard, although a prior poor functional condition, metastatic disease and the SOFA score upon admission may worsen the prognosis, this should not be allowed to condition patient admission to the ICU.
Authorship/collaborationsDomingo Díaz-Díaz: study design, data compilation, analysis and interpretation of results, drafting of the manuscript.
Mercedes Vilanova-Martínez: study design, data compilation, analysis and interpretation of results, review and approval of the final manuscript.
Eduardo Palencia-Herrejón: study design, review and approval of the final manuscript.
Conflicts of interestNone.
Please cite this article as: Díaz-Díaz D, Villanova Martínez M, Palencia Herrejón E. Pacientes oncológicos ingresados en Unidad de Cuidados Intensivos. Análisis de factores predictivos de mortalidad. Med Intensiva. 2018;42:346–353.