In this point of view, the implementation of muscular and diaphragmatic ultrasound is proposed for diagnosis purposes and nutritional therapy in critical patients.
The assessment of body composition, both at admission and during the length of ICU stay is a crucial process that must be performed to determine, along with severity scores, the survival possibilities of these patients. In fact, promising scores such as the Nutri-score, developed with the intention of identifying patients who would require more “aggressive” nutritional support, included traditional assessment parameters, such as the Apache II or SOFA scores, which eventually helped determine the most appropriate therapies, especially for critically ill patients with a grim prognosis and metabolic stress at admission. These patients clearly needed nutritional therapy in accordance with the latest national or international guidelines.1
However, the issue of body composition in our patients persisted, and the medical literature available often referred to the “gold standard,” which was the Subjective Global Assessment (SGA) initially described generically for malnourished patients by Detsky and later applied to various diseases, including critical ill patients.2 Then, came the GLIM criteria for malnutrition elaborated by the European Society for Clinical Nutrition and Metabolism, including 2 of its etiological criteria that almost all of our patients meet (reduced intake or inflammation), and 2 phenotypic criteria that our patients can also present at admission: weight loss, low body mass index, and reduced muscle mass.3 Initially, the detailed reading of the GLIM criteria included, among the etiological criteria, diagnostic methods to assess the musculoskeletal mass. However, the ultrasound analysis of muscle mass was conspicuously absent. In addition to the more traditional anthropometric measurements, other methods were proposed, such as computed tomography, typically at L3 level, or magnetic resonance imaging, typically at L2-L3 level, dual-energy X-ray absorptiometry, or bioelectrical impedance analysis (BIA)—of limited use—in critical patients. However, a “working group” was created, and in a subsequent publication, they included what many of us were already doing and hoping to see: the inclusion of muscular ultrasound among these globally disseminated criteria.4
On the other hand, a very interesting study was published that validated the GLIM criteria with a good area under the ROC curve of 0.85 (P < .001), comparing these criteria, among others, with SGA in critical patients.5 This, along with the studies conducted by Puthucheary et al.6 using ultrasound and muscle biopsies, mainly in the rectus femoris muscle, which correlated their findings in critical patients, along with studies on the loss of diaphragmatic muscle thickness and decreased diaphragm excursion with ultrasound control,7 completely changed the landscape of body composition assessment in our patients, both during the ICU admission and during their course of care.
The study examined the loss of mass and function in various muscles, and as expected, the use of ultrasound began to be standardized in these patients. Initially, the ultrasound was primarily focused on assessing the loss of muscle mass. However, its focus gradually shifted towards assessing the quality of the muscle tissue.8 We have transitioned from considering muscular ultrasound in critically ill patients as a “promising technique” to a routine clinical tool in real-world scenarios, both at the ICU admission and during the patient's disease progression to adapt nutritional therapy to our patients’ ever-changing needs. Additionally, recent medical literature from various international research groups has been discussing the use of muscular ultrasound as a key tool to assess the therapeutic decisions made in the context of critically ill patients.9
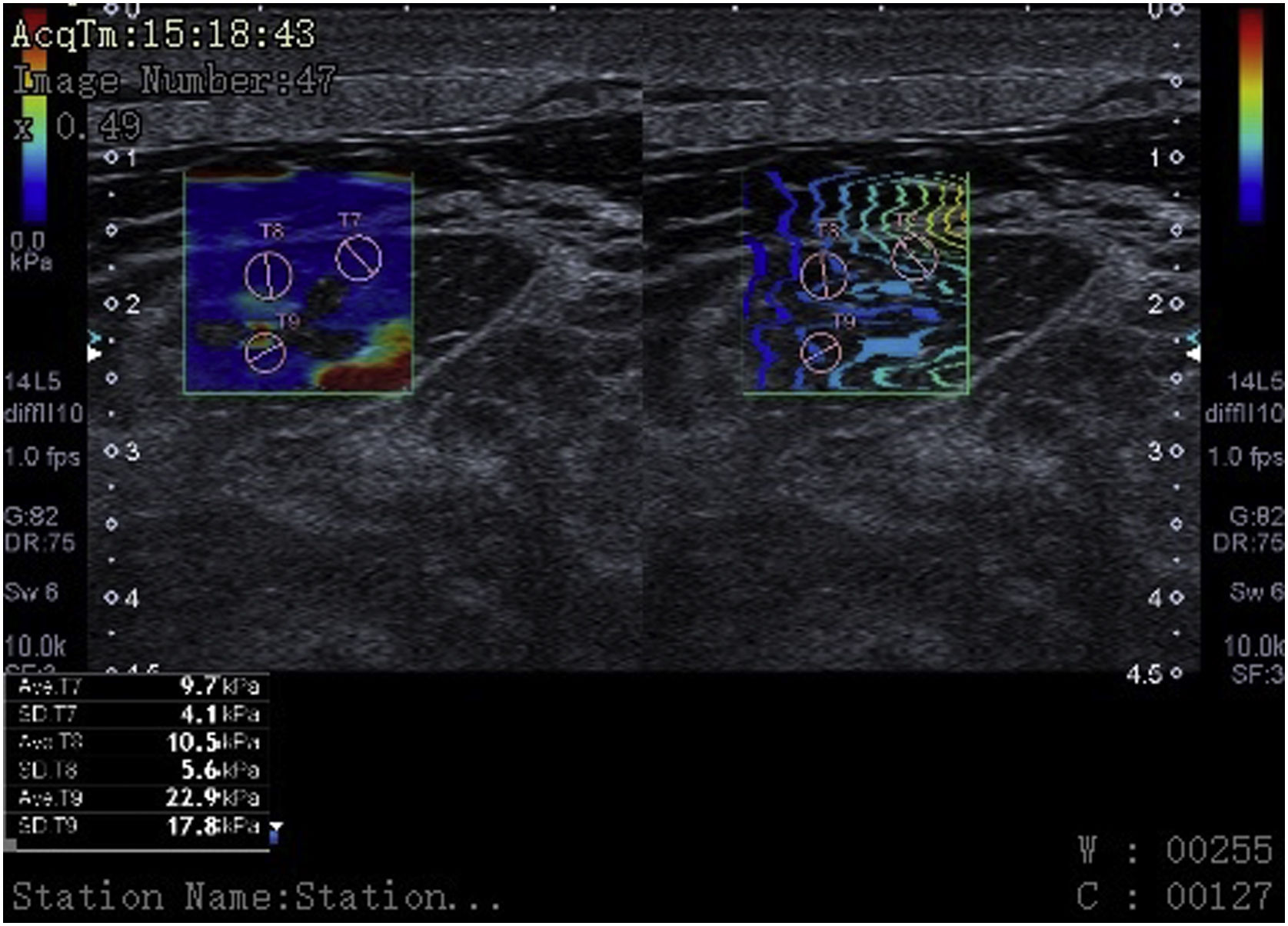
Finally, we have described and implemented highly innovative ultrasound techniques for critical patients with excellent diagnostic capabilities. However, they are still underused and further implementation is required.10 A clear example of this is the use of the “M-mode” ultrasound to analyze muscle fasciculations, color Doppler, the use of contrast-enhanced ultrasound (CEUS) to study muscle perfusion, and the visualization of microvascularization using special software (SMI) to demonstrate muscle flow unseen with color Doppler, or the use of elastography to assess muscle elasticity or rigidity in the diagnosis and follow-up of critical patients, with an area under the ROC curve of 0.972 (95%CI, 0.916–1) (Fig. 1).
Elastography: cross-sectional view of the rectus femoris muscle in a 55-year-old patient with multiple organ failure showing kilopascal values that translate into muscle stiffness in various regions of interest (ROIs) (pink circles) at the bottom of the image. The presence of interfascial and intramuscular fluid becomes evidente here too.
The current problem is how, who, and what can be done to implement the so-called “nutritional ultrasound” in settings other than ICU setting. The “how” can only be answered with training. Training courses for this type of ultrasound exist and have become a common thing. However, they are still scarce in our field, mainly due to the lack of teachers who are knowledgeable of the techniques and have enough training to disseminate them.
The “who” is more complex and depends on the level of interest of each center and development of these ultrasound techniques. Dietitians in other countries are already adding ultrasound measurements of muscle mass loss, along with other traditional anthropometric measurements. In other contexts, it will be radiologists, ultrasound experts, or physicians from several specialties the health professionals involved. In our country, endocrinologists, geriatricians, or internists are the ones often involved in the implementation of the so-called “nutritional ultrasound”. However, we believe that it is a highly operator-dependent technique, being radiologists the most experienced ones because they can implement the new aforementioned qualitative muscle methods, which have a significant impact not only on diagnosis but also on prognosis and the patient's disease progression.
In conclusion, muscular ultrasound, primarily of the rectus femoris and diaphragm muscles, whether to consider the possibility of weaning from mechanical ventilation or to initiate or make evolutionary adjustments of nutritional therapy, has already been standardized and is definitely here to stay. It is with this perspective in mind that we wish a “long life” to muscular ultrasound and a rapid implementation in critical patients.
FundingNone declared.
Conflicts of interestNone declared.