To estimate the impact of smart pump implementation in a pediatric intensive care unit in terms of number and type of administration errors intercepted.
DesignObservational, prospective study carried out from January 2010 to March 2015 with syringe and great volumen infusion pumps available in the hospital.
SettingA tertiary level hospital pediatric intensive care unit.
ParticipantsInfusions delivered with infusion pumps in all pediatric intensive care unit patients.
InterventionsDesign of a drug library with safety limits for all intravenous drugs prescribed.
Main variablesUsers’ compliance with drug library as well as number and type of errors prevented were analyzed.
ResultsTwo hundred and eighty-three errors were intercepted during 62 months of study. A high risk drug was involved in 58% of prevented errors, such as adrenergic agonists and antagonists, sedatives, analgesics, neuromuscular blockers, opioids, potassium and insulin. Users’ average compliance with the safety software was 84%.
ConclusionsSmart pumps implementation has proven effective in intercepting high risk drugs programming errors. These results might be exportable to other critical care units, involving pediatric or adult patients. Interdisciplinary colaboration is key to succeed in this process.
Estimar el impacto de la implantación de bombas de infusión inteligentes en una unidad de cuidados intensivos pediátricos en cuanto al número y tipo de errores de administración interceptados.
DiseñoEstudio observacional, prospectivo, realizado de enero de 2010 a marzo de 2015 con las bombas volumétricas y de jeringa disponibles en el hospital.
ÁmbitoUnidad de Cuidados Intensivos Pediátricos de un hospital general de tercer nivel.
ParticipantesTodas las infusiones programadas con bomba de infusión en los pacientes ingresados en la Unidad de Cuidados Intensivos Pediátricos.
IntervencionesElaboración de una biblioteca de fármacos con límites de seguridad a través de la cual se programarían todas las infusiones intravenosas prescritas.
Variables principalesSe analizó la adherencia a la biblioteca de fármacos y el número y tipo de errores evitados según las alarmas generadas en el sistema.
ResultadosSe interceptaron 283 errores reales de programación durante los 62 meses que duró el estudio. En el 58% de los errores estuvo implicado un fármaco de alto riesgo, como agonistas y antagonistas adrenérgicos, sedantes, analgésicos, bloqueantes neuromusculares, opiáceos, potasio e insulina. Durante este período, la adherencia media de los usuarios al software de seguridad fue del 84%.
ConclusionesLa implantación de bombas de infusión inteligentes ha demostrado ser eficaz en la intercepción de errores de programación relacionados con fármacos de alto riesgo. Esta herramienta es susceptible de implantarse en otras unidades de pacientes críticos, tanto adultos como pediátricos. La colaboración multidisciplinar es clave para el éxito del proceso.
The concept of safety in drug use has experienced changes in recent years. The errors that occur in any of the phases of the drug utilization circuit result in important patient morbidity–mortality.1,2 In this regard, administration errors are the errors that prove most difficult to intercept,3 and their potential impact upon the patient depends on the administration route involved, the drug type and dose, and the characteristics of the patient. When high risk drugs are administered via the intravenous route in critically ill pediatric patients, the likeliness of damage in the event of error is seen to multiply.4 Guaranteeing safety in this scenario therefore should constitute a priority concern, and in this regard the use of smart infusion pumps (SIPs) may play an important role.
An intelligent (smart) infusion system is a conventional infusion system equipped with safety software that contains a drug library specific of each Unit, and which constitutes a list of drugs with concrete concentration, maximum and minimum dose, and infusion time specifications for each of them. The relationship between the dose and infusion time determines the administration rate, for which absolute and relative limits (both upper and lower) are established with the purpose of intercepting errors attributable to over- and under-dosing, respectively. In this way, if programming error breaches a relative limit, the user is alerted to the fact that the infusion rate might not be adequate for a given patient. However, the alert can be ignored and infusion can be continued, after checking that programming has indeed been correct. In contrast, the accidental breaching of an absolute limit generates an alert that cannot be obviated, and the user in this case must reprogram administration of the drug.5
Different organizations acknowledge the increased safety afforded by SIP technology, and advocate replacing conventional infusion systems with smart systems.6–8
However, the published information on the true impact of these systems in terms of the interception of programming error is still limited.8
The present study examines the impact of implementing SIP technology in the administration phase of intravenous drugs in a Pediatric Intensive Care Unit (PICU) in terms of the number and types of programming errors intercepted.
Patients and methodsStudy design and settingA prospective, observational interventional study with analytical components was carried out on the prevalence of programming errors. The study began in January 2010 and ended in March 2015, and was carried out in the PICU of the Maternal and Child Hospital pertaining to Gregorio Marañón University General Hospital in Madrid (Spain).
The Maternal and Child Hospital has a total of 231 beds, of which 150 correspond to pediatric patients and 81 to Obstetrics and Gynecology. The beds are distributed into a total of 5 hospital wards. The Hospital possesses an electronic prescription program associated to 17 automatic dispensation systems.
Specifically, the PICU has 11 beds, with an annual admission volume of approximately 450 patients, of which 70% correspond to cardiovascular disease (including the postoperative period of heart surgery), while the remaining 30% correspond to other postoperative cases or medical patients. The nurse/patient ratio is 1.
Working teamA multidisciplinary team was created, composed of two pediatric intensivists, two clinical pharmacists, and the nursing supervisor. All of the members of the team actively participated in each of the phases of the implementation process.
Selection of systemsThe study was carried out with volumetric infusion pumps (12 sets) and syringes (60 sets) available in the hospital through public concourse at the time of the study.
Migration from the conventional systems to the smart systems implied no added costs for the hospital.
These systems were chosen due to their precision in administering drugs at low flow rates (as is common practice in pediatric patients), in preference to other systems available on the market that offer connectivity advantages9 but are not suitable for use in pediatric patients.
Creation of the drug libraryFrom June to December 2009, the multidisciplinary team, coordinated by a pharmacist, developed the first version of the drug library that was subsequently incorporated to the infusion systems.
A list of the drugs used in the PICU was initially obtained through the drug management program of the Department of Pharmacy (FarHos Gestión®, Madrid, Spain). A selection was made of the drugs administered via the intravenous route by means of infusion pumps, as well as of the most commonly used drugs, those classified as high risk substances, and those which may pose administration problems because of limited experience with their use.
Once the drug list was defined, we identified the literature sources most widely used by the PICU and the Department of Pharmacy as regular consulting material,10–15 in order to define concentrations, minimum and maximum doses, and recommended administration times and rates. These data were then used to establish the relative and absolute limits, with only some relative lower limits, since the editing software used did not contemplate the inclusion of absolute lower limits.
We decided to standardize concentrations in order to minimize the possibility of error related to variability in the preparation of the intravenous mixtures, and to establish the bases for future centralization of the preparation of intravenous mixtures in the Departments of Pharmacy.
The literature was consulted to define standard concentrations,10–15 and the information was adapted to the experience and specific needs of the Unit.
In concordance with the recommendations of the literature,16,17 we defined the smallest possible number of standard concentrations.
The need to incorporate new drugs, add concentrations and modify limits in order to better adapt them to routine practice made it necessary to periodically update the drug library. During the first two years of implementation, these updates were carried out on a trimestrial basis, though as of the year 2013 a single annual update proved sufficient. The changes introduced were reported within the Unit through sessions programmed with the same periodicity.
At present, the version used in the infusion systems is version 11, which contains 105 drugs – representing 100% of the substances administered by means of pump systems in the PICU.
In parallel to development of the drug library, an intravenous drug administration guide was created, with information on the preparation of the mixtures, stability and preservation,18 that was updated with the same periodicity as the drug database. Likewise, a compatibilities table was produced referred to drugs amenable to Y-site coadministration in continuous perfusion.19 Both documents were drafted as consulting material and support for the nursing personnel.
Since the selected systems were already available in the hospital and the nursing personnel were familiarized with their use, a one-week training period covering all the shifts was enough to allow them to learn infusion programming through the drug library.
Analysis of the stored informationAll the information stored in the devices during use was systematically analyzed by a pharmacist and pediatric intensivist using the data processing program associated to the infusion systems. The information was downloaded at the same time as the drug library updates, and was reported with the same periodicity to the Unit personnel members.
The analysis of the information allowed us not only to identify opportunities for improvement and which led to the different versions of the drug library,20 but also to detect training needs among the users.21 On the other hand, we were able to determine the total number of programming errors avoided, based on the alerts generated by the safety software analyzed in the present study.
As inclusion criterion in this error interception study, we considered all programmed infusion pump administrations using the drug library in the course of routine clinical practice in patients admitted to the PICU. We excluded those programmed administrations not based on the drug library and which were therefore external to the safety network and could not be analyzed, since the technology used only allows the description of events occurring after infusion programming with the mentioned drug library – identifying each programming event with a concrete drug at a certain concentration and infusion rate.
The following technology evaluation indicators were defined and subjected to descriptive statistical analysis:
- •
Quality of use indicators
- o
Adherence to the drug library: number of infusions programmed through the drug library×100/total number of infusions programmed.
- o
Ratio of the number of alarms generated number of infusions started through the drug library: total number of alerts per drug×100/total number of infusions programmed through the drug library.
- o
Percentage of alerts ignored: total number of alerts per drug ignored×100/total number of relative limit alerts.
- o
- •
Effectiveness indicators
- o
Total number of programming errors avoided based on the alerts detected.
- o
After identifying the errors intercepted following implementation of the technology, we classified them according to their risk profile, based on the list of high risk drugs proposed by the Institute for Safe Medication Practices (ISMP)22 or its equivalent adapted to pediatric patients and published in the official journal of the Spanish Association of Pediatrics (Asociación Española de Pediatría).23
Patient informed consent was not considered necessary, since the study subjects were the infusion systems – a technology commonly used in pediatric patients – and conduction of the study did not alter patient treatment in any way.
The study was approved by the Clinical Research Ethics Committee of the hospital.
ResultsAn exhaustive review of the alerts generated during the 62 months of utilization of the SIPs in the PICU identified 283 real programming errors that led to cancelation and/or reprogramming of the drug infusion – with the consequent avoidance of potential adverse effects in the patient.
The greatest incidence of alerts per hour of the day corresponded to the intervals 11:00–14:00h and 17:00–18:00h. With regard to the distribution per month of the year, the periods from June to August, December and January, as well as March and April yielded the largest number of alerts due to programming errors.
Percentage utilization of the safety software for the programming of infusions during the entire study period was 84% (840,223 infusions were started through the drug library out of a total of 997,723 programmed infusions using volumetric pumps and syringe pumps). Programmed administrations with volumetric pumps represented 11% of the total, while the remaining 89% involved the use of syringe pumps. Adherence to the drug library was 49.6% in the case of the volumetric pumps and 89.4% in the case of the syringe pumps, with a variation of ±1% over the entire study period.
The ratio between the number of alerts and the number of programmed infusions was 0.76%, while the percentage of alerts due to ignored relative limits was 68%, versus a 32% incidence of alerts due to relative limits that implied reprogramming of the infusion.
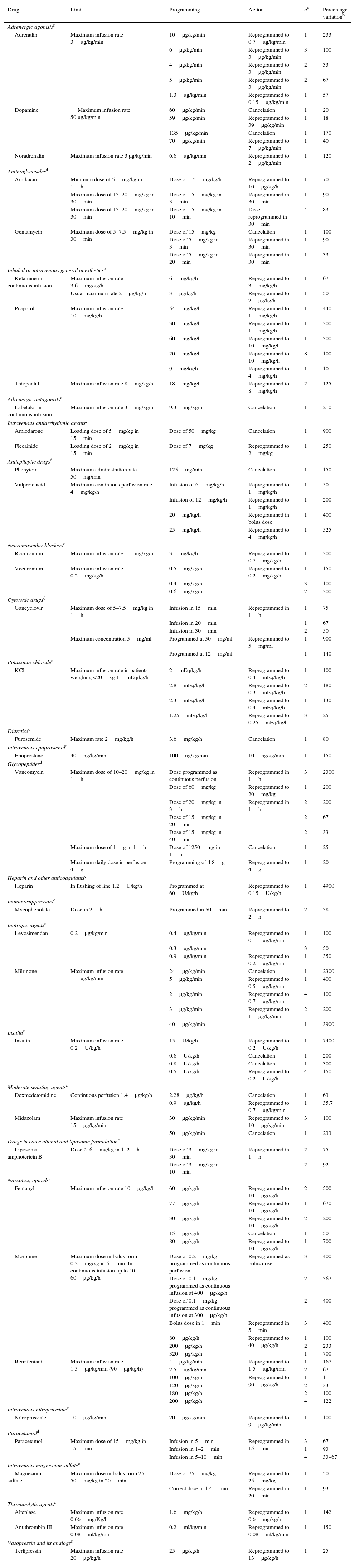
High risk drugs were implicated in 164 of the 283 intercepted programming errors (58%), involving mainly attempts to exceed an absolute upper limit (Table 1).
High risk drugs implicated in the intercepted programming errors.
| Drug | Limit | Programming | Action | na | Percentage variationb |
|---|---|---|---|---|---|
| Adrenergic agonistsc | |||||
| Adrenalin | Maximum infusion rate 3μg/kg/min | 10μg/kg/min | Reprogrammed to 0.7μg/kg/min | 1 | 233 |
| 6μg/kg/min | Reprogrammed to 3μg/kg/min | 3 | 100 | ||
| 4μg/kg/min | Reprogrammed to 3μg/kg/min | 2 | 33 | ||
| 5μg/kg/min | Reprogrammed to 3μg/kg/min | 2 | 67 | ||
| 1.3μg/kg/min | Reprogrammed to 0.15μg/kg/min | 1 | 57 | ||
| Dopamine | Maximum infusion rate 50 μg/kg/min | 60μg/kg/min | Cancelation | 1 | 20 |
| 59μg/kg/min | Reprogrammed to 39μg/kg/min | 1 | 18 | ||
| 135μg/kg/min | Cancelation | 1 | 170 | ||
| 70μg/kg/min | Reprogrammed to 7μg/kg/min | 1 | 40 | ||
| Noradrenalin | Maximum infusion rate 3 μg/kg/min | 6.6μg/kg/min | Reprogrammed to 2μg/kg/min | 1 | 120 |
| Aminoglycosidesd | |||||
| Amikacin | Minimum dose of 5mg/kg in 1h | Dose of 1.5mg/kg/h | Reprogrammed to 10μg/kg/h | 1 | 70 |
| Maximum dose of 15–20mg/kg in 30min | Dose of 15mg/kg in 3min | Reprogrammed in 30min | 1 | 90 | |
| Maximum dose of 15–20mg/kg in 30min | Dose of 15mg/kg in 10min | Dose reprogrammed in 30min | 4 | 83 | |
| Gentamycin | Maximum dose of 5–7.5mg/kg in 30min | Dose of 15mg/kg | Cancelation | 1 | 100 |
| Dose of 5mg/kg in 3min | Reprogrammed in 30min | 1 | 90 | ||
| Dose of 5mg/kg in 20min | Reprogrammed in 30min | 1 | 33 | ||
| Inhaled or intravenous general anestheticsc | |||||
| Ketamine in continuous infusion | Maximum infusion rate 3.6mg/kg/h | 6mg/kg/h | Reprogrammed to 3mg/kg/h | 1 | 67 |
| Usual maximum rate 2μg/kg/h | 3μg/kg/h | Reprogrammed to 2μg/kg/h | 1 | 50 | |
| Propofol | Maximum infusion rate 10mg/kg/h | 54mg/kg/h | Reprogrammed to 1mg/kg/h | 1 | 440 |
| 30mg/kg/h | Reprogrammed to 1mg/kg/h | 1 | 200 | ||
| 60mg/kg/h | Reprogrammed to 10mg/kg/h | 1 | 500 | ||
| 20mg/kg/h | Reprogrammed to 10mg/kg/h | 8 | 100 | ||
| 9mg/kg/h | Reprogrammed to 4mg/kg/h | 1 | 10 | ||
| Thiopental | Maximum infusion rate 8mg/kg/h | 18mg/kg/h | Reprogrammed to 8mg/kg/h | 2 | 125 |
| Adrenergic antagonistsc | |||||
| Labetalol in continuous infusion | Maximum infusion rate 3mg/kg/h | 9.3mg/kg/h | Cancelation | 1 | 210 |
| Intravenous antiarrhythmic agentsc | |||||
| Amiodarone | Loading dose of 5mg/kg in 15min | Dose of 50mg/kg | Cancelation | 1 | 900 |
| Flecainide | Loading dose of 2mg/kg in 15min | Dose of 7mg/kg | Reprogrammed to 2mg/kg | 1 | 250 |
| Antiepileptic drugsd | |||||
| Phenytoin | Maximum administration rate 50mg/min | 125mg/min | Cancelation | 1 | 150 |
| Valproic acid | Maximum continuous perfusion rate 4mg/kg/h | Infusion of 6mg/kg/h | Reprogrammed to 1mg/kg/h | 1 | 50 |
| Infusion of 12mg/kg/h | Reprogrammed to 1mg/kg/h | 1 | 200 | ||
| 20mg/kg/h | Reprogrammed in bolus dose | 1 | 400 | ||
| 25mg/kg/h | Reprogrammed to 4mg/kg/h | 1 | 525 | ||
| Neuromuscular blockersc | |||||
| Rocuronium | Maximum infusion rate 1mg/kg/h | 3mg/kg/h | Reprogrammed to 0.7mg/kg/h | 1 | 200 |
| Vecuronium | Maximum infusion rate 0.2mg/kg/h | 0.5mg/kg/h | Reprogrammed to 0.2mg/kg/h | 1 | 150 |
| 0.4mg/kg/h | 3 | 100 | |||
| 0.6mg/kg/h | 2 | 200 | |||
| Cytotoxic drugsd | |||||
| Gancyclovir | Maximum dose of 5–7.5mg/kg in 1h | Infusion in 15min | Reprogrammed in 1h | 1 | 75 |
| Infusion in 20min | 1 | 67 | |||
| Infusion in 30min | 2 | 50 | |||
| Maximum concentration 5mg/ml | Programmed at 50mg/ml | Reprogrammed to 5mg/ml | 1 | 900 | |
| Programmed at 12mg/ml | 1 | 140 | |||
| Potassium chloridec | |||||
| KCl | Maximum infusion rate in patients weighing <20kg 1mEq/kg/h | 2mEq/kg/h | Reprogrammed to 0.4mEq/kg/h | 1 | 100 |
| 2.8mEq/kg/h | Reprogrammed to 0.3mEq/kg/h | 2 | 180 | ||
| 2.3mEq/kg/h | Reprogrammed to 0.4mEq/kg/h | 1 | 130 | ||
| 1.25mEq/kg/h | Reprogrammed to 0.25mEq/kg/h | 3 | 25 | ||
| Diureticsd | |||||
| Furosemide | Maximum rate 2mg/kg/h | 3.6mg/kg/h | Cancelation | 1 | 80 |
| Intravenous epoprostenolc | |||||
| Epoprostenol | 40ng/kg/min | 100ng/kg/min | 10ng/kg/min | 1 | 150 |
| Glycopeptidesd | |||||
| Vancomycin | Maximum dose of 10–20mg/kg in 1h | Dose programmed as continuous perfusion | Reprogrammed in 1h | 3 | 2300 |
| Dose of 60mg/kg | Reprogrammed to 20mg/kg | 1 | 200 | ||
| Dose of 20mg/kg in 3h | Reprogrammed in 1h | 2 | 200 | ||
| Dose of 15mg/kg in 20min | 2 | 67 | |||
| Dose of 15mg/kg in 40min | 2 | 33 | |||
| Maximum dose of 1g in 1h | Dose of 1250mg in 1h | Cancelation | 1 | 25 | |
| Maximum daily dose in perfusion 4g | Programming of 4.8g | Reprogrammed to 4g | 1 | 20 | |
| Heparin and other anticoagulantsc | |||||
| Heparin | In flushing of line 1.2U/kg/h | Programmed at 60U/kg/h | Reprogrammed to 0.15U/kg/h | 1 | 4900 |
| Immunosuppressorsd | |||||
| Mycophenolate | Dose in 2h | Programmed in 50min | Reprogrammed to 2h | 2 | 58 |
| Inotropic agentsc | |||||
| Levosimendan | 0.2μg/kg/min | 0.4μg/kg/min | Reprogrammed to 0.1μg/kg/min | 1 | 100 |
| 0.3μg/kg/min | 3 | 50 | |||
| 0.9μg/kg/min | Reprogrammed to 0.2μg/kg/min | 1 | 350 | ||
| Milrinone | Maximum infusion rate 1μg/kg/min | 24μg/kg/min | Cancelation | 1 | 2300 |
| 5μg/kg/min | Reprogrammed to 0.5μg/kg/min | 1 | 400 | ||
| 2μg/kg/min | Reprogrammed to 0.7μg/kg/min | 4 | 100 | ||
| 3μg/kg/min | Reprogrammed to 1μg/kg/min | 2 | 200 | ||
| 40μg/kg/min | 1 | 3900 | |||
| Insulinc | |||||
| Insulin | Maximum infusion rate 0.2U/kg/h | 15U/kg/h | Reprogrammed to 0.2U/kg/h | 1 | 7400 |
| 0.6U/kg/h | Cancelation | 1 | 200 | ||
| 0.8U/kg/h | Cancelation | 1 | 300 | ||
| 0.5U/kg/h | Reprogrammed to 0.2U/kg/h | 4 | 150 | ||
| Moderate sedating agentsc | |||||
| Dexmedetomidine | Continuous perfusion 1.4μg/kg/h | 2.28μg/kg/h | Cancelation | 1 | 63 |
| 0.9μg/kg/h | Reprogrammed to 0.7μg/kg/min | 1 | 35.7 | ||
| Midazolam | Maximum infusion rate 15μg/kg/min | 30μg/kg/min | Reprogrammed to 10μg/kg/min | 3 | 100 |
| 50μg/kg/min | Cancelation | 1 | 233 | ||
| Drugs in conventional and liposome formulationc | |||||
| Liposomal amphotericin B | Dose 2–6mg/kg in 1–2h | Dose of 3mg/kg in 30min | Reprogrammed in 1h | 2 | 75 |
| Dose of 3mg/kg in 10min | 2 | 92 | |||
| Narcotics, opioidsc | |||||
| Fentanyl | Maximum infusion rate 10μg/kg/h | 60μg/kg/h | Reprogrammed to 10μg/kg/h | 2 | 500 |
| 77μg/kg/h | Reprogrammed to 10μg/kg/h | 1 | 670 | ||
| 30μg/kg/h | Reprogrammed to 10μg/kg/h | 2 | 200 | ||
| 15μg/kg/h | Cancelation | 1 | 50 | ||
| 80μg/kg/h | Reprogrammed to 10μg/kg/h | 1 | 700 | ||
| Morphine | Maximum dose in bolus form 0.2mg/kg in 5min. In continuous infusion up to 40–60μg/kg/h | Dose of 0.2mg/kg programmed as continuous perfusion | Reprogrammed as bolus dose | 3 | 400 |
| Dose of 0.1mg/kg programmed as continuous infusion at 400μg/kg/h | 2 | 567 | |||
| Dose of 0.1mg/kg programmed as continuous infusion at 300μg/kg/h | 2 | 400 | |||
| Bolus dose in 1min | Reprogrammed in 5min | 3 | 400 | ||
| 80μg/kg/h | Reprogrammed to 40μg/kg/h | 1 | 100 | ||
| 200μg/kg/h | 2 | 233 | |||
| 320μg/kg/h | 1 | 700 | |||
| Remifentanil | Maximum infusion rate 1.5μg/kg/min (90μg/kg/h) | 4μg/kg/min | Reprogrammed to 1.5μg/kg/min | 1 | 167 |
| 2.5μg/kg/min | 2 | 67 | |||
| 100μg/kg/h | Reprogrammed to 90μg/kg/h | 1 | 11 | ||
| 120μg/kg/h | 2 | 33 | |||
| 180μg/kg/h | 2 | 100 | |||
| 200μg/kg/h | 4 | 122 | |||
| Intravenous nitroprussiatec | |||||
| Nitroprussiate | 10μg/kg/min | 20μg/kg/min | Reprogrammed to 9μg/kg/min | 1 | 100 |
| Paracetamold | |||||
| Paracetamol | Maximum dose of 15mg/kg in 15min | Infusion in 5min | Reprogrammed in 15min | 3 | 67 |
| Infusion in 1–2min | 1 | 93 | |||
| Infusion in 5–10min | 4 | 33–67 | |||
| Intravenous magnesium sulfatec | |||||
| Magnesium sulfate | Maximum dose in bolus form 25–50mg/kg in 20min | Dose of 75mg/kg | Reprogrammed to 25mg/kg | 1 | 50 |
| Correct dose in 1.4min | Reprogrammed in 20min | 1 | 93 | ||
| Thrombolytic agentsc | |||||
| Alteplase | Maximum infusion rate 0.66mg/Kg/h | 1.6mg/kg/h | Reprogrammed to 0.6mg/kg/h | 1 | 142 |
| Antithrombin III | Maximum infusion rate 0.08ml/kg/min | 0.2ml/kg/min | Reprogrammed to 0.08ml/kg/min | 1 | 150 |
| Vasopressin and its analogsc | |||||
| Terlipressin | Maximum infusion rate 20μg/kg/h | 25μg/kg/h | Reprogrammed to 13μg/kg/h | 1 | 25 |
Table 2 (online supplementary material) shows the remaining drugs, not regarded as high risk substances, related to programming errors.
DiscussionGuaranteeing safe drug use in patients is one of the main responsibilities of healthcare professionals. In order to assess the impact of the introduction of SIP technology in relation to the safety of intravenous drug administration, a distinction must be made between “medication error” and “adverse event”24,25:
Medication error is understood to be “any avoidable incident that can cause damage to the patient or give rise to inadequate medication use”.
An adverse event is defined as “any damage, whether mild or severe, caused by the use of a medication, or any damage resulting from the clinical use of a medication”. Adverse events in turn can be classified as avoidable (associated to medication error) or unavoidable (related to adverse reactions).
It should be noted that the programming errors intercepted by SIP technology reported in this study were avoidable incidents that did not reach the patient, but which could have caused an adverse event had they reached the patient.
In order to guarantee the contribution of this technology to safe drug use, a continuous evaluation program should be established in order to identify opportunities for improvement and thus optimize each phase of the project.26,27
The introduction of SIP technology in our PICU has achieved a drug library adherence rate of 84%, which is similar to the 92% adherence rate reported by some authors28 and far higher than the rates described in other studies (in the range of 30–40%).26,29
It is important to note that the drug library was the same with both the volumetric pumps and the syringe pumps, with the sole exception of fluid therapy, which was only available for the volumetric pumps, since these are the main systems used in the administration of fluids. In this regard, and because of problems in configuring the fluid therapy profile, for some time we were unable to start infusions through the database, and adherence with these devices was very low (49.6%). This had a negative impact upon overall adherence of SIP technology, since the safety software adherence rate was 89.4% when only considering the syringe pumps.
The fact that a drug library is incorporated to the infusion systems does not oblige the user to program administration through the library, and this is the most important limitation of the technology. However, in a certain sense the possibility of deciding whether or not to use the drug library may be an advantage in some situations, such as for example when intending to infuse a medication not included in the drug library, or in emergency scenarios requiring rapid patient stabilization, where we do not have the seconds needed to search the database for the drug to be programmed. Thus, reinforcing training and promoting safety culture among the nursing personnel are key elements for improving adherence to SIP technology.21
With regard to the distribution of alerts according to the time of day, the hourly intervals exhibiting the largest number of alerts, namely 11:00–14:00h and 17:00–18:00h, coincide with the hours in which medication is administered in the Unit, and where the probability of error is therefore greater. Fanikos et al.30 found the time interval with the largest number of alerts to be between 14:00–16:00h, coinciding with the changing of shift in the Unit.
On the other hand, an analysis of the distribution of alerts over the different months of the year shows the vacation months to accumulate a greater proportion of alerts. This is probably explained by the incorporation of substituting personnel members who are less familiarized with the systems and the protocols used in the Unit.
In our study, the percentage reprogramming rate due to the breaching of relative limits was very high when compared with the 4–6% reported by Breland,26 but lower than the 43.2% documented by Fanikos et al.30
In any case, and as also evidenced by other authors,31 the data obtained question the usefulness of relative limits, since they tend to be ignored. This is in contrast to absolute limits, which oblige infusion reprogramming and are therefore more directly implicated in the interception of errors.29,32 In our study, the breaching of relative limits did not result in potentially serious errors (referred to either the magnitude of the error or the type of drug involved). Nevertheless, periodic revision of these limits makes it possible to optimize their performance.
The implementation of this technology allowed the interception of 283 programming errors of different kinds during 62 months of SIP use in our PICU.
Estimation of the potential seriousness of an intercepted error and of the probability of causing an adverse event if the error had reached the patient is usually based on clinical judgment. The lack of a validated universal method for establishing the potential or real causal relationship of a medication error and of an adverse event often results in great variability in the classifications.33 Since these are avoided medication errors, it is difficult to estimate the damage they would have caused had they reached the patient. Nevertheless, the fact that 58% of the drugs implicated in these errors were high risk substances reasonably suggests that the potential damage would have been greater. In this regard, it must be taken into account that the high risk drug classification published by the ISMP makes no distinction between the adult and pediatric populations; as a result, there is an information gap regarding the safety profile of drugs used in children. With the purpose of casting some light on this issue, in 2013 the official journal of the Spanish Association of Pediatrics published a list of high risk drugs used in the pediatric population. In addition to the drugs cited by the ISMP, the list also contains some other substances of special interest in the pediatric population, such as paracetamol, antiepileptic drugs, immunosuppressors, diuretics and aminoglycoside and glycopeptide antibiotics.23 According to the information published in this article, the percentage of intercepted errors involving a high risk drug increased from 43.8% when only considering the ISMP classification to 58% when also considering high risk drugs in the pediatric population.
Likewise, the age of the patients (pediatric subjects in our case) is crucial for determining the potential seriousness of the intercepted administration errors, as evidenced by Folli et al.34 The administration of incorrect doses as a consequence of overdose was the most frequent error in the mentioned article, in coincidence with our own study, where most intercepted errors corresponded to the programming of doses or infusion rates far higher than those recommended for a given patient.
The main pharmacotherapeutic groups associated to programming error were adrenergic agonists and antagonists, sedating agents, analgesics, neuromuscular blockers and opioid narcotics, together with insulin and potassium.
In relation to antibiotics, it should be mentioned that although most of them are not included in the proposed lists of high risk drugs, they are associated to a large number of programming errors (representing 33.6% of the errors involving drugs not considered to be high risk substances).
Although most of the intercepted errors corresponded to overdose or the infusion of the correct dose in less than the recommended time, we found that a dose lower than the recommended dose had been programmed in two cases, corresponding to acyclovir and amikacin.
Other drugs not considered to be high risk substances, but which were related to programming errors intercepted in our study were metamizol, octreotide, omeprazole and ranitidine.
Practically all the programming errors identified in our study corresponded to alerts produced by the breaching of absolute upper limits. Other investigators have described programming errors due to the breaching of relative upper limits, with consequent reprogramming of the infusion.26,35 The drugs involved include propofol, fentanyl and vecuronium, i.e., analgesic-sedating agents; inotropic drugs such as dopamine and noradrenalin; insulin; and antibiotics such as vancomycin and linezolid. This error profile referred to the type of drug is consistent with that recorded in our own study. Fanikos et al.30 in turn underscored the importance of implementing SIP technology with a view to reducing anticoagulant drug programming errors.
Although some studies point to the potential of SIP technology in reducing the incidence of administration errors due to incorrect programming,31,36 some authors question its usefulness. Rothschild et al. concluded that the implementation of SIPs in critical care has no effect upon the number of avoided serious errors. The reason for this apparent failure of SIP technology to lower the number of errors is the scant utilization of the safety software observed by the reports. In this case, in the event of an error in programming an infusion, the system would not generate the corresponding alert, and the medication error could therefore reach the patient.29 The authors considered that despite the promising error-detecting potential of these systems, the factor limiting their success is acceptance of such technology by the nursing personnel – these being the professionals that handle such systems on a daily basis.
Likewise, different studies have reported that when SIP technology is used on an isolated basis in the healthcare setting, its capacity to detect errors is limited.37,38 It must be taken into account that of the “5 correct criteria” that must be met to guarantee safety of the drug administration process (i.e., correct patient, drug, dose, administration route and timing), only correct dosing can be ensured with this technology,39 and possible errors, such as inadequate identification of the patient, administration of the wrong medication, or the use of an incorrect administration route or timing, can still happen.
As long as those hospitals that use SIPs for the administration of intravenous drugs fail to develop an interface to connect the different technologies, certain types of errors will continue to occur within the drug utilization circuit – with possible consequences for the patients.
LimitationsSmart infusion pumps are not “smart” in themselves, and it must be taken into account that this technology has its limitations. The possibility of programming infusions outside the context of the drug library, together with limited capacity to intercept errors other than “incorrect dosing” when used in isolated settings, are two of the fundamental limitations of this technology, as explained above.
On the other hand, despite the global prospective design of our study, the infusion systems used did not allow real-time analysis of the results obtained, and this retrospective nature of data exploitation complicated access to the details that developed after the canceled programmings described both in our study and in that of Fanikos et al.30
The availability of systems affording greater connectivity, with data flow in a wireless environment, could facilitate prospective and per patient analysis of the stored information; offer a more precise estimate of the true impact of this technology; and, in future, contribute to integration with the rest of the hospital technologies to ensure patient safety in all the stages of the drug utilization circuit.40
ConclusionsThe implementation of SIP technology in a PICU has been shown to have a great impact in our hospital, since it is able to intercept programming errors related to high risk drugs.
The results obtained in our study may serve as the basis for extending this technology to other Units (with both adult and pediatric patients) that might benefit from it in the same measure as in the PICU. Nevertheless, due consideration is required of the limitations of this technology when it is used on an isolated basis. Collaboration among the different healthcare professionals – physicians, pharmacists, nursing personnel – and computing and software specialists is essential in order to guarantee the success of the project and make this tool easy to use and effective in avoiding errors.
Conflicts of interestThe authors agree with the contents of the present manuscript and declare that they have no conflicts of interest.
The authors express their sincere gratitude to all the professionals of the Pediatric Intensive Care Unit for their collaboration and implication in the development and implementation of this technology.
Please cite this article as: Manrique-Rodríguez S, Sánchez-Galindo AC, Fernández-Llamazares CM, Calvo-Calvo MM, Carrillo-Álvarez Á, Sanjurjo-Sáez M. Administración segura de medicamentos intravenosos en pediatría: 5 años de experiencia de una Unidad de Cuidados Intensivos Pediátricos con bombas de infusión inteligentes. Med Intensiva. 2016;40:411–421.