Edited by: Federico Gordo - Medicina Intensiva del Hospital Universitario del Henares (Coslada-Madrid)
Last update: December 2023
More infoSelective digestive decontamination (SDD) is a prophylactic strategy aimed at preventing or eradicating bacterial overgrowth in the intestinal flora that precedes the development of most infections in the Intensive Care Unit. SDD prevents serious infections, reduces mortality, is cost-effective, has no adverse effects, and its short- or long-term use is not associated with any significant increase in antimicrobial resistance. SDD is one of the most widely evaluated interventions in critically ill patients, yet its use is not widespread. The present article offers a narrative review of the most relevant evidence and an update of the pathophysiological concepts of infection control supporting the use of SDD.
La descontaminación digestiva selectiva (DDS) es una estrategia profiláctica cuyo objetivo es prevenir o erradicar el sobrecrecimiento bacteriano en la flora intestinal que precede al desarrollo de la mayoría de las infecciones en la UCI. La DDS previene infecciones graves, reduce la mortalidad, es coste-efectiva, no tiene efectos adversos, y su uso a corto o largo plazo no muestra un aumento significativo de la resistencia antimicrobiana. La DDS es una de las intervenciones más evaluadas en pacientes críticos, a pesar de lo cual su uso no se ha generalizado. El objetivo de este artículo es presentar una revisión narrativa de la evidencia más relevante y una actualización de los conceptos fisiopatológicos de control de la infección en los que se fundamenta el uso de la DDS.
The prevention, diagnosis and treatment of infections is one of the main challenges in the management of critical patients. Infections in the Intensive Care Unit (ICU) have been associated with increased morbidity, mortality and healthcare costs.1–3 Selective digestive decontamination (SDD) is a preventive strategy for critical patients involving the use of an oropharyngeal paste and an enteral suspension containing non-absorbable antimicrobial agents, along with the administration of an intravenous antibiotic, during the first four days of ICU stay, with the collection of surveillance samples of the oropharyngeal and intestinal flora, and observation of the recommendations referred to hygiene. SDD aims to prevent or eradicate bacterial overgrowth of the intestinal flora and reduce the incidence of infections in the ICU. Selective digestive decontamination has been widely evaluated over almost 40 years in more than 70 clinical trials. However, although SDD is now routinely used in ICUs throughout Europe, its application has not become generalized in clinical practice,4 despite the evidence of its efficacy and safety.
The present narrative review summarizes the main evidence on the impact of SDD on respiratory infections, bacteremia and mortality in patients subjected to mechanical ventilation, and upon antimicrobial resistance, and addresses the concept of nosocomial infection control. In addition, recommendations for the correct management of SDD in critical patients are established.
A structured literature search was carried out in MEDLINE/PubMed using the keywords selective digestive or oropharyngeal decontamination, selective decontamination of the digestive tract, intensive care, critically ill patients, infections and antibiotic prophylaxis or prevention. Articles published in English or Spanish between 1983 and 2022 were included.
OriginsThe concept of SDD originated from several key observations made in the 1960s and 1970s. Johanson reported that the digestive flora of patients changes after a few days of hospital admission, with a predominance of gram-negative bacteria (GNB), and that the main factor underlying this change is disease severity.5 Later studies showed these GNB to be the cause of a significant percentage of the infections suffered by critical patients, particularly pneumonia.6
In the mid-1970s, Bodey7 showed that many systemic antimicrobials can sterilize the lungs, blood and bladder, but are usually unable to eliminate such GNB from the oropharynx and/or intestine. He found that the enteral administration of non-absorbable antibiotics can eliminate the GNB from the gastrointestinal tract, as a result of the high drug concentrations reached in the intestinal lumen.8 The combination of polymyxin E (colistin) and tobramycin was chosen given its efficacy against GNB, including Pseudomonas spp., and because it constitutes a synergic combination "in vitro".
Selective digestive decontamination in critical patients was first described by Stoutenbeek et al. in 1984.9 The pathogens selected for prevention were Enterobacteria, Pseudomonas aeruginosa and Staphylococcus aureus. The intestinal flora was considered to be the source of these microorganisms for colonization of the upper respiratory tract during hospital stay. The combination of tobramycin and colistin was administered as an oropharyngeal paste in the oral cavity, and as a solution administered through the nasogastric tube (both four times a day). The small initial studies carried out (mainly in trauma patients) led to two modifications of the strategy. Amphotericin B was incorporated to avoid intestinal yeast overgrowth, and four days of intravenous prophylactic treatment with cefotaxime were added. Cefotaxime was chosen because it was assumed that at the time of injury and admission to the hospital, trauma patients would have a normal respiratory tract flora, sensitive to a third-generation cephalosporin (Supplementary material).
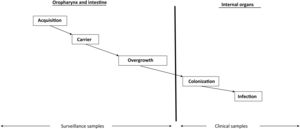
Overgrowth: risk factor for infection and the development of antimicrobial resistanceCritical disease has a strong qualitative and quantitative impact on the digestive flora, favoring a switch from "normal" potentially pathogenic microorganism (PPM) carrier status to "abnormal" PPM carrier status, and from a low bacterial burden (<105 PPMs per milliliter or per gram of digestive tract secretions) to a high bacterial burden, with concentrations regarded as constituting overgrowth (≥105 PPMs per milliliter or per gram of digestive tract secretions)11 (Supplementary material).
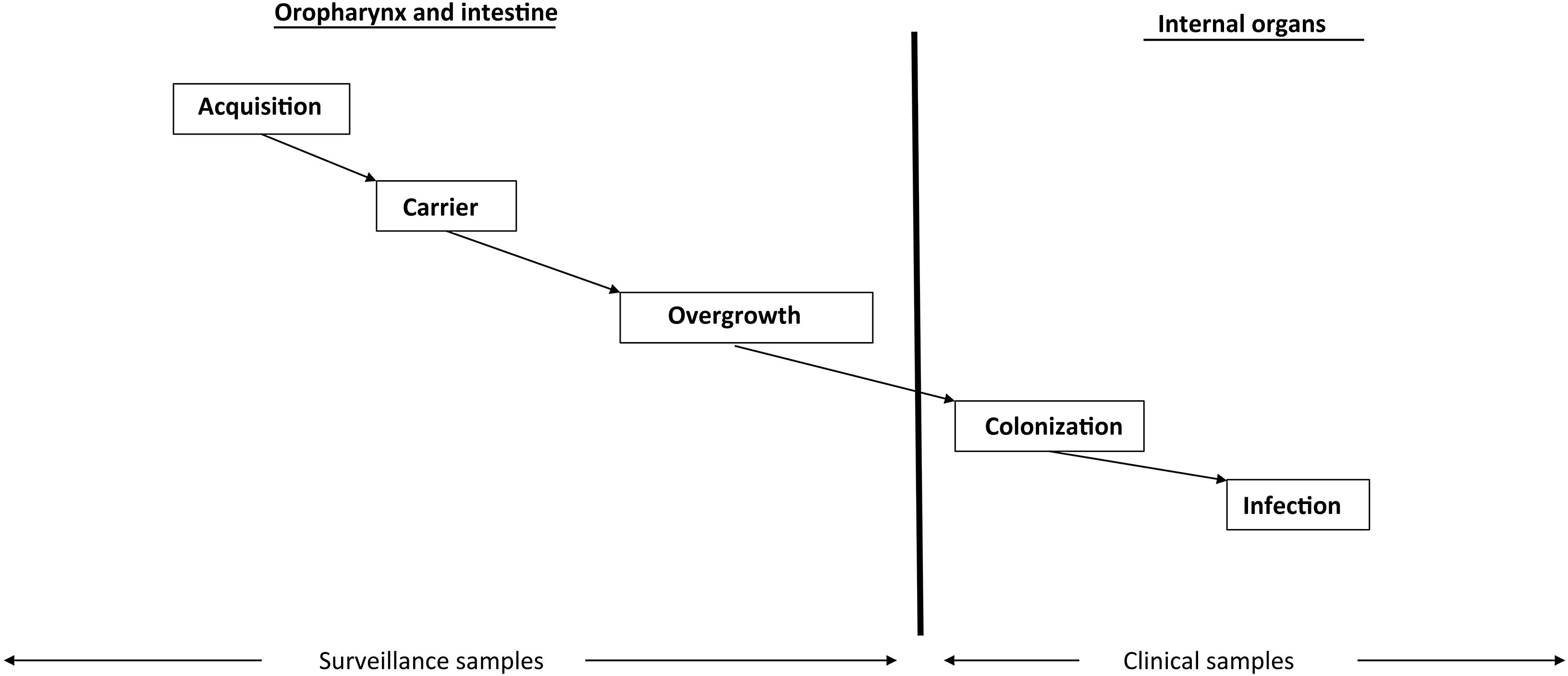
Intestinal overgrowth precedes the development of most infections in the ICU (Fig. 1). The PPMs present at overgrowth concentrations in the oropharynx and intestine and which migrate towards the lower respiratory tract and bladder in turn causing respiratory and urinary colonization and infection, respectively.12
Intestinal microbial overgrowth in the critical patient is moreover a key risk factor for the development of antimicrobial resistance (Supplementary material). The administration of systemic antimicrobials, which are excreted in low concentrations into the intestinal lumen and eradicate the most sensitive flora, in turn favors the selection of more resistant flora.11,13,14
Overall, the prevention of overgrowth in the intestine is essential to avoid infection and the appearance of resistances in critical patients.15
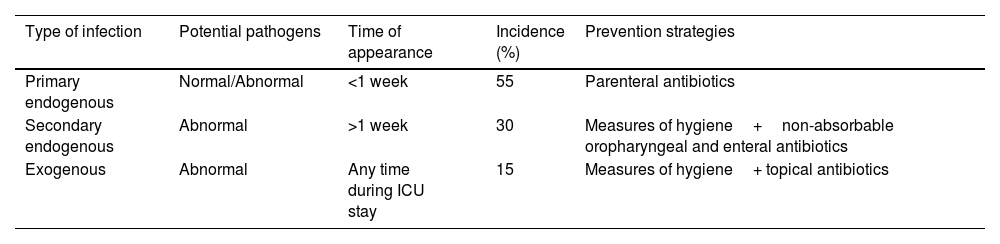
Control of infection: selective digestive decontamination (SDD)Infections in the ICU are classified according to the carrier status of the patient (Supplementary material). The surveillance samples from the oropharynx and rectum, along with the diagnostic samples, allow us to differentiate three types of infection: primary endogenous, secondary endogenous and exogenous infections, and to develop an effective specific control program for each type of infection (Table 1). Although the prevention of transmission from live (patient) and inert reservoirs (devices) is essential and requires strict compliance with the usual measures of hygiene (hands, wearing of gloves and isolation), it is ineffective for controlling endogenous infections,1 which account for approximately 85% of all infections in critical patients.16–18
Types of infections and their prevention.
| Type of infection | Potential pathogens | Time of appearance | Incidence (%) | Prevention strategies |
|---|---|---|---|---|
| Primary endogenous | Normal/Abnormal | <1 week | 55 | Parenteral antibiotics |
| Secondary endogenous | Abnormal | >1 week | 30 | Measures of hygiene+non-absorbable oropharyngeal and enteral antibiotics |
| Exogenous | Abnormal | Any time during ICU stay | 15 | Measures of hygiene+ topical antibiotics |
Selective digestive decontamination seeks to reduce endogenous infections by preventing or eradicating potentially pathogenic flora carrier status in the patient, and consists of four components: (1) a short parenteral antibiotic cycle after admission to the ICU; (2) the administration of non-absorbable antibiotics in the oropharynx and digestive tract; (3) strict compliance with the measures of hygiene; and (4) flora surveillance samples (oropharyngeal exudate and rectal swabs) to monitor the efficacy of SDD, bacterial overgrowth, and the possible appearance of resistant microorganisms.9,10,19–22
Parenteral antibioticsThe administration of parenteral antibiotics in the first four days of ICU stay allows us to control primary endogenous infections - fundamentally respiratory infections - produced by PPMs present in the flora and which colonize the oropharynx of patients upon admission to the ICU, and often also the tracheobronchial tree after intubation. Cefotaxime is the parenteral antibiotic of choice in the SDD strategy, since it is effective against the normal PPMs and is excreted in saliva, where the drug reaches bactericidal concentrations. Cefotaxime can be replaced by other antibiotics if the patient is already receiving parenteral antibiotics that are effective against the microorganisms causing an infection, or if it is known or suspected that the patient carries oropharyngeal flora that might not be sensitive to cefotaxime (multidrug-resistant GNB [MRGNB] or methicillin-resistant Staphylococcus aureus [MRSA]).23,24
Non-absorbable antibioticsThe administration of non-absorbable antibiotics in the form of an oropharyngeal paste and enteral suspension can prevent and treat carrier status. Enteral antibiotics aim to eradicate or avoid the overgrowth of abnormal PPMs and thus prevent the colonization and infection of normally sterile internal organs (Fig. 1). The administered antibiotics must meet several criteria: they must be non-absorbable, treat flora sensitive to such antibiotics, reach bactericidal levels within the digestive tract, preserve (as far as possible) the autochthonous anaerobic flora needed to control colonization, and be safe and inexpensive. The enteral antimicrobial combination of colistin (polymyxin E), tobramycin and amphotericin B (or nystatin) complied with these criteria when it was first described,7 but the appearance of outbreaks and endemics due to MRSA and MRGNB in some cases makes it necessary to modify the original combination. The use of nystatin in European ICUs, in place of amphotericin B, is due to a lack of supply of amphotericin B in powder form for inclusion in the SDD composition. Oropharyngeal decontamination is achieved after 48−72hours. Intestinal decontamination takes longer, between 5 and 7 days after the start of SDD, provided intestinal motility is intact (Supplementary material).
Measures of hygieneThe enteral and parenteral administration of antibiotics does not prevent exogenous infections. Only strict compliance with the measures of hygiene can prevent such infections.25,26 Tracheotomized patients may acquire abnormal PPMs directly through the tracheotomy, in the absence of a previous oropharyngeal carrier status. Some exogenous infections, such as respiratory infections in tracheotomized individuals, can be controlled through the application of the oropharyngeal paste in the stoma.27
Surveillance samplesThe use of SDD must be accompanied by surveillance of the microbiological flora, collecting oropharyngeal samples and rectal swabs on the day of admission and twice a week. The obtainment of diagnostic samples for microbiological cultures, such as tracheal aspirates and urine samples, only confirms the clinical diagnosis of the infection and the causal microorganism. The surveillance samples are the only samples allowing us to detect overgrowth11 and thus assess the efficacy of the enteral antibiotics in eradicating normal and abnormal PPMs.4 The results obtained from the surveillance samples also serve as an early alert for detecting PPMs resistant to the antibiotics, thereby allowing us to establish isolation and contact measures to avoid cross-contamination (through the hands of the healthcare professionals, patient-to-patient, patient-to-devices, or vice versa), and to adjust the enteral and parenteral antibiotics to the sensitivities of the isolated PPMs.28–35
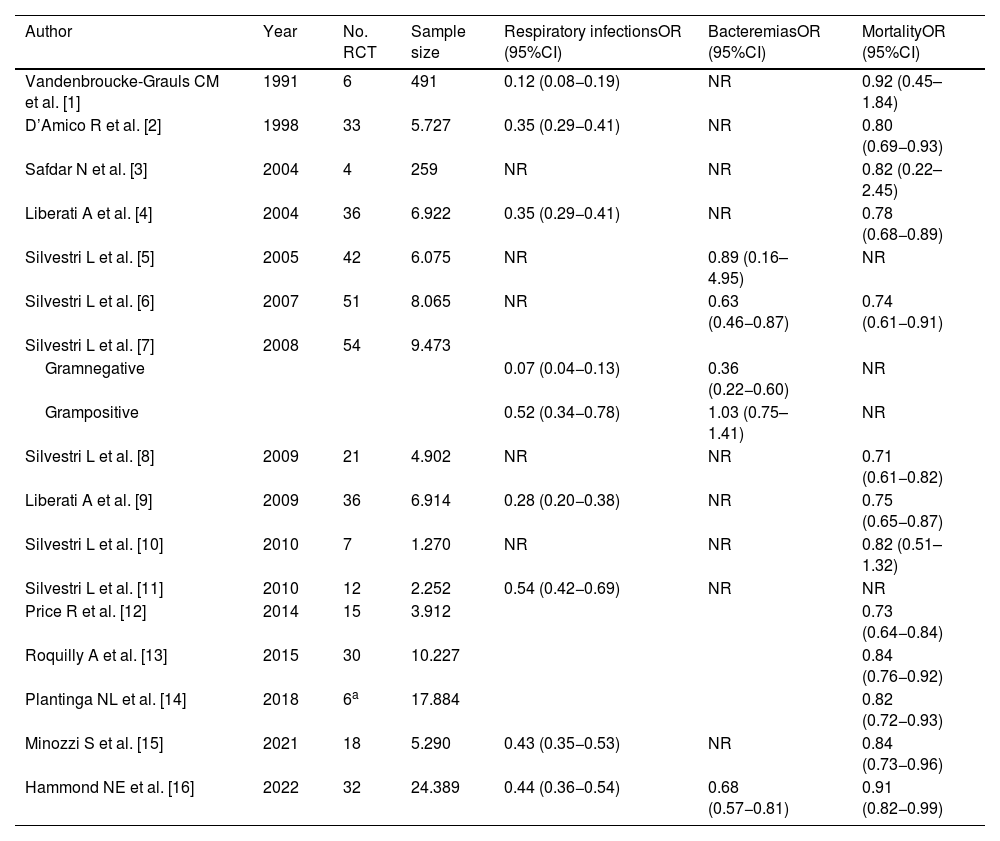
Clinical impact of SDDMany studies and meta-analyses in critical patients have shown SDD to prevent serious infections, reduce mortality, and prove cost-effective36–50 (Table 2) (references of Table S2 in Supplementary material).
Summary of the impact of SDD on respiratory infections, bacteremias and mortality in patients subjected to mechanical ventilation: 16 meta-analyses comprising a total of 73 clinical trials.
| Author | Year | No. RCT | Sample size | Respiratory infectionsOR (95%CI) | BacteremiasOR (95%CI) | MortalityOR (95%CI) |
|---|---|---|---|---|---|---|
| Vandenbroucke-Grauls CM et al. [1] | 1991 | 6 | 491 | 0.12 (0.08−0.19) | NR | 0.92 (0.45–1.84) |
| D’Amico R et al. [2] | 1998 | 33 | 5.727 | 0.35 (0.29−0.41) | NR | 0.80 (0.69−0.93) |
| Safdar N et al. [3] | 2004 | 4 | 259 | NR | NR | 0.82 (0.22–2.45) |
| Liberati A et al. [4] | 2004 | 36 | 6.922 | 0.35 (0.29−0.41) | NR | 0.78 (0.68−0.89) |
| Silvestri L et al. [5] | 2005 | 42 | 6.075 | NR | 0.89 (0.16–4.95) | NR |
| Silvestri L et al. [6] | 2007 | 51 | 8.065 | NR | 0.63 (0.46−0.87) | 0.74 (0.61−0.91) |
| Silvestri L et al. [7] | 2008 | 54 | 9.473 | |||
| Gramnegative | 0.07 (0.04−0.13) | 0.36 (0.22−0.60) | NR | |||
| Grampositive | 0.52 (0.34−0.78) | 1.03 (0.75–1.41) | NR | |||
| Silvestri L et al. [8] | 2009 | 21 | 4.902 | NR | NR | 0.71 (0.61−0.82) |
| Liberati A et al. [9] | 2009 | 36 | 6.914 | 0.28 (0.20−0.38) | NR | 0.75 (0.65−0.87) |
| Silvestri L et al. [10] | 2010 | 7 | 1.270 | NR | NR | 0.82 (0.51–1.32) |
| Silvestri L et al. [11] | 2010 | 12 | 2.252 | 0.54 (0.42−0.69) | NR | NR |
| Price R et al. [12] | 2014 | 15 | 3.912 | 0.73 (0.64−0.84) | ||
| Roquilly A et al. [13] | 2015 | 30 | 10.227 | 0.84 (0.76−0.92) | ||
| Plantinga NL et al. [14] | 2018 | 6a | 17.884 | 0.82 (0.72−0.93) | ||
| Minozzi S et al. [15] | 2021 | 18 | 5.290 | 0.43 (0.35−0.53) | NR | 0.84 (0.73−0.96) |
| Hammond NE et al. [16] | 2022 | 32 | 24.389 | 0.44 (0.36−0.54) | 0.68 (0.57−0.81) | 0.91 (0.82−0.99) |
SDD, selective digestive decontamination; RCT, randomized clinical trial; No., number; OR, odds ratio; CI, confidence interval; NR, not reported.
Study references: see Supplementary Material.
The latest update of the Cochrane review36 included 17 controlled clinical trials (RCTs) with 2951 patients subjected to mechanical ventilation, to evaluate the effect of SDD upon respiratory infections. A 57% decrease in the relative risk (RR) of suffering respiratory infections was observed (RR 0.43 [95%CI 0.35−0.53]); accordingly, one respiratory infection was avoided for every 4.6 treated patients.
Likewise, a recent systematic review and meta-analysis37 has been published, involving 22 studies with 3619 patients, in which SDD was seen to be associated with a decrease in the risk of ventilator-associated pneumonia (VAP)(RR 0.44 [95%CI 0.36−0.54])(Supplementary material).
Impact upon the incidence of bacteremiaDifferent studies have evaluated the effect of SDD on the incidence of bacteremia. In a recent systematic review, Hammond et al.37 (Supplementary material) found the use of SDD to reduce the risk of bacteremia in the ICU (RR 0.68 [95%CI 0.57−0.81]). Previously, in another systematic review, Silvesti et al.38 found that in the group of patients with catheter-associated bacteremia (9 RCTs with 1276 patients) (odds ratio [OR] 0.74 [95%CI 0.45–1.20]) and in the group of patients with non-catheter-associated bacteremia caused by gram-positive microorganisms (16 RCTs with 2097 patients) (OR 1.06 [95%CI 0.77–1.47]), SDD showed no significant effect. However, in the group of patients with non-catheter-associated bacteremia caused by GNB, the use of SDD resulted in a significant decrease in the incidence of bacteremia (OR 0.39 [95%CI 0.24−0.63]).
The lack of effect of SDD upon intravascular catheter-associated bacteremia supports the hypothesis that infections of this kind are mainly exogenous. This hypothesis is strengthened by the observed decrease in the incidence of catheter-associated bacteremia when only measures of hygiene are applied.26
More recently, Wittekamp et al.51 have reported the failure of the antimicrobial combination originally described for the prevention of bacteremias. In a cluster randomized RCT involving a large sample size, SDD was unable to reduce the incidence of bacteremias caused by MRGNB versus standard care in 4333 patients; the adjusted hazard ratio (HR) was 0.70 (95%CI 0.43–1.14). The published criticisms of this study were the lack of routine use of systemic antibiotics during the first days of ICU stay, and failure to adapt the composition of SDD to the specific sensitivity patterns of the isolated microorganisms, which would explain the high prevalence (14.8%) of rectal cultures with GNB growth after 14 days of administration of SDD.52,53
Impact upon mortality in patients subjected to mechanical ventilationThe latest Cochrane review36 that analyzed the effect of SDD on mortality included 18 studies with 5290 patients. The patients subjected to SDD showed a significant decrease in mortality versus placebo or no treatment (RR 0.84 [95%CI 0.73−0.96]); accordingly, one death was avoided for every 26 treated patients.
Previously, de Jonge et al.,39 in 934 patients, estimated the effect of SDD upon mortality in the ICU (RR 0.65 [95%CI 0.49−0.85]) and in hospital (RR 0.78 [95%CI 0.63−0.96]). A total of 12.3 patients in the ICU and 14.3 patients in the hospital had to be treated with SDD to avoid one death. On the other hand, de Smet et al.,40 in a cluster randomized RCT involving 4035 patients, estimated the mortality risk after 28 days between subjects treated with SDD and patients receiving standard care. After adjusting for covariables, the OR was 0.83 (95%CI 0.72−0.97).
However, in the most recent and last RCT published to date,45 involving 5982 patients, the use of SDD did not significantly reduce hospital mortality versus standard care (27% versus 29.1%, respectively; OR 0.91 [95%CI 0.82–1.02]; p=0.12). This result reflects a decrease in mortality of 1.7% in the patients subjected to SDD (95%CI −4.8% to 1.3%). Although not statistically significant, it allowed the authors to conclude that the confidence interval around the estimation of effect includes clinically important benefits (Supplementary material).
Furthermore, the most recent systematic review37 involved 32 studies, including those with the largest sample size,39,51 and incorporated the data from the last published RCT,45 with a total of 24,389 patients. This review concluded that the estimated RR of mortality among the patients subjected to mechanical ventilation and treated with SDD versus those receiving standard care was 0.91 (95%CI 0.82−0.99), with a posterior 99.3% probability that SDD is associated with decreased hospital mortality (bayesian analysis) (95%CI 1 %–18 %). Beneficial effects were only obtained by grouping the studies in which SDD included the intravenous component (RR 0.84 [95%CI 0.74−0.94]). The inclusion of clusters may underestimate the overall effect of SDD.54
Adverse effectsThe administration of SDD is safe. The latest Cochrane review36 established that no conclusions can be drawn regarding the adverse effects of SDD (gastrointestinal disorders or allergic reactions), since few were reported, and the data were scarce (Supplementary material).
Antimicrobial resistanceControl of infection outbreaks and endemics (modified SDD)The first clinical trial on the control of outbreaks with SDD was published in 1989.55 In this study, an endemic caused by multidrug-resistant K. pneumoniae was controlled using neomycin, polymyxin E and nalidixic acid as a decontaminating formula.
In persistent carriers of PPMs resistant to polymyxin or tobramycin or both, the topical antimicrobial formula of SDD must be adjusted, adding paromomycin, amikacin or another aminoglycoside in which the antibiogram evidences a more favorable minimum bactericidal concentration (MBC) for eradicating MRGNB.28–30,35 The isolation of MRSA from diagnostic and surveillance samples requires the oropharyngeal and enteral administration of vancomycin.31–34 Topical and enteral vancomycin added to the antimicrobials of SDD reduces colonization and morbidity and mortality, with no reported association between colonization by vancomycin-resistant enterococci (VRE) and the use of vancomycin.33,34,56 Cerdá et al.32 estimated the effect of vancomycin on the control of an MRSA endemic in a major burn unit for 9 years. During this period the authors documented four VRE carriers in which eradication was achieved without modifying the enteral and oropharyngeal administration of vancomycin.
This SDD strategy modified in accordance with the prevalent flora allows the eradication of MRSA or MRGNB carrier status, and has been successfully used in controlling outbreaks due to these resistant microorganisms.28–35,55,57
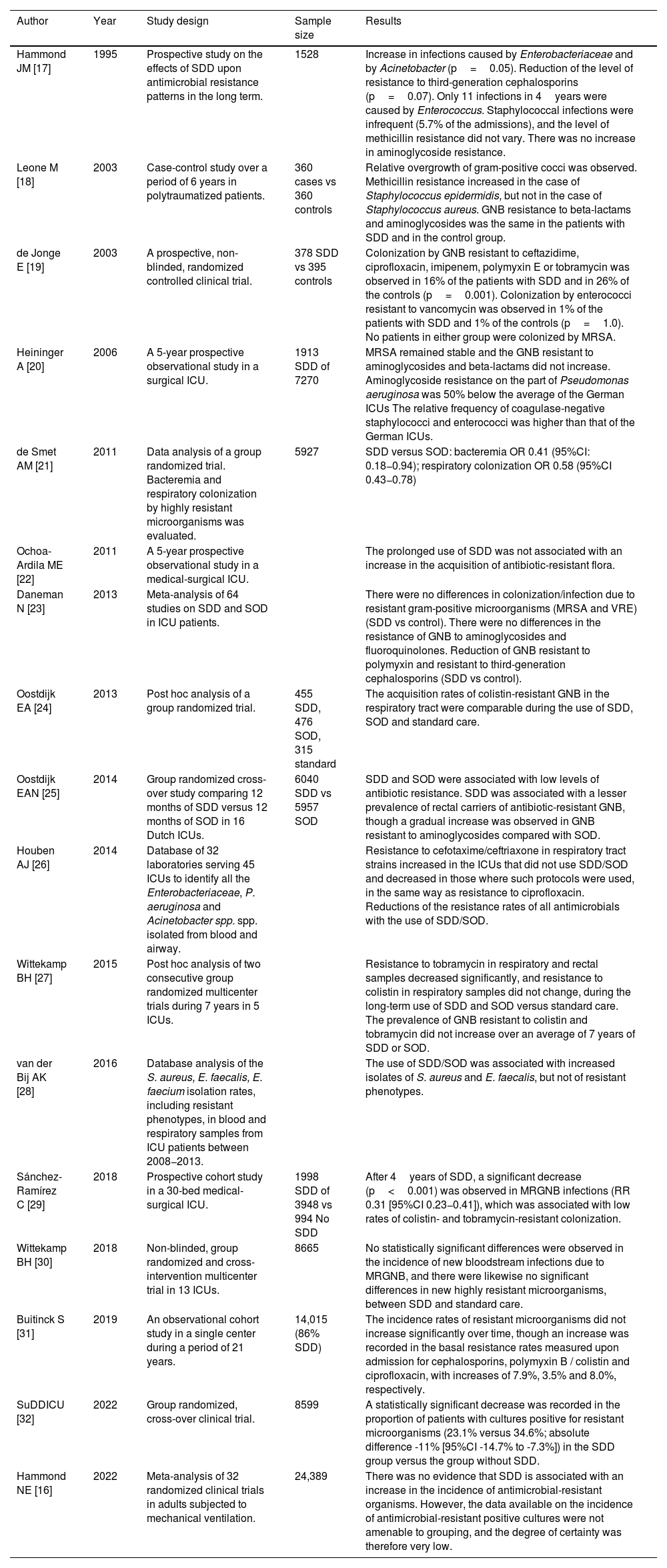
Impact of SDD upon antimicrobial resistanceEcological studies involving large sample sizes, meta-analyses and longitudinal studies with long follow-up periods have shown that the routine use of SDD is not associated with increased antibiotic resistance37,39,45,58–67 (Table 3) (references of Table S3 in Supplementary material).
Summary of the impact of SDD upon antimicrobial resistance.
| Author | Year | Study design | Sample size | Results |
|---|---|---|---|---|
| Hammond JM [17] | 1995 | Prospective study on the effects of SDD upon antimicrobial resistance patterns in the long term. | 1528 | Increase in infections caused by Enterobacteriaceae and by Acinetobacter (p=0.05). Reduction of the level of resistance to third-generation cephalosporins (p=0.07). Only 11 infections in 4years were caused by Enterococcus. Staphylococcal infections were infrequent (5.7% of the admissions), and the level of methicillin resistance did not vary. There was no increase in aminoglycoside resistance. |
| Leone M [18] | 2003 | Case-control study over a period of 6 years in polytraumatized patients. | 360 cases vs 360 controls | Relative overgrowth of gram-positive cocci was observed. Methicillin resistance increased in the case of Staphylococcus epidermidis, but not in the case of Staphylococcus aureus. GNB resistance to beta-lactams and aminoglycosides was the same in the patients with SDD and in the control group. |
| de Jonge E [19] | 2003 | A prospective, non-blinded, randomized controlled clinical trial. | 378 SDD vs 395 controls | Colonization by GNB resistant to ceftazidime, ciprofloxacin, imipenem, polymyxin E or tobramycin was observed in 16% of the patients with SDD and in 26% of the controls (p=0.001). Colonization by enterococci resistant to vancomycin was observed in 1% of the patients with SDD and 1% of the controls (p=1.0). No patients in either group were colonized by MRSA. |
| Heininger A [20] | 2006 | A 5-year prospective observational study in a surgical ICU. | 1913 SDD of 7270 | MRSA remained stable and the GNB resistant to aminoglycosides and beta-lactams did not increase. Aminoglycoside resistance on the part of Pseudomonas aeruginosa was 50% below the average of the German ICUs The relative frequency of coagulase-negative staphylococci and enterococci was higher than that of the German ICUs. |
| de Smet AM [21] | 2011 | Data analysis of a group randomized trial. Bacteremia and respiratory colonization by highly resistant microorganisms was evaluated. | 5927 | SDD versus SOD: bacteremia OR 0.41 (95%CI: 0.18−0.94); respiratory colonization OR 0.58 (95%CI 0.43−0.78) |
| Ochoa-Ardila ME [22] | 2011 | A 5-year prospective observational study in a medical-surgical ICU. | The prolonged use of SDD was not associated with an increase in the acquisition of antibiotic-resistant flora. | |
| Daneman N [23] | 2013 | Meta-analysis of 64 studies on SDD and SOD in ICU patients. | There were no differences in colonization/infection due to resistant gram-positive microorganisms (MRSA and VRE)(SDD vs control). There were no differences in the resistance of GNB to aminoglycosides and fluoroquinolones. Reduction of GNB resistant to polymyxin and resistant to third-generation cephalosporins (SDD vs control). | |
| Oostdijk EA [24] | 2013 | Post hoc analysis of a group randomized trial. | 455 SDD, 476 SOD, 315 standard | The acquisition rates of colistin-resistant GNB in the respiratory tract were comparable during the use of SDD, SOD and standard care. |
| Oostdijk EAN [25] | 2014 | Group randomized cross-over study comparing 12 months of SDD versus 12 months of SOD in 16 Dutch ICUs. | 6040 SDD vs 5957 SOD | SDD and SOD were associated with low levels of antibiotic resistance. SDD was associated with a lesser prevalence of rectal carriers of antibiotic-resistant GNB, though a gradual increase was observed in GNB resistant to aminoglycosides compared with SOD. |
| Houben AJ [26] | 2014 | Database of 32 laboratories serving 45 ICUs to identify all the Enterobacteriaceae, P. aeruginosa and Acinetobacter spp. spp. isolated from blood and airway. | Resistance to cefotaxime/ceftriaxone in respiratory tract strains increased in the ICUs that did not use SDD/SOD and decreased in those where such protocols were used, in the same way as resistance to ciprofloxacin. Reductions of the resistance rates of all antimicrobials with the use of SDD/SOD. | |
| Wittekamp BH [27] | 2015 | Post hoc analysis of two consecutive group randomized multicenter trials during 7 years in 5 ICUs. | Resistance to tobramycin in respiratory and rectal samples decreased significantly, and resistance to colistin in respiratory samples did not change, during the long-term use of SDD and SOD versus standard care. The prevalence of GNB resistant to colistin and tobramycin did not increase over an average of 7 years of SDD or SOD. | |
| van der Bij AK [28] | 2016 | Database analysis of the S. aureus, E. faecalis, E. faecium isolation rates, including resistant phenotypes, in blood and respiratory samples from ICU patients between 2008−2013. | The use of SDD/SOD was associated with increased isolates of S. aureus and E. faecalis, but not of resistant phenotypes. | |
| Sánchez-Ramírez C [29] | 2018 | Prospective cohort study in a 30-bed medical-surgical ICU. | 1998 SDD of 3948 vs 994 No SDD | After 4years of SDD, a significant decrease (p<0.001) was observed in MRGNB infections (RR 0.31 [95%CI 0.23−0.41]), which was associated with low rates of colistin- and tobramycin-resistant colonization. |
| Wittekamp BH [30] | 2018 | Non-blinded, group randomized and cross-intervention multicenter trial in 13 ICUs. | 8665 | No statistically significant differences were observed in the incidence of new bloodstream infections due to MRGNB, and there were likewise no significant differences in new highly resistant microorganisms, between SDD and standard care. |
| Buitinck S [31] | 2019 | An observational cohort study in a single center during a period of 21 years. | 14,015 (86% SDD) | The incidence rates of resistant microorganisms did not increase significantly over time, though an increase was recorded in the basal resistance rates measured upon admission for cephalosporins, polymyxin B / colistin and ciprofloxacin, with increases of 7.9%, 3.5% and 8.0%, respectively. |
| SuDDICU [32] | 2022 | Group randomized, cross-over clinical trial. | 8599 | A statistically significant decrease was recorded in the proportion of patients with cultures positive for resistant microorganisms (23.1% versus 34.6%; absolute difference -11% [95%CI -14.7% to -7.3%]) in the SDD group versus the group without SDD. |
| Hammond NE [16] | 2022 | Meta-analysis of 32 randomized clinical trials in adults subjected to mechanical ventilation. | 24,389 | There was no evidence that SDD is associated with an increase in the incidence of antimicrobial-resistant organisms. However, the data available on the incidence of antimicrobial-resistant positive cultures were not amenable to grouping, and the degree of certainty was therefore very low. |
SDD: selective digestive decontamination; GNB: gram-negative bacteria; MRSA: methicillin-resistant S. aureus; ICU: Intensive Care Unit; SOD: selective oropharyngeal decontamination; OR: odds ratio; CI: confidence interval; MRGNB: multidrug-resistant gram-negative bacteria.
The references of the studies are found in the Supplementary material.
In a cluster randomized study involving 13 ICUs,58 information was collected over two years from 1868 patients receiving SDD and 1837 patients receiving standard care. Selective digestive decontamination was associated with fewer bacteremias, and specifically bacteremias caused by multidrug-resistant flora (RR 0.41 [95%CI 0.18−0.94]). A decrease in multidrug-resistant flora in respiratory samples was also observed (RR 0.58 [95%CI 0.43−0.78]), without the acquisition of cefotaxime-resistant GNB. Resistance to colistin was less in the patients treated with SDD.
In a systematic review of 64 studies,59 no differences were recorded in the prevalence of colonization or infection due to resistant gram-positive organisms between patients treated with SDD and the controls (MRSA, OR 1.46 [95%CI 0.90–2.37]; VRE, OR 0.63 [95%CI 0.39–1.02]). Likewise, no differences were observed with regard to GNB resistant to aminoglycosides (OR 0.73 [95%CI 0.51–1.05]) or fluoroquinolones (OR 0.52 [95%CI 0.16–1.68]). A decrease was recorded in GNB resistant to polymyxin E (OR 0.58 [95%CI 0.46−0.72]) and third-generation cephalosporins (OR 0.33 [95%CI 0.20−0.52]) among the patients that received SDD.
Several later studies have evaluated the long-term impact of SDD upon the acquisition of resistance.60–67 In a study60 carried out in a medical-surgical ICU with 1588 patients and a follow-up period of 5 years, the incidence of resistant PPM carriers remained stable at 18.9 per 1000 patient-days. The incidence of Enterobacteria resistant to the antimicrobials of SDD was not modified, and a significant decrease was observed in the resistance of Pseudomonas aeruginosa to tobramycin and amikacin. In the longest cohort study,61 evaluating the continued use of SDD over a period of 21 years in 12,053 patients, the incidence of resistant microorganisms acquired in the ICU did not increase significantly over time, even though the basal rates of resistant strains measured upon admission increased over time. Thus, the prolonged use of SDD is not associated with an increase in resistant flora.
These findings are consistent with the recent evidence provided by the last published RCT45 and the last systematic review,37 in which the introduction of SDD did not result in any negative impact on the ecology of resistance. The last trial,45 recorded a statistically significant decrease in the proportion of patients with cultures positive for resistant microorganisms (23.1% versus 34.6%; absolute difference -11% [95%CI −14.7% to −7.3%]) in the SDD group versus the group without SDD.
Selective oropharyngeal decontamination (SOD) versus selective digestive decontamination (SDD)Selective oropharyngeal decontamination (SOD) is a modification of the SDD strategy without the parenteral and intestinal component. Different studies have shown SDD to be more effective than SOD in preventing infections in the ICU40,41,58,68 and in reducing mortality,40–42,68 and it is also more cost-effective.69,70 Likewise, the intestinal burden and the resistant GNB acquisition rate after discharge from the ICU are lower when SDD is used.40,41,58,71
In a cluster randomized trial,40 both SDD and SOD significantly reduced mortality versus standard care (adjusted OR 0.83 [95%CI 0.72−0.99] and adjusted OR 0.86 [95%CI 0.69−0.99], respectively), with an absolute mortality decrease of 3.5% and 2.9% (corresponding to relative reductions of 13% and 11%) on day 28 with SDD and SOD, respectively. This clinical trial was the first to demonstrate a survival benefit with the use of SOD. The patients receiving SDD had a lesser incidence of bacteremia (OR 0.44 [95%CI 0.34−0.57]) and candidemia (OR 0.65 [95%CI 0.49−0.85]) than the patients that received SOD. This finding was due to the decrease in Enterobacteria and Candida spp. carriers with the use of SDD - an effect not observed with SOD.
More recently, a cluster randomized study42 compared the effects of SDD (n=6116) and SOD (n=5881) upon mortality, the incidence of bacteremia and the acquisition of resistance in 16 ICUs. In the corrected version following revision of the first version, SDD showed a significant decrease in mortality versus SOD on day 28 (23.8% versus 25.7%; adjusted OR 0.84 [95%CI 0.75−0.93]), ICU mortality (18.4% versus 20%; adjusted OR 0.86 [95%CI 0.78−0.94]) and hospital mortality (28.2% versus 26.3%; adjusted OR 0.85 [95%CI 0.79−0.93]). Selective digestive decontamination was moreover associated with lower bacteremia and candidemia rates, and a lesser prevalence of rectal colonization by antibiotic-resistant GNB. However, although the percentage of bacteremias caused by aminoglycoside-resistant PPMs was lower with SDD, the increase in aminoglycoside resistance over time was greater in the SDD group (0.7% versus 0.4% at one month).
In a subsequent analysis70 based on data corresponding to individual patients in the study published by Smet et al.40 and in the study of Oostdijk et al.,42 SDD was associated with significantly lower hospital mortality and similar costs versus SOD.
In conclusion, critical disease and the medical interventions used to treat it favor the overgrowth of pathogenic intestinal flora, colonization and infection. In critical patients, SDD makes it possible to eradicate PPMs from the intestinal tract, reduce the incidence of infections and lessen patient mortality. The use of SDD is therefore advised in critical patients subjected to mechanical ventilation for over 48h, based on the correct application of its four components. Modification of the protocol by excluding one or more of its components is not consistent with the definition of SDD and reduces its efficacy. Since the traditional SDD protocol is not targeted to MRSA and VRE, it is advisable to adjust the antimicrobial drug profile of SDD by adding vancomycin in ICUs with strong endemicity of such gram-positive infections. Likewise, modification of the SDD formula is recommended in carriers of resistant PPMs, in accordance with the prevalent flora.
However, one of the main barriers to the adoption of SDD72,73 (Supplementary material) is the fact that the generalized use of antibiotics may favor the appearance of resistant organisms, even though the available evidence does not warrant this concern. Thus, future studies should investigate how the intestinal and pulmonary microbiota of critical patients subjected to SDD differs from that of patients who only receive parenteral antibiotics for the treatment of nosocomial infections, non-critical hospitalized patients, and the healthy population. Likewise, the way in which the composition of the intestinal and pulmonary microbiota evolves following patient reincorporation into the community needs to be addressed.
Authors’ contributionsAll the authors comply with the conditions for authorship, and state that they have participated in the study sufficiently to officially accept responsibility for its contents - including participation in study conception, design, analysis, and writing or review of the manuscript.
We thank doctors van Saene and Rommes for their important scientific contributions over the years, which have consolidated the routine use of selective digestive decontamination.