To describe the use of extracorporeal membrane oxygenation (ECMO) in refractory respiratory failure.
DesignA prospective, observational, multi-center study was carried out.
SettingIntensive Care Units (ICU) in 148 Spanish hospitals.
PatientsSubjects admitted during epidemic weeks 50–52 of 2010 and weeks 1–4 of 2011, receiving respiratory support with ECMO.
Main variables of interestClinical and blood gas features, complications and survival of patients with ECMO.
ResultsOut of 300 ICU admitted patients, 239 (79.6%) were mechanically ventilated. ECMO was available in only 5 ICUs. Nine patients were treated with ECMO (3% of the total and 3.2% of the ventilated patients). In 77.7% of the cases some hypoxemia rescue technique was previously used. ECMO was initiated when ARDS proved refractory to standard treatment. ECMO therapy was started a median of 4.5 days after the onset of mechanical ventilation. The median duration of ECMO was 6 days. Veno-venous (VV) ECMO was the most frequent cannulation mode (88.9%). Four patients had complications associated with ECMO therapy. The median ICU and hospital stay was 17 and 29 days, respectively. In five patients (55.5%), ECMO assistance was satisfactory suspended. The ICU and hospital survival rate was 44.4%.
ConclusionsThe use of ECMO in refractory respiratory failure in patients with influenza A (H1N1) is rare in Spain. The hospital survival achieved with its use allows it to be regarded as a possible rescue technique in these patients.
Describir la utilización de la oxigenación por membrana extracorpórea (ECMO) en la insuficiencia respiratoria refractaria.
DiseñoEstudio prospectivo, observacional y multicéntrico.
ÁmbitoServicios de Medicina Intensiva (SMI) de 148 hospitales españoles.
PacientesEnfermos ingresados entre las semanas 50-52 del 2010 y la 1-4 del 2011 con el diagnóstico de gripe A (H1N1) que recibieron soporte respiratorio con ECMO.
Principales variables de interéscaracterísticas clínicas, gasométricas, complicaciones y supervivencia de los pacientes con ECMO.
ResultadosIngresaron 300 pacientes y se ventilaron 239 (79,6%). Sólo cinco SMI disponían de la técnica. Se indicó la ECMO en nueve (3% del total y 3,2% de los ventilados). En el 77,7% se empleó previamente alguna técnica de rescate frente a la hipoxemia. La canulación mayoritaria fue veno-venosa (88,9%). Su colocación fue precoz, tras una mediana de 4,5 días de ventilación mecánica. La duración mediana de la asistencia fue de seis días. Cuatro pacientes presentaron complicaciones asociadas a la ECMO. La mediana de estancia en el SMI y hospitalaria fue 17 y 29 días respectivamente. En cinco pacientes (55,5%) se pudo retirar la asistencia con la ECMO. La supervivencia tanto del SMI como hospitalaria fue del 44,4%.
ConclusionesEl uso de la ECMO en la insuficiencia respiratoria refractaria en pacientes con gripe A (H1N1) es poco frecuente en nuestro país. La supervivencia hospitalaria lograda con su uso permite considerarla como una posible técnica de rescate en estos pacientes.
The salient characteristic of the pandemic caused by the influenza A (H1N1) virus is the frequent appearance of acute respiratory failure episodes, associated to high mortality rates.1–3 The principal underlying etiology in both the Spanish national and international clinical series is rapidly progressive viral pneumonia—this being the leading reason for admission to Intensive Care among such patients.4–8 The seriousness of the patient condition, often characterized by hypoxemia refractory to conventional treatment, has led to the adoption of rescue treatment measures in such cases, with both drugs (e.g., corticosteroids) and non-pharmacological measures, including different supportive and ventilation strategies (alveolar recruitment maneuvers, ventilation in prone decubitus, nitric oxide (NO), etc.). Extracorporeal membrane oxygenation (ECMO) is an example of such supportive therapy.9,10 The present article describes the experience gained with the utilization of ECMO in patients admitted to the Spanish Intensive Care Units (ICUs) during the seasonal influenza outbreak of the year 2010, produced by the influenza A (H1N1) virus.8
Material and methodsThis prospective and observational study of patients admitted to the ICU was carried out in a total of 148 Spanish hospitals. The data were obtained from a voluntary registry created and auspiced by the Spanish Society of Intensive Care Medicine and Coronary Units (Sociedad Española de Medicina Intensiva Crítica y Unidades Coronarias, SEMICYUC), the Spanish Research Network in Infectious Disease (REIPI), and the Networked Research Center of Respiratory Diseases (CIBERES). The study was approved by the Ethics Committee of Juan XXIII University Hospital in Tarragona (Spain) (IRB NEMAGRIP/11809). Patient identity was kept confidential, and no informed consent was required, given the observational nature of the study. The data were reported by the physician attending the patients. Information was collected on all the patients consecutively admitted with a diagnosis of influenza A (H1N1) between weeks 50–52 of the year 2010, and weeks 1–4 of the year 2011. All included patients were ≥15 years of age. In all cases, the diagnosis of influenza A (H1N1) infection was confirmed by real time, reverse transcription polymerase chain reaction (RT-PCR) testing of nasopharyngeal secretions and/or tracheal secretions requested by the attending physician upon admission to the ICU. The determination was carried out in each participating hospital center or in a core laboratory when the test was not locally available. Cases were defined by the presence of an acute respiratory condition with laboratory RT-PCR confirmation. The registry only included confirmed cases.
The criteria for admission to the ICU, the management regimens, including the need for intubation and mechanical ventilation, and the antibiotic or antiviral treatments were not protocolized and were established by the physician attending the patient. Systemic corticosteroids were prescribed when the patient developed septic shock refractory to usual treatment (hydrocortisone) or as coadjuvant treatment in pneumonia (methylprednisolone). Antiviral therapy consisted of oral oseltamivir (75mg/12h or 150mg/12h) or zanamivir via the intravenous route (600mg/12h), according to the criterion of the supervising physician. The decision regarding the indication of ECMO as ventilatory support in situations of refractory hypoxemia was not standardized, and was established by the physician attending the patient. Likewise, there was no consensus-based protocol referred to the indications, contraindications and therapeutic objectives of ECMO ventilatory support. Five hospital centers implemented ECMO: La Fe University Hospital (Valencia), Reina Sofia University Hospital (Cordoba), Marqués de Valdecilla University Hospital (Santander), Gregorio Marañón University Hospital (Madrid), and the Clinic University Hospital (Barcelona). In all cases ECMO pertained to the hospital reporting the cases, with the exception of one patient in which the intensivists and heart surgeon of La Fe University Hospital (Valencia) visited the ICU of Játiva Hospital (Valencia) where the patient had been admitted. Following implantation of the device, the patient was transferred to the reference hospital center under ECMO ventilatory support in a medicalized ambulance.
DefinitionsPrimary viral pneumonia was defined as the presence of a clinical condition consisting of acute respiratory failure and alveolar condensations on the chest X-rays in at least two lung lobes, with negative respiratory and blood sample cultures during the acute phase of the influenza infectious process.3
Community acquired respiratory co-infection (CARC) was defined as any infection diagnosed within the first two days of admission to hospital.11 Infections occurring at a later point were regarded as nosocomial infections. The definition of hospital-acquired pneumonia was based on the current criteria of the American Thoracic Society and Infectious Disease Society of America.12 Obesity was considered in the presence of a body mass index (BMI) of >30kg/m2. Vaccinated patients were defined as those who had received the monovalent or seasonal vaccine for influenza A (H1N1) corresponding to 2009, or the seasonal vaccine for influenza 2010–2011. The presence of renal failure was defined according to the criteria of the Acute Kidney Injury Network (AKIN).13
Statistical analysisDiscrete variables are reported as numbers (percentages), while continuous variables are expressed as the median (25–75% interquartile range).
Data analysis was carried out using the SPSS version 15.0 statistical package for MS Windows (SPSS, Chicago, IL, USA).
ResultsAnalysis was limited to the data of the first 300 adults admitted to the 148 Spanish ICUs during the seasonal influenza outbreak of the winter of 2010–2011. In all cases the cause of the respiratory disease was the influenza A (H1N1) virus, confirmed by RT-PCR testing.
A total of 239 patients (79.7%) required intubation and invasive mechanical ventilation (IMV). ECMO was indicated as ventilatory support in 9 patients (3% of the total and 3.8% of the ventilated subjects). In each of the 5 hospital centers ECMO was used in two patients, except in the Clinic Hospital in Barcelona, where the technique was used in a single patient. The patients were young, with a median age of 36 years (27.5–42.0), and 58% were males. The median APACHE II and SOFA scores were 15.0 (12.5–24.0) and 5.5 (4.0–8.8), respectively. Comorbidities were present in four patients (44.4%)—the most prevalent condition being chronic renal failure requiring conventional renal filtration in two cases (22.2%). No patient had been vaccinated. In all cases the clinical condition corresponded to viral pneumonia, and in two cases the latter was associated to bacterial coinfection produced by Pseudomona aeruginosa. The median stay of the total cases in both the ICU and in hospital was long—17 (13.5–38.5) and 29 (13.5–44.5) days, respectively—and longer in those who survived than in the patients who died (19.5 versus 15 days in the ICU and 15 versus 29 day in hospital). All of the patients received antiviral treatment with oseltamivir, with a delay of 5 days from symptoms onset. Corticosteroids were used in 6 patients (66.7%), fundamentally due to septic shock refractory to the usual management measures. Vasopressors proved necessary in 7 patients (77.8%). Seven patients presented or developed renal failure. Of these, 5 were subjected to continuous renal replacement therapy while two were subjected to both continuous techniques and conventional hemodialysis.
All patients were intubated and subjected to mechanical ventilation. In three subjects noninvasive mechanical ventilation (NIMV) had been previously attempted but failed. The invasive mechanical ventilation (IMV) modalities, the rescue measures in relation to refractory hypoxemia, and the blood gas parameters prior to the introduction of ECMO are described in Table 1. The most widely used invasive mechanical ventilation modality was the pressure-controlled technique. Some hypoxemia rescue measure was used in 7 of the patients (77.7%) – the most frequently used option being ventilation in prone decubitus and the utilization of nitric oxide in 55.5% of the patients. The severity of respiratory failure prior to ECMO implantation was evidenced by the need for high oxygen concentrations and positive end-expiratory pressures (PEEP) in the respirator, as well as by hypercapnia, the lowering of pH and the low PaO2/FiO2 ratio, with a median value of 66.
Mechanical ventilation modalities, refractory hypoxemia rescue techniques, blood gas parameters before implantation, and characteristics of support with ECMO.
| Variable | ECMO (n=9) |
| Invasive mechanical ventilation (IMV) mode | |
| Pressure-controlled IMV, n (%) | 6 (66.7) |
| Volume-controlled IMV, n (%) | 3 (33.3) |
| Refractory hypoxemia rescue techniques | |
| Prone decubitus, n (%) | 5 (55.5) |
| Nitric oxide, n (%) | 5 (55.5) |
| Prostaglandins, n (%) | 2 (22.2) |
| High-frequency ventilation, n (%) | 0 (0) |
| Ventilator and pre-ECMO blood gas parameters | |
| FiO2, median (IQR) | 1.0 (0.7–1.0) |
| PEEP, median (IQR) | 14.0 (8.5–15.5) |
| PaO2, median (IQR) | 66.0 (64.0–73.5) |
| PaCO2, median (IQR) | 54.0 (42.4–66.5) |
| pH, median (IQR) | 7.30 (7.22–7.37) |
| PaO2/FiO2, median (IQR) | 66.0 (64.0–102.1) |
| Characteristics of ECMO support | |
| Days of pre-ECMO IMV, median (IQR) | 4.5 (1.7–7.0) |
| Type of ECMO | |
| Venous–venous, n (%) | 7 (77.8) |
| Venous–arterial, n (%) | 1 (11.1) |
| Venous–venous→venous–arterial, n (%) | 1 (11.1) |
| Duration of ECMO, in days, median (IQR) | 6.0 (5.0–22.0) |
| Complications of ECMO | |
| Patients with at least one complication, n (%) | 4 (44.4) |
| Bleeding at cannulation site, n (%) | 3 (33.3) |
| Pump dysfunction, n (%) | 3 (33.3) |
| Thrombocytopenia, n (%) | 1 (11.1) |
IQR, interquartile range 25–75; %, percentage; IMV, invasive mechanical ventilation.
The characteristics of ECMO (Table 1) show insertion of the device to be early (after a median of 4.5 days of invasive mechanical ventilation). As expected, most were venous–venous procedures (88.9%), though posteriorly conversion to the venous–arterial technique was required in one patient due to the appearance of left ventricle dysfunction. In only one case venous–arterial cannulation was carried out from the start as ventilatory and circulatory support. The median duration of assistance was 6 days. Complications were relatively frequent, with at least one complication in 44.4% of the cases. The most common problems were pump dysfunction and bleeding at the cannulation points, requiring pump replacement and revision of the cannula insertion points. In no case did the complications lead to patient death.
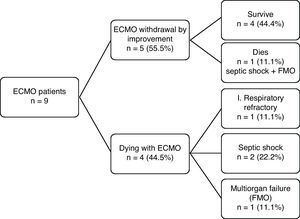
The results in terms of patient outcome are shown in Fig. 1. Of the 9 patients subjected to ECMO, the device could be removed in 5 cases (55.5%) as a result of improvement of the respiratory condition, and four of these patients (44.4%) were discharged from the ICU and hospital. The remaining patient died 7 days after suspending ECMO, as a result of septic shock and multiorgan failure (MOF). The other four patients died while receiving ECMO (due to septic shock in two cases, multiorgan failure in one, and refractory respiratory failure in another).
DiscussionThis is the first article to describe the experience in Spain with ECMO in critical patients admitted to the ICU during the seasonal influenza A (H1N1) epidemic in the winter of 2010–2011.
Our findings are similar to those reported by other international registries and publications (Table 2). The availability of the technique is generally limited to third level hospitals with adequate technological and human resources. In this country only 5 of the 148 participating ICUs (3.4%) incorporated ECMO, in concordance with similar data from registries in countries such as Australia–New Zealand14 or Canada,15 with healthcare systems similar to our own, and where the percentage of ICUs with the technique was reported to be 8% (15/187) and 11.8% (4/34), respectively. The same resource-concentrating policy referred to the availability of ECMO is also recommended by the health authorities in Italy,16 with the accreditation of only 14 ECMO-equipped ICUs in the entire country. The concentration of ECMO in a few reference hospital centers is not an insurmountable barrier against application of the technique to seriously ill patients admitted to other centers and who cannot be moved to reference hospitals. Although in our series only one patient was subjected to ECMO in another hospital lacking the technique, followed by transfer with the device to the corresponding reference hospital, extensive experience has been gained in countries such as Australia,14 Italy16 or Sweden,17 where 55.9% (36/68), 46% (28/60) and 92.3% (12/13) of the devices were implanted in patients admitted to hospitals lacking ECMO—followed by transfer to the reference centers. This approach moreover appears safe and without major complications, as reflected in numerous series of up to 40 patients transferred with ECMO.18 The creation of a multidiscipline hospital ECMO team with the participation of an intensivist, and the development of protocols referred to the indications and contraindications of the technique, allow adequate selection of the patients and their safe transfer.16,18,19
Clinical characteristics and survival of patients with influenza A (H1N1) infection admitted to the ICU published in different studies.
| ANZIC7 ANZ-ECMO14 | Freed DH15 | Patroniti N16 | NicolayN20 | Holzgraefe B17 | |
| ICU included in the registry (n) | 187 | 34 | na | 30 | na |
| ICU with ECMO, n (%) | 15 (8.0) | 4 (11.8) | 14 in all Italy | 1 (3.3) | 2 in all Sweden |
| Patients with influenza A (H1N1) | |||||
| With IMV (n) | 133 | 162 | na | 51 | na |
| With IMV and ECMO (n) | 68 | 6 | 49 | 4 | 12 |
| Place of ECMO application | |||||
| In reference center, n (%) | 32 (44.1) | 6 (100) | 32(53) | 4 (100) | 1(8.3) |
| In other hospital, n (%) | 36 (55.9) | 0 (0) | 28 (47) | 0 (0) | 12 (91.7) |
| Pre-ECMO rescue techniques | |||||
| Ventilation in prone decubitus, n (%) | 12 (17.6) | 2 (33.3) | 13 (26.5) | na | na |
| Nitric oxide, n (%) | 20 (29.4) | 6 (100) | 7 (14.3) | na | na |
| High-frequency ventilation, n (%) | 3 (4.4) | 3 (50) | 2 (4.1) | na | na |
| Prostaglandins, n (%) | 14 (20.6) | 0 | 0 | na | na |
| Pre-ECMO values | |||||
| FiO2, median (IQR) | 1 (1–1) | 1 (1–1) | 1 (1–1) | na | 1 (1–1) |
| PEEP, median (IQR) | 18 (15–20) | 20 (99)b | 16 (14–19) | na | 17 (15–20) |
| PaO2, median (IQR) | na | 58 (17)b | na | na | 52 (38–58) |
| PaCO2, median (IQR) | 69 (54–83) | na | 57 (47–71) | na | 47 (41–57) |
| pH, median (IQR) | 7.2 (7.1–7.3) | 7.31 (0.05)b | 7.3 (7.2–7.4) | na | 7.3 (7.3–7.4) |
| PaO2/FiO2, median (IQR) | 56 (48–63) | 58 (17)b | 63 (56–79) | 49 (37–67) | 7.3 (7.3–7.4) |
| Days IMV pre-ECMO, median (IQR) | 2 (1–5) | 5 (2–8) | 2 (1–5) | na | 1 (0.5–7) |
| Days assistance with ECMO, median (IQR) | 10 (7–15) | 15 (14–15) | 10 (7–17) | 53 (11–120) | 16 (9–30) |
| ICU stay, median (IQR) | 22 (13–32) | 28 (18–37) | 22 (14–37) | 96 (74–98) | na |
| Hospital stay, median (IQR) | 28 (15–43) | na | 39 (22–50) | na | na |
| ICU survival | |||||
| ECMO, n (%) | 48 (70.6) | 4 (66.6) | 35 (71.4) | 3 (75.0) | 11 (91.7) |
| IMV, n (%) | 121 (91.0) | 140 (86.4) | na | 39 (76.4) | na |
| Hospital survival | |||||
| ECMO, n (%) | 32 (47.0)a | 4 (66.6) | 35 (71.4) | 3 (75.0) | 11 (91.7) |
| IMV, n (%) | 116 (87.2) | 140 (86.4) | na | 39(76.4) | na |
IQR, interquartile range; FiO2, fraction of inspired oxygen; na, data not available; PEEP, positive end-expiratory pressure; IMV, invasive mechanical ventilation; %, percentage.
22 of the 68 patients were still in hospital.
Mean (standard deviation).
The recorded 3% ECMO utilization rate in patients with influenza A (H1N1) admitted to the ICU is lower than that reported by the first Australian registries (11.6%),7 and is closer to the 4.2% reported in Canada,6 5.2% in Ireland20 or 6.4% in Chile.21 There are also multicenter registries of critical patients with influenza A in Mexico22 and Argentina5 where no ECMO devices have been implanted as respiratory assists. The variations in ECMO utilization rates are related to the type of healthcare system and the healthcare technological development of the different countries.
ECMO, as an invasive and complex technique requiring trained personnel, is not regarded as the initial rescue option in patients with severe and refractory hypoxemia. In most of our patients (77.7%), the technique was preceded by at least one ventilatory maneuver or drug measure such as ventilation in prone decubitus, the administration of nitric oxide, or the use of prostaglandins. This approach is standard practice, with percentages in the Canadian,6 Australian14 and Italian16 series of between 28.6% and 81%. Ventilation in prone decubitus has been the most widely used initial measure in all the series (20–55% of the total patients), followed by nitric oxide (17.32%). High-frequency ventilation in turn was the least used option (0–5%).
Despite the lack of a uniform and consensus-based protocol referred to the indications of ECMO, the patient population appears to have been selected according to the international recommendations of the Extracorporeal Life Support Organization (ELSO).23 These are young patients, with severe respiratory failure defined by the high oxygen concentrations and PEEP values of the ventilators, and the hypoxemia, hypercapnia and respiratory acidosis findings of the blood gas determinations. The timing of ECMO implantation also appears to have been correct, i.e., early, after a median of 4.5 days of invasive mechanical ventilation, before the patients develop lung fibrosis and multiorgan failure. Promptness in introducing ECMO appears to result in increased survival in these patients.14,15 In this context, the Italian registry16 reports that those patients who survived received ECMO after a median of one day on mechanical ventilation, versus 5 days in those who died. Moreover, each day of mechanical ventilation before introducing ECMO resulted in a significant increase in mortality risk [OR 1.29 (95%CI 1.092–1.527)].
The hospital survival rate in our series, i.e., 44.4% (4/9), is somewhat lower than the percentages reported by the larger series: 71% (48/68) for ICU and 47% (32/68) for hospital survival in Australia,14 71% (35/49) for both ICU and hospital survival in Italy,16 and 66.6% (4/6) in Canada.15 Comparatively better results have also been reported by the international ELSO registry24 of adult patients with influenza A subjected to ECMO, with a survival rate of 64.3% (153/238). In contrast, our survival rate does not differ from that described by the Chilean multicenter survey,21 with a hospital survival rate of 50% (2/5), and is also in line with the data published by some French hospital centers with extensive experience in the use of ECMO, with a 44.4% survival rate (4/9).25 These results cannot be attributed to a greater rate of complications of the procedure, which was similar to those reported by other series,14,15 though they can be partially attributed to the small size of our series and to the learning curve associated with all new techniques—since to date the Spanish hospital with the greatest experience has not used ECMO in more than two patients with influenza A and refractory acute respiratory distress syndrome (ARDS).
The only randomized study26 comparing conventional treatment versus ECMO in patients with severe and refractory ARDS, which moreover did not include patients with influenza A (H1N1) infection, reported that the healthcare costs in the ECMO treatment group doubled those in the conventional treatment group. Analysis of the cost of each QALY (quality-adjusted life year) gained yielded a figure of 19,252 English pounds per year, which was considered to be cost-effective and assimilable by the national healthcare system in the United Kingdom.27 In addition, it should be mentioned that the number needed to treat (NNT) in order to save a life was found to be low (only 6 patients). To our knowledge, no studies have been made of the healthcare costs of incorporating ECMO to patients with influenza A. The data supplied by one of the participating Spanish hospitals estimated the expendable materials cost of ECMO (pump, oxygenator and circuit), assuming an average of 15 days of ventilatory and/or circulatory support, to be 300–565 euros/day, depending on the ECMO model used. We therefore believe that at the present time, and based on the available information, ECMO can be viewed as a treatment of reasonable cost, assimilable by the national healthcare system, and with an acceptable cost-efficacy ratio. Here again it must be underscored that this appraisal of the technique and its possibilities are applicable provided ECMO is concentrated in only a few hospitals, with the option of implanting the device in patients in second and third level centers that lack the technique, followed by transfer with ECMO to the reference hospitals, in accordance with the protocol of the CESAR study26 and the recommendations of both the Italian16 and the Australian health authorities.18
The small sample size of our study is one of its main limitations. In the influenza A (H1N1) pandemic of the autumn–winter of 2010, ECMO use was merely anecdotal in Spain (three patients out of 968 admissions to the ICU). Despite this small sample size, however, we wish to stress that this is the first and only series published in this country to date, and describes the experience of those Spanish hospitals that implant ECMO in patients with these characteristics. The data obtained therefore cannot be extrapolated to other countries or to the pediatric population. Another weakness of the study is the lack of a uniform and consensus-based protocol establishing the indications of ECMO, its contraindications and the clinical and analytical objectives of the technique. The different types of assistance provided in the small number of patients involved may also have distorted the mentioned results to some extent.
Although there is presently not enough scientific evidence to recommend the utilization of ECMO in patients with ARDS secondary to influenza A (H1N1), the use of ECMO should be considered in patients with hypoxemia refractory to other “less invasive” rescue measures such as ventilation in prone decubitus or the administration of nitric oxide or prostaglandins. The clinical results obtained in our series are satisfactory, with hospital survival in the order of 50%. The clinical data derived from large patient series should be used to establish uniform criteria regarding the adequate selection of those patients who are most likely to benefit from the technique, and the best timing of the introduction of ECMO. Due to the complexity involved and the need for considerable resources, and with a view to ensuring better care and cost-effectiveness results, the technique should be implemented in adequately equipped reference hospitals with the possibility of forming multidisciplinary teams capable of applying ECMO in other centers and of transferring patients from such centers to the reference hospital.
FundingThis study was partially supported by the Instituto de Salud Carlos III (Spanish ministry of Innovation and Science).
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Bonastre J, et al. Uso de oxigenador de membrana extracorpóreo en pacientes con insuficiencia respiratoria aguda grave refractaria en la epidemia de gripe estacional 2010–2011 por influenza A (H1N1) en España. Med Intensiva. 2012;36:193–9.