To evaluate the prevalence of vitamin D deficiency in critically ill patients upon admission to an Intensive Care Unit (ICU) and its prognostic implications.
DesignA single-center, prospective observational study was carried out from January to November 2015. Patients were followed-up on until death or hospital discharge.
SettingThe department of Critical Care Medicine of a university hospital.
PatientsAll adults admitted to the ICU during the study period, without known factors capable of altering serum 25(OH)D concentration.
InterventionsDetermination of serum 25(OH)D levels within the first 24h following admission to the ICU.
Main variables of interestPrevalence and mortality at 28 days.
ResultsThe study included 135 patients, of which 74% presented deficient serum 25(OH)D levels upon admission to the ICU. Non-survivors showed significantly lower levels than survivors (8.14ng/ml [6.17–11.53] vs. 12ng/ml [7.1–20.30]; p=.04), and the serum 25(OH)D levels were independently associated to mortality (OR 2.86; 95% CI 1.05–7.86; p=.04). The area under the ROC curve was 0.61 (95% CI 0.51–0.75), and the best cut-off point for predicting mortality was 10.9ng/ml. Patients with serum 25(OH)D<10.9ng/ml also showed higher acute kidney injury rates (13 vs. 29%; p=.02).
ConclusionVitamin D deficiency is highly prevalent upon admission to the ICU. Severe Vitamin D deficiency (25[OH]D<10.9ng/ml) upon admission to the ICU is associated to acute kidney injury and mortality.
Determinar la prevalencia de hipovitaminosis D al ingreso en el Servicio de Medicina Intensiva (SMI), así como su asociación con el pronóstico del paciente crítico.
DiseñoAnálisis observacional prospectivo llevado a cabo desde enero a noviembre de 2015. Los pacientes incluidos fueron seguidos hasta su fallecimiento o alta hospitalaria.
ÁmbitoSMI polivalente de un hospital universitario.
PacientesTodos los individuos adultos que ingresaron en el SMI durante el periodo de estudio y que no presentaban factores conocidos que pudieran alterar los valores sanguíneos de 25(OH)D.
IntervencionesDeterminación de los niveles séricos de 25(OH)D en las primeras 24h de ingreso en el SMI.
Principales variables de interésPrevalencia de hipovitaminosis D al ingreso en UCI y mortalidad a los 28 días.
ResultadosSe incluyeron 135 individuos. El 74% de los pacientes presentó niveles bajos de 25(OH)D en el momento de su ingreso en el SMI. El grupo de pacientes que fallecieron presentaba niveles significativamente inferiores al grupo de pacientes que sobrevivieron (8,14ng/mL [6,17-11,53] vs. 12ng/mL [7,1-20,30], p=0,04) y el valor en sangre de 25(OH)D al ingreso se mostró como factor de riesgo independiente en el análisis multivariado (OR 2,86; IC 95% 1,05-7,86, p=0,04). La curva ROC fue de 0,61 (IC 95% 0,51-0,75) y el mejor punto de corte para predecir mortalidad fue de 10,9ng/mL. Los pacientes con valores de 25(OH)D<10,9ng/mL también presentaron mayores tasas de fracaso renal agudo (13 vs. 29%, p=0,02).
ConclusiónExiste una elevada prevalencia de hipovitaminosis D en el momento de ingreso en el SMI. La hipovitaminosis D severa (25[OH]D<10,9ng/mL) al ingreso en el SMI se asocia a mayor incidencia de fracaso renal agudo y mayor mortalidad.
Vitamin D is a liposoluble vitamin fundamentally produced in the skin as a result of the action of ultraviolet B radiation. Its native forms (vitamins D2 and D3) are transported in plasma bound to albumin or to the vitamin D binding proteins, and must undergo two hydroxylation reactions to become active. The first reaction takes place in the liver and produces 25(OH)D. The second reaction takes place in the kidneys, with the mediation of the enzyme alpha-1-hydroxylase, and produces 1,25(OH)D, which exerts its function by binding to the vitamin D receptors.1 Although 1,25(OH)D is the metabolically active form, it is considered that the blood 25(OH)D levels offer a better indication of vitamin D status in the body.2,3 The classical functions of vitamin D have been studied for decades, and much is known about its effects upon mineral and bone metabolism. However, other non-classical functions have also been identified that are related to the presence of alpha-1-hydroxylase and of vitamin D receptors in many body tissues,2 and to the capacity of vitamin D to modulate the expression of certain genes mediated by binding to these receptors—exerting an effect upon the regulation of hormone secretion, control of the innate and adaptive immune response, and on cell proliferation and differentiation.1 In this way it has been possible to establish associations between hypovitaminosis D and cancer4 and cardiovascular diseases.5
A high prevalence of hypovitaminosis D has been observed in the last decade in the general population, with the identification of geographical location and solar exposure as some of the conditioning factors.6–9 A number of studies have reported a particularly high prevalence of hypovitaminosis D in critically ill patients.10–14 However, the relationship between vitamin D deficiency and morbidity–mortality in terms of organ failure or infections in critical patients remains the subject of debate.12,15–17
Furthermore, although different recent randomized clinical trials that have evaluated the administration of vitamin D in critical patients have demonstrated normalization of the vitamin D levels as a result of supplementing,18 discordant results have been obtained in terms of the effect upon patient survival or duration of admission,19–24 and two recent meta-analyses have even published contradictory findings that currently preclude the recommendation of systematic supplementing in critical patients with vitamin D deficiency.25,26
The objectives of the present study are: (a) to determine the vitamin D levels upon admission to a Department of Intensive Care Medicine (DICM) belonging to a hospital in the south of Europe during the course of one year; and (b) to establish the relationship between these levels and the prognosis of critical patients, as well as determine whether there are total 25(OH)D levels in blood capable of predicting increased mortality after 28 days of admission to the DICM.
Patients and methodsA prospective observational study was carried out during a period of 11 months (from January to November 2015) in the DICM of a university tertiary hospital (Hospital del Mar, Barcelona, Spain). The study was approved by the Clinical Research Ethics Committee, and written informed consent was obtained for the processing of biological samples and the use of clinical and laboratory test data from all the patients (or their representatives, in the event the patients were unable to give consent).
We included all Caucasian patients ≥18 years of age admitted during the study period. Pregnant women were excluded, as were patients with a history of admission to the DICM in the last year, those admitted to hospital for ≥7 days before admission to the DICM, patients with malabsorption (including post-gastrectomy or bariatric surgery patients, and individuals with inflammatory bowel disease), patients diagnosed with hyperparathyroidism, hyperthyroidism or chronic renal failure, and individuals with granulomatous disorders. We likewise excluded patients receiving antiepileptic or antiretroviral therapy, vitamin D, or drugs interfering with bone metabolism (bisphosphonates).
Upon admission to the DICM, we recorded demographic information and clinical data, including patient age, gender, race, comorbidities, treatment received, the APACHE II27 and SOFA scores,28 and the disease indicating patient admission. The patients were classified into three groups: medical patients, surgical patients and trauma patients.
Within the first 24h of admission to the DICM we recorded the serum values corresponding to 25(OH)D, glucose, parathyroid hormone (using chemiluminescence–immunoassay techniques [Siemens, Berlin, Germany]), total and ionic calcium, albumin, prealbumin, creatinine, sodium, potassium, phosphorus, magnesium, C-reactive protein, procalcitonin, lactate, as well as liver function parameters, blood count and coagulation function. All the laboratory test parameters were determined with the methods commonly used in the hospital, and the 25(OH)D levels were determined by radioimmunoassay (Liaison®; DiaSorin, Stillwater, MN, USA)—the results being expressed in ng/ml.29
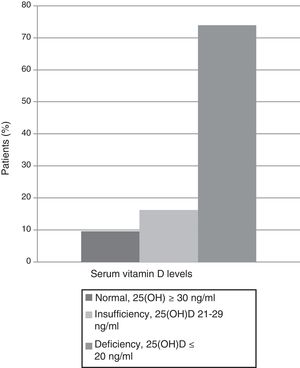
Patient stratification according to serum 25(OH)D levels was based on the guidelines of the United States Endocrine Society (2011), which define vitamin D deficiency as serum total 25(OH)D≤20ng/ml, while vitamin D insufficiency is defined as concentrations between 21 and 29ng/ml, and normal levels are defined as ≥30ng/ml.2,30
Acute renal failure (ARF) was defined according to the criteria of the Acute Kidney Injury Network,31 based on the clinical and laboratory test data at the time of admission to the DICM (the laboratory tests were performed within the first 24h of stay in the DICM).
Mortality was defined as death after 28 days of admission to the DICM.
The univariate analysis was based on the chi-squared test or Fisher exact test as applicable in the case of categorical variables, while the nonparametric Mann–Whitney U-test was used in the case of quantitative variables. The results were reported as the median and interquartile range (IQR), or as percentages. Receiver operating characteristic (ROC) curves were used to establish the best cut-off point for vitamin D according to mortality. Lastly, binary logistic regression analysis was performed to generate a multivariate model, entering those variables with p<0.1 in the univariate analysis. Variables not found to be significant were discarded on a stepwise basis in order to establish a final model offering the best mortality discriminating capacity. Statistical significance was considered for p<0.05.
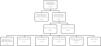
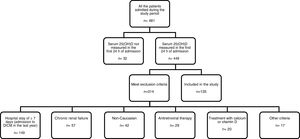
ResultsA total of 481 patients were admitted to the DICM during the study period. Of these, 314 presented exclusion criteria and 32 were excluded because plasma vitamin D had not been determined in the first 24h of admission. Fig. 1 describes the reasons for patient exclusion.
A total of 135 patients were finally analyzed. Table 1 shows the demographic characteristics of the patients.
Demographic characteristics of the patients included in the study.
| Age, years | 59 (49–74) |
|---|---|
| Gender | |
| Males | 89 (65.9) |
| Type of patient | |
| Medical | 96 (71.1) |
| Surgical | 23 (17) |
| Trauma | 16 (11.9) |
| APACHE II score | 17 (10–23) |
| SOFA score | 5 (3–8) |
| Mortality after 28 days | 25 (18.5) |
| DICM stay, days | 6 (3–14) |
| Hospital stay, days | 16 (7–27) |
APACHE: Acute Physiology and Chronic Health Evaluation; DICM: Department of Intensive Care Medicine; SOFA: Sequential Organ Failure Assessment.
Values expressed as median (percentile 25–75) and n (percentage).
The mean 25(OH)D level upon admission was 11ng/ml (range 7–19.8), and 13 of the patients (9.6%) had normal vitamin D levels, while 100 patients (74%) presented vitamin D deficiency or insufficiency (Fig. 2). No patient received vitamin D supplements during admission. Table 2 describes the biochemical parameters and markers of the included patients.
Biochemical parameters and markers of the patients included in the study.
| Variable | Results | Normal range |
|---|---|---|
| 25(OH)D (ng/ml) | 11 (7–19.78) | 30–150 |
| PTH (pg/ml) | 83 (46–161) | 14–72 |
| Creatinine (mg/dl) | 0.8 (0.6–1.26) | 0.5–1.2 |
| Lactate (mmol/L) | 1.7 (1–3.2) | 0.5–2.2 |
| CRP (mg/dl) | 5.1 (1.1–14) | 0.0–0.5 |
| Procalcitonin (ng/ml) | 0.29 (0.05–4.48) | 0–0.05 |
| Ionic calcium (mg/dl) | 1.05 (1.0–1.09) | 1.16–1.34 |
| Phosphorus (mg/dl) | 3 (2.4–3.7) | 2.5–4.8 |
| Magnesium (mg/dl) | 1.9 (1.66–2.2) | 1.6–2.5 |
| Albumin (mg/dl) | 3.1 (2.7–3.5) | 3.8–5.1 |
CRP: C-reactive protein; PTH: parathyroid hormone; 25(OH)D: 25-hydroxyvitamin D.
Values expressed as median (percentile 25–75).
The normal range is based on the reference values of the hospital laboratory.
On comparing survivors versus non-survivors in the univariate analysis (Table 3), no significant differences were observed in terms of age or gender. As expected, the non-survivors presented higher APACHE II and SOFA prognostic scores upon admission, and also exhibited higher lactate concentrations. There were no differences between the two groups in terms of other variables such as creatinine, albumin or ions.
Univariate analysis comparing survivors versus non-survivors.
| Variable | Survivors | Non-survivors | p |
|---|---|---|---|
| Age, years | 58 (41.5–72) | 63 (53.5–75) | 0.10 |
| Gender | |||
| Males | 75 (68.2) | 14 (56) | 0.24 |
| Type of patient | |||
| Medical | 77 (70) | 19 (76) | 0.93 |
| Surgical | 19 (17.3) | 4 (16) | 0.93 |
| Trauma | 14 (12.7) | 2 (8) | 0.93 |
| APACHE II score | 15 (9–22) | 20 (18–26.5) | 0.00 |
| SOFA score | 4 (3–7.25) | 7 (5.50–10.50) | 0.00 |
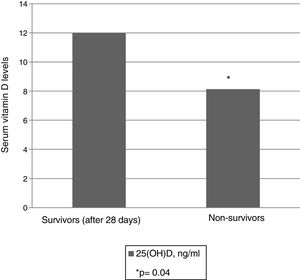
| 25(OH)D (ng/ml) | 12 (7.15–20.31) | 8.14 (6.17–11.53) | 0.04 |
| Creatinine (mg/dl) | 0.77 (0.57–1.15) | 0.98 (0.66–1.57) | 0.12 |
| Ionic calcium (mg/dl) | 1.05 (1.01–1.09) | 1.04 (0.94–1.09) | 0.45 |
| Phosphorus (mg/dl) | 3.00 (2.4–3.6) | 3.1 (2.4–4.08) | 0.65 |
| Magnesium (mg/dl) | 1.9 (1.68–2.20) | 2.00 (1.63–2.30) | 0.32 |
| Albumin (g/dl) | 3.15 (2.70–3.50) | 3 (2.80–3.50) | 0.90 |
| CRP (mg/dl) | 5.05 (0.90–13.85) | 6.60 (1.9–14.55) | 0.65 |
| Procalcitonin (ng/ml) | 0.19 (0.05–4.83) | 0.49 (0.05–4.00) | 0.80 |
| Lactate (mmol/L) | 1.6 (0.98–3.00) | 2.3 (1.4–3.8) | 0.01 |
| PTH (pg/ml) | 83 (46.00–162.00) | 82 (43–193.50) | 0.92 |
| DICM stay, days | 6 (3.00–15.00) | 6 (2.00–11.50) | 0.33 |
| Hospital stay, days | 18 (10.00–31.25) | 6 (2.00–11.50) | 0.00 |
APACHE: Acute Physiology and Chronic Health Evaluation; CRP: C-reactive protein; PTH: parathyroid hormone; SOFA: Sequential Organ Failure Assessment; DICM: Department of Intensive Care Medicine; 25(OH)D: 25-hydroxyvitamin D.
Values expressed as median (percentile 25–75) and n (percentage).
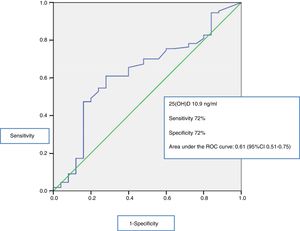
The serum 25(OH)D levels were significantly lower in the group of non-survivors than among the survivors (8.14ng/ml [6.17–11.53] and 12ng/ml [7.1–20.30], respectively, p=0.04), as can be seen in Fig. 3. Receiver operating characteristic (ROC) curves were plotted to determine which 25(OH)D value upon admission was associated to increased mortality after 28 days. The area under the curve (AUC) was 0.61 (95%CI 0.51–0.75), and the cut-off point corresponding to maximum sensitivity and specificity was 10.9ng/ml, with a sensitivity of 72%, a specificity of 61%, a negative predictive value of 91%, and a positive predictive value of 21% (Fig. 4).
The multivariate analysis included those variables with p<0.1 in the univariate analysis (SOFA, APACHE II, lactate and vitamin D; in the case of vitamin D we entered 25[OH]D<10.9ng/ml as variable, based on the cut-off value obtained from the ROC curve). The analysis identified serum total 25(OH)D upon admission<10.9ng/ml (OR 2.86; 95%CI 1.05–7.86; p=0.04) and the APACHE II score upon admission (OR 1.1; 95%CI 1.02–1.14) as independent predictors of mortality risk.
Using this value of 10.9ng/ml as cut-off point, we compared the 61 patients with 25(OH)D<10.9ng/ml versus the 74 patients with 25(OH)D≥10.9ng/ml, and analyzed the possible differences in terms of DICM and hospital stay, ARF and sepsis. We only observed statistically significant differences between the two groups in terms of the presence of ARF upon admission. Of the patients with total 25(OH)D<10.9ng/ml upon admission, 29% suffered ARF versus 13% of those with 25(OH)D≥10.9ng/ml (p=0.02). As commented, there were no differences between these two groups in terms of DICM or hospital stay, or as regard the diagnosis of sepsis upon admission (Table 4).
Comparative analysis between the group with vitamin D<10.9ng/ml and the group with vitamin D≥10.9ng/ml.
| Variable | Total 25(OH)D in blood<10.9ng/ml (n=58) | Total 25(OH)D in blood≥10.9ng/ml (n=77) | p |
|---|---|---|---|
| APACHE II | 19 (12–27) | 16 (8–20) | <0.01 |
| SOFA | 7 (4–9) | 4 (2–7) | <0.01 |
| Sepsis upon admission | 12 (20.7) | 13(16.9) | 0.36 |
| ARF upon admission | 17 (29) | 10 (13) | 0.02 |
| Mortality | 15(25) | 10(13) | 0.04 |
| DICM stay, days | 6 (2.5–14.5) | 6 (2.75–14) | 0.94 |
| Hospital stay, days | 16 (7–30.75) | 16 (8–27) | 0.96 |
APACHE: Acute Physiology and Chronic Health Evaluation; ARF: acute renal failure; DICM: Department of Intensive Care Medicine; SOFA: Sequential Organ Failure Assessment; 25(OH)D: 25-hydroxyvitamin D.
Values expressed as median (percentile 25–75) and n (percentage).
This is the first prospective study carried out in southern Europe covering the entire year, i.e., involving different solar exposures, and in which a high prevalence of hypovitaminosis D is confirmed, as well as the association between severe vitamin D deficiency (25[OH]D<10.9ng/ml) and ARF and mortality among critically ill patients in our setting.
The results obtained show 74% of the patients admitted to our DICM to have deficient 25(OH)D levels. The factors historically associated to hypovitaminosis D in both the general population and in critical patients include geographical setting—with altitude and solar exposure being regarded as determinant parameters in the development of vitamin D deficiency. However, there are also studies reporting a high prevalence of hypovitaminosis D in the general population in areas with high solar exposure, and even in zones where supplementing programs are in force.7 In this regard, our study, which is the only publication in southern Europe involving patients included in all four seasons of the year, has recorded hypovitaminosis D levels upon admission to the DISM identical to those reported in other geographical settings with a climate very different to our own, in which 60–80% of all patients presented hypovitaminosis D upon admission.12,16
In the last decade, a number of studies have examined the possible association between low vitamin D levels and morbidity–mortality among critical patients, with contradictory results.11,12,16,32–34 Although the two meta-analyses published in 201435,36 appear to support an association between hypovitaminosis D and mortality, these findings must be viewed with caution, since most of the analyzed studies were of a retrospective nature, and both meta-analyses had important limitations in terms of the heterogeneity of the included publications, sample size, the definition of hypovitaminosis D, and the prognostic variables studied. Furthermore, these studies were conducted at an earlier date and did not take into account publications such as the FINNAKI trial 201617—a prospective study involving over 600 septic critical patients that recorded no increased mortality among patients with vitamin D deficiency. In this regard, the authors considered vitamin D deficiency to be an indicator of patient severity rather than a direct contributing factor to patient outcome. In contrast to the above article, our prospective study was not limited to septic patients and did observe a clear association between vitamin D deficiency and mortality among the critically ill, even after risk adjustment for other mortality-associated variables. However, in our opinion, demonstrating that vitamin D deficiency is correlated to increased mortality is not the only important issue: we also must determine the degree of deficiency (i.e., the cut-off point) at which such a correlation becomes established. In our series we identified a cut-off point of 10.9ng/ml as the best predictor of mortality—this figure being concordant with other studies in which the critical 25(OH)D levels were seen to be between 10 and 12ng/ml.12,16 Our study was based on the criteria of the United States Endocrine Society30 for grouping the patients according to their vitamin D values upon admission, as this is currently the most widely used classification.
Considering the difficulties there have always been in adequately defining hypovitaminosis D and the results obtained in our study, we believe that the mentioned classification—derived from studies and recommendations referred to the general population—is not applicable to critical patients. This suggests the need for classifications more closely adjusted to or specific of certain types of patients, such as those admitted to the DICM, and which are particularly important if we wish to use them as a guide in deciding the administration of vitamin D supplements. This treatment option—supplementing in the case of vitamin D deficiency in critical patients—is also subject to debate. While the study of Amrein et al. (2014),19 involving 475 patients, does appear to demonstrate that systematic supplementing in critical patients reduces in-hospital mortality in cases of severe vitamin deficiency, other smaller studies have only been able to normalize serum vitamin D concentration or shorten hospital stay—with no impact upon mortality.22,36 Even the two recent meta-analyses on this subject have yielded contradictory results.25,26 We believe that the disparity of findings and the scant existing evidence are probably a consequence of the heterogeneity of the studies regarding the type of vitamin D prescribed, the administration route employed, and the doses used. All this makes their analysis very difficult.
Our study also found an association between severe hypovitaminosis D and ARF upon admission to the DICM, with renal failure being significantly more frequent in patients with 25(OH)D<10.9ng/ml than in those with concentrations of ≥10.9ng/ml (29% vs. 13%). In a retrospective study published in 2012, Braun et al.37 also recorded an association between vitamin D deficiency and ARF in the critical patient, which they attributed to the important role played by vitamin D in the inflammation seen in ARF, in view of its effects upon the immune system. This issue is still subject to debate, and knowing whether the relationship between vitamin D deficiency and mortality and ARF is of a causal nature—playing a significant role in the physiopathology of organ failure—or simply a marker of patient severity, remains to be clarified. Nevertheless, taking into account the pleiotropic actions of this vitamin, it seems logical that it may be implicated in the pathogenesis of organ dysfunction in the critical patient, though our observational study does not allow the drawing of conclusions in this respect.
Our study has some limitations. A first limitation is that it is a single-center study with a relatively limited number of patients. Furthermore, and in order to ensure a homogeneous sample of patients and eliminate possible external confounding factors, we adopted very strict exclusion criteria that caused us to lose part of the sample, and which limit the possibility of reproducing the results obtained. The other two main limitations are the same as those which affect most studies in this field. In order to know vitamin D status among the included patients, we determined the total 25(OH)D levels (regarded as the gold standard to date), and this determination was made using immunoassay techniques, without taking into account that critical patients experience changes in albumin concentration and vitamin D binding proteins secondary to the inflammatory process, intensive fluid resuscitation measures, and renal losses of albumin and vitamin D binding proteins. These factors can generate technical bias, and in this regard mass spectrophotometry would be more indicated for measurement purposes in this patient population.38,39 Another point of controversy is whether the total 25(OH)D levels are truly the best indicator of vitamin D status in the body. In this regard, it seems logical that the free and albumin-bound fractions (i.e., the genuinely bioavailable fractions) would constitute a better indicator than total levels in blood.40
In conclusion, our series confirms the high prevalence of hypovitaminosis D upon admission to the DICM and demonstrates the association between severe vitamin D deficiency and mortality and ARF in the critical patient. In addition, we have identified the cut-off point in serum total 25(OH)D that best predicts mortality in this patient population (<10.9ng/ml), demonstrating that the definitions of hypovitaminosis D contained in the currently most widely used guides are not appropriate for use in the critically ill.
We consider that well designed randomized studies involving a large number of patients are needed to evaluate the usefulness of vitamin D supplementing in patients admitted to the DICM, though the high prevalence of deficiency and its association to ARF suggest that critical patients with ARF would be the best candidates for supplementation of this kind.
Authors’ contributionAll the authors have made a substantial contribution to the conception and conduction of the study or to interpretation of the data, as well as to the drafting, revision or definitive approval of the manuscript.
Drs. Nolla, Zapatero and Dot have participated in the conception and design of the study.
Drs. Nolla, Zapatero, Dot and Masclans have participated in major drafting of the original manuscript, while Drs. Gracia, Diaz and Pérez-Terán have participated in some points of the preparation of the manuscript.
All the authors have participated in compilation of the database and in identification of the patients, though Dr. Climent has played a particularly relevant role in this respect.
Drs. Nolla, Zapatero, Dot and Masclans have participated in the revision indicated by the reviewers and in major drafting of the manuscript and in the reply to reviewers. Drs. Gracia, Diaz and Pérez-Terán have also participated in the reply to reviewers.
All the authors have participated in the final approval of the study.
Conflicts of interestThe authors declare that they have no conflicts of interest in relation to the present study.
Please cite this article as: Zapatero A, Dot I, Diaz Y, Gracia MP, Pérez-Terán P, Climent C, et al. La hipovitaminosis D grave al ingreso en el paciente crítico se asocia a fracaso renal agudo y mal pronóstico. Med Intensiva. 2018;42:216–224.