Since one of the main challenges in treating acute burn injuries is preventing infection, early excising of the eschar and covering of the wound becomes critical. Non-viable tissue is removed by initial aggressive surgical debridement. Many surgical options for covering the wound bed have been described, although split-thickness skin grafts remain the standard for the rapid and permanent closure of full-thickness burns.
Significant advances made in the past decades have greatly improved burns patient care, as such that major future improvements in survival rates seem to be more difficult. Research into stem cells, grafting, biomarkers, inflammation control, and rehabilitation will continue to improve individualized care and create new treatment options for these patients.
La escarectomía precoz y la cobertura de la quemadura son fundamentales en la prevención de la infección en el paciente quemado agudo. Mediante un desbridamiento quirúrgico intensivo inicial se retira el tejido no viable. Se han descrito múltiples opciones quirúrgicas para cobertura del lecho cutáneo, aunque los injertos de piel parcial continúan siendo el estándar para una cobertura rápida y permanente de las quemaduras de espesor total.
Los grandes avances que han tenido lugar en décadas anteriores han mejorado de forma sustancial los cuidados del paciente quemado de tal forma que parece difícil que pueda haber mejoras importantes en las tasas de supervivencia futuras. Técnicas tales como la investigación en células madre, injertos, biomarcadores, control de la inflamación y rehabilitación contribuirán a mejorar unos planes de cuidados individualizados y a crear nuevas opciones de tratamiento para estos pacientes.
Burns are common injuries with considerable morbidity and mortality. Early excision and grafting has been the standard care for decades. Since mid 70s most studies have shown that excision within 24–48h after injury is associated with decreased blood loss, infection, length of hospital stay and mortality, and increased graft take,1–4 although mortality reductions may only occur in patients without inhalation injury.5,6
The current therapy of the acutely burned patient is based on adequate resuscitation, early wound debridement and closure, support of post burn hypermetabolic response and control of infection. Therefore, since one of the main challenges in treating acute thermal injuries is preventing infection, excising the eschar and covering the wound as early as possible is critical. By early, aggressive surgical debridement, non-viable tissue is removed and hence the wound bed is relatively infection free. Furthermore, the removal of dead tissue has the potential to reduce the generation of chemical mediators that stimulate the inflammatory cascade leading to remote and multisystem organ failure.
Need for surgical intervention/debridement depends on the depth of the injury.
- •
Full-thickness burns destroy all of the dermal elements; hence there are no epidermal cells left to regenerate the injured area.
- •
Partial thickness injuries allow epidermal cells to survive in the dermal elements, such as sweat glands or hair follicles to repopulate the injured area.
In general, partial thickness and second-degree burns are used interchangeably, while full thickness and third degree are synonymous.
Complete debridement should proceed at the earliest possible opportunity, even if donor sites are insufficient to provide total wound coverage. In this case, biological dressings (preferably cadaveric donor allograft) should be used to cover the remaining wounds. Excision of burn wounds requires large volumes of blood for transfusion (approximately 1cc/cm2 to be excised).7 Another factors leading to blood loss in burn patients are old age, male gender and extensive total body surface areas (TBSA) burnt. Blood loss can be minimized by the use of excision to the level of fascia or tourniquets when performing tangential excisions of the extremities. Moreover adrenaline-lidocaine subcutaneous infiltration solutions can be used in both burned areas and donor sites.8 Besides, thrombin spray, fibrin glue, alginate dressings and topically applied adrenaline gauzes may also help dealing with bleeding.7,8
Tangential excision gives a better cosmetic outcome by leaving subcutaneous fat but blood loss is greater. On the contrary, fascial excision presents lower bleeding rates but worse cosmetic results. The latter is indicated in burns comprising extense TBSA and critical patients1–3 (Table 1). The depth of excision is judged on the degree of bleeding and on visual inspection of the excised bed, both of which require the technical expertise of an experienced burn surgeon. Equally, to resolve the depth of the injury often relies on clinical examination and experienced judgment. In addition to examining burn wounds directly another potential method to determine the ability of burn wounds to heal is non-invasive imaging.9 Recently, a number of non-invasive imaging techniques have been investigated for their use in determining burn depth. Such techniques include terahertz imaging, spatial-frequency-domain imaging, near-infrared spectroscopic imaging, and reflectance-mode confocal microscopy, among others.10,11 Many of these techniques have not been yet refined sufficiently for clinical application. Nowadays, laser Doppler imaging provides the most evidence for accurately assessing burn severity,12 but it has been shown to be superior to visual assessment only 48h after thermal injury.13
Types of surgical excisions.
| Advantages | Disadvantages | Clinical applications | |
|---|---|---|---|
| Tangential excision | - Improved esthetic outcomes | -Difficult to assess good plane for grafting -Blood loss | More common |
| Fascial excision | - Delivery of a well vascularized plane for grafting. -Lower bleeding rates | Unpleasant esthetic outcome | Reserved for larger deeper burn injuries |
The Versajet hydrosurgery system (Smith & Nephew, London, UK) is a device based on the Venturi effect, able to cut and aspirate debris contemporarily.14 While in full thickness burns the sharp debridement is advisable, the Versajet shows its benefits in the treatment of partial thickness burns. Especially for debridement of difficult to treat areas such as the face, neck, lips, fingers, interdigital spaces, convex and concave areas. With the Versajet System, tissue excision is precise; moreover it helps to avoid the damage of viable tissue and its vascular supply. Is suggested by some to have facilitated a paradigm shift in wound management by allowing debridement of undesirable tissue while accurately preserving viable structures.15
Enzymatic debridementEnzymatic debridement is considered one promising alternative to surgical excision and has driven motivated clinicians and researchers for half a century to investigate the potential of various non-surgical debriding agents and enzymatic debridement means for burns, even with slow and inefficient enzymes. Therefore, until recently, results proved to be highly variable.16
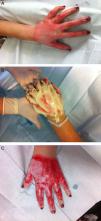
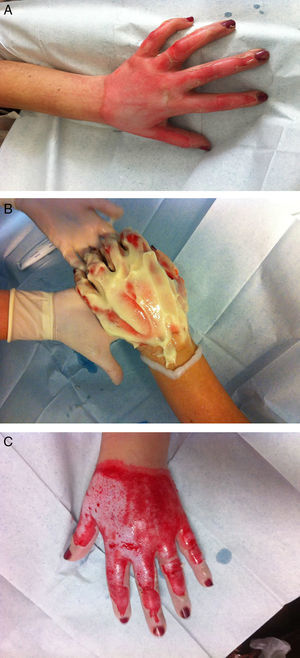
Hand burns have a special place in the field of burn care, demanding special attention and treatment. Hands are involved in 30–60% of all burn patients. Due to the anatomy of the hand (important and delicate structures crowded in a small limited space without subdermal soft tissue), surgical debridement of the burned tissue is technically difficult and may cause considerable complications17 (Fig. 1).
Lately, enzymatic debridement studies have been reported with promising results.18,19 Application is possible outside the operation room as long as sufficient analgesia is ensured during this otherwise painful debridement procedure. In a subgroup of hand burns, additional benefits were seen as these patients exhibited earlier time to wound closure and better long-term results.18 Similar findings had previously been reported in a retrospective data analysis of 69 hand burns.20 To date, other than pain, no enzyme-related adverse event or complications that jeopardize burn healing process have been reported.19
EscharotomyWhen the burn eschar circumferentially surrounds any body structure (specially digits, extremities, abdomen, chest, or neck) the tissues within are subject to increasing interstitial pressures exacerbated by tissue edema developed during the acute phase of burn resuscitation in the first 48h after injury. As the interstitial pressure rises there is initially impairment of venous outflow followed by diminution of arterial inflow. This condition will cause dysfunction, ischemia, or necrosis within or distal to that body structure, often within hours. In the limbs, nerve and muscle death may occur causing permanent functional impairment or even the need for amputation. In the abdomen, the impaired blood supply to the bowels, kidneys and other internal organs results in the rapid onset of hepatic and renal failure, intestinal ischemia, and restriction of diaphragmatic excursion.
Escharotomy releases the constricting tissue allowing the body tissues and organs to maintain their normal perfusion and function. In most cases a single incision is inadequate to provide release of the constricting burn eschar. Escharotomy incisions are routinely performed on both sides of the torso or the medial and lateral sides of each affected limb. For the abdomen and chest, transverse incisions are often required to permit restoration of respiratory movement. Delayed primary closure of escharotomy incisions may produce better functional and cosmetic results than those achieved if the escharotomies are allowed to close by secondary intention.5
Skin graftsThe standard for rapid and permanent closure of full-thickness burns is a split-thickness skin graft from an uninjured donor site on the same patient. Two types of autografts used for permanent wound coverage have been used:
- -
Sheet graft is a piece of donor skin harvested from an unburned area of the body. Size of the donor skin is about the same as the burn wounds. This occurs because the donor skin used in sheet grafts does not usually stretch. When the TBSA burned is large, sheet grafts are usually saved for the face, neck and hands, making the most visible parts of the body appear less scarred. When a burn is small and there is plenty of donor skin available, a sheet graft can be used to cover the entire burned area. The disadvantages of sheet grafts comprise both higher rates of postoperative hematoma and larger donor site than meshed skin. Sheet grafts are usually more durable and scars less.
- -
Meshed skin grafts. As the size of injury increases, there is proportionately less donor site for autografting, so alternative techniques are required. Split-thickness skin grafts can be meshed with variable expansion ratios to increase the coverage area. Common mesh ratios include 1:1, 1.5:1, 2:1, 3:1, and 4:1. The larger the ratio, the more area of excised burn wound can be covered by any given piece of donor skin. Meshing allows blood and body fluids to drain from under the skin grafts, therefore preventing graft loss. However, the larger the ratio, the less the wound is actually covered and the more hypertrophic scarring results during the slow process of epithelial cell migration. Besides, some concerns remain over the effect that meshing has on range of motion21 and the graft site healing rate resulting in less satisfactory functional and cosmetic result. Moreover, donor sites can be very painful and add more wound-healing areas to the patient.22 Various dressings have been used to cover donor sites during healing, with variable results.23
In 1958 a remarkable technique for expanding autografts was described by the American surgeon C.P. Meek. With a so-called ‘MEEK-WALL’ dermatome postage stamp autografts were obtained and expanded with a ratio 1:9 using double pleated gauzes. The method however required too much skill and it became eclipsed by the introduction of mesh skin grafts by Tanner in 1964. However, lack of autograft donorsites is still encountered as a limiting factor in achieving wound closure in case of extensive burns. The meshgraft technique requires donorsites of suitable size and shape and epithelialization may be delayed in case of expansion ratios greater than 1:4. Encouraging results are reported in combinations of the new MEEK technique with other methods for wound healing in burns, like dermal generating templates and cell spraying methods.24,25
Skin grafts will not take on an infected wound, over a hematoma, or on a wound with necrotic material. Adequate debridement and recipient-site preparation are essential for skin graft take. Skin graft procedures performed early after the burn injury with meticulous technique and postoperative splinting will often prevent subsequent contractures.
Temporary wound coveragePatients with more extensive burns often require temporary coverage with an allograft, xenograft, skin substitute, or dermal analog due to insufficient or unavailable donor sites. Besides, temporary skin substitutes are used when the patient is too ill to undergo the creation of another wound that results when skin is harvested from a donor site, in cases regarding the viability of the recipient bed, or when there is a concern regarding potential infectious complications. Allografts, or tissue taken from a living or deceased human donor, and xenografts, taken from a different species, promote re-epithelialization and prepare the wound bed for autograft, increasing the healing rate when compared with traditional dressings.26
The gold standard temporary skin substitute is cadaver allograft. Besides, it is also the preferred material for protection of widely meshed autografts (3:1 or higher meshing ratios) during healing. In that setting, the allograft is applied over the meshed autograft as a sandwich.
Allograft skin from living donors is not widely accepted worldwide and hence raises the ethical dilemma and medico-legal implications of this technique. Taking into account the religious and ethical considerations, improvements and major developments in burns treatment and the availability of cadaveric, porcine or synthetic skin substitutes, the option of using live allograft has been long abandoned.27 However in developing countries, synthetic skin options are either not available or are very expensive and would be a huge burden of cost to the health system. Besides, the use of cadaveric donor skin in those countries is not acceptable due to religious and cultural beliefs, and relatives would not consent to skin harvesting from cadavers. They also lack the facilities for storage and screening of cadaveric skin, hence the option of live siblings’ allograft is considered as a viable option despite the limited literature available.28,29
A variety of different skin substitutes and dermal analogs are available in different regions of the world30,31 (Table 2). They can be broadly divided into those which replace the epidermis or replace the dermis.31 Epidermal substitutes are normally only a few cell layers thick and lack normal dermal components. Collagen-based dermal substitutes are porous matrices that act as templates for dermal regeneration. Like skin grafts, these products become revascularized over time and then can be covered with a thin epidermal graft to complete the coverage. Among the advantages of these products are decreased donor-site morbidity and elasticity and skin feeling patient satisfaction when compared with conventional meshed split-thickness skin grafts.32 Small areas of bone, joint capsule, and tendon can also be covered. Disadvantages include cost and an increased susceptibility to infection compared with split-thickness skin grafts. These products have also been successful in treating reconstructive problems such as hypertrophic scarring and joint contractures.33 Commercially available dermal substitutes include acellular matrices, commonly from human or other sources.34
Skin substitutes and coverage options.
| Product name | Classification | Characteristics |
|---|---|---|
| EpiDex | Autologous | Keratinocyte-based |
| Dermagraft | Cellular | Bioabsorbable polyglactin mesh scaffold with human fibroblasts (neonatal origin) |
| Epicel | Cellular | Keratinocyte-based cultured epidermal autograft |
| Recell | Cellular | Autologous cell suspension of keratinocytes, fibroblasts, Langerhans cells and melanocytes Sprayable after culture |
| Alloderm | Acellular | Human origin |
| Integra | Acellular | Bovine/shark origin Bilayer matrix |
| Biobrane | Acellular | Biocomposite dressing, nylon fibers in silicone with collagen |
Biobrane (Smith & Nephew, London, UK) is a silicone membrane pressed onto a flexible nylon fabric which has been coated with porcine dermal collagen. It is considered a dressing used for temporary closure of superficial burns and donor sites.31,35 Nowadays there are different products under development that integrate the concept of dermal scaffolds actively promoting revascularization by incorporating stem cells and growth factors to recreate a favorable cellular microenvironment.34
Cultured epithelial cellsAlternatively, cell-based techniques for more permanent coverage have made progress. Both widely expanded meshed autograft and cultured epithelial cells have particular advantages and disadvantages (Table 3).
Expanded meshed autograft versus cultured epithelial autograft.
| Advantage | Disadvantage | |
|---|---|---|
| Meshed autograft | Low cost/complexity | Limited donor site |
| Durable | Multiple surgeries | |
| Cultured epithelium | Cover large wounds | High cost |
| Useful in limited donor areas | Long term fragility | |
| Lack of dermis | ||
| Increased scar contracture |
Research on cultured epithelial cells has made advancements, especially with respect to culture time. Culture-based options use a small biopsy of the patient's skin to provide keratinocytes, which are expanded over 2–3 weeks. Some commercial products are available for this goal such as Epicel o ReCell.36,37 These techniques may reduce the amount of donor skin needed for large burns treatment, significantly reducing the healing time of both the donor and the burn sites, and increasing overall graft success and scar quality.38 However, it shows some limitations including sensitivity to infection and the lack of a dermis, which leads to fragility of the healed skin and severe scarring. Therefore its use in our clinical environment is usually restricted to large burned patients with no other therapeutical options.
Flap solutionsIncisional and excisional release and skin grafting remain the mainstays of post burn reconstruction. However, on mobile areas such as joints, neck, and axilla, skin-grafted wounds can contract, leading to secondary contractures. Local flaps, when available, provide an excellent choice to supply stable coverage and minimize joint contractures. Flaps do not need rigorous post-operative physiotherapy or splintage and grow with age, especially in children. These local flaps can be classified as advancement flaps, rotation flaps, and transposition flaps.
Harvesting a tissue from the burnt area is possible, but there are increased chances of flap failure. For cases where traditional flaps are not easily designed because of extensive burn injury, propeller flaps may be an excellent choice.39 The original propeller flap uses intact skin in the fossa of the elbow or axilla elevated on a central subcutaneous pedicle. More recently, these flaps have been designed as perforator flaps.39 Other advances include multilobed propeller flaps, keystone flaps and square flaps.40,41
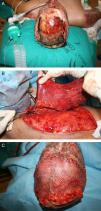
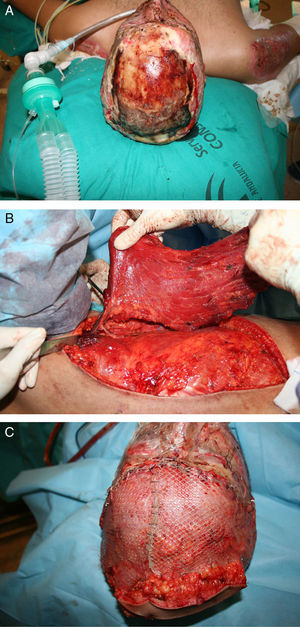
Traditionally, free flaps were used in burn reconstruction when there was exposed bone, tendon, or cartilage present and local or regional flaps were inadequate. To reduce donor-site morbidity, perforator flaps such as anterolateral thigh flaps or conventional flaps such as groin flaps and scapular flaps are useful42 (Fig. 2).
Composite tissue allotransplantation (CTA)For very significant deformities that result from injuries to extremities and the face, CTA offers a solution that can restore significant esthetic and functional units in a single surgical procedure. Hand and face allotransplantation are demanding and challenging operations. To our knowledge only three of the 19 face CTA accomplished have been performed in burn patients, and one of them died.43–46
The potential high risk of lifetime immunosuppression remains a significant barrier to the widespread use of this technology.47 Another particular concern specific to the burn patient population regarding CTA is the high prevalence of psychiatric comorbidity and drug abuse, as well as high risk of septic complications.43,45 Therefore, CTA is still regarded as an experimental procedure. Due to the complexity involved, its indications appear to be currently limited to only severely disfiguring facial defects in burns (involving the peri-orbital and/or peri-oral regions) and bilateral hand amputation, with severe functional and cosmetic impairment.45–47
Future researchWound healing is a complex process centered on cellular proliferation, matrix deposition, and tissue remodeling. Studies have attempted to delineate the influence of fat grafts on wound area and volume reduction. The current literature reports significant functional and esthetic improvements after fat grafting in the setting of burn and radiation injuries. In vivo studies demonstrating the regenerative capacities of adipose derived stem cells have proven to be generalizable to a clinical setting, given the extent of improvement in tissue quality noted after fat grafting in numerous patient population.48
In porcine models the speed and quality of wound healing was statistically better in the models that received an adipose graft when compared to those receiving only growth factors.49 Moreover, human adipose stem cells that would normally be discarded have recently been isolated from debrided burn eschar tissue50 and used to generate a tri-layered, vascularized construct.51 Promising data with nonembryonic stems cells have stimulated interest into future applications and development. Likely further investigations will produce exciting results.
Keratinocytes also play a vital role in wound closure. Retrospective analyses showed that cultured allogeneic or autologous keratinocytes may accelerate wound healing.52 Studies evaluating both keratinocytes expansion on human fibroblasts following trypsin extraction, and engineered skin with keratinocytes on a fibrin matrix, have demonstrated improvements in wound healing.53 Taken together, the future impact of keratinocyte-mediated cell coverage options is promising. Nevertheless more research is needed before broad conclusions can be made.
Pain managementPain is the most frequent complaint of burn-injured patients. It is known that persistent pain of a peripheral origin may induce pathological changes on a spinal and supraspinal level, leading to central sensitization, mechanical and thermal skin hyperalgesia and pain maintenance.54 These patients usually present a hyperaroused state that augments the probability of defence programs activation.55
Recent publications report unacceptably high pain ratings for procedural and chronic pain 4 months after injury.56,57 Clinical observations in different burn units may confirm that these reports are not unusual. The discrepancy between patients and staff assessments of pain severity is constant in burn patient's literature.58 Those who specialize in these patients care may become accustomed to the expectation that pain will not be controlled, particularly during procedures. Burn Unit personal may rationalize that it will only hurt for a brief period of time therefore underestimating procedural pain.
Usually pain-relieving protocols include the systematic use of opioids, with the dispensable use of nonsteroidal anti-inflammatory analgesics and anxiolytics as adjuvant drugs. All of them administered either by oral or intravenous access. Patient-controlled analgesia (PCA) is used for patients upon hospital admission and after surgical procedures. Moreover, sedation with propofol or midazolam is used very frequently for extensive burn-dressing changes. The use of fentanyl administered during treatments and procedures has shown a significant difference in pain reduction post-procedure, with minimal presence of adverse events arising from sedo-analgesia. Therefore fentanyl offers a potentially significant improvement showing it is a safe and effective way to control procedural pain.59
Ketamine is a non-competitive antagonist of NMDA receptors and may be used for conscious sedation during dressing changes in burn patients.60 It induces a state of dissociative anesthesia with intravenous doses of 1mg/kg. As an advantage, it maintains the airway reflexes, blood pressure, and heart rate by indirect release of norepinephrine. The occurrence of hallucinations, a significant adverse effect, may be attenuated by concomitant administration of benzodiazepines or propofol. Furthermore, ketamine was effective as rescue medication in case of pain less responsive to opioids. It also appears to promote some action in reducing hiperalgesia.61
Additionally, a number of non-pharmacological therapies have been described. Qualitative research has shown humor as a coping approach during their injuries acceptance process.62 Additionally, other non-pharmacological interventions such as relaxation techniques,63 music therapy,64 or more recently techniques using virtual reality devices65 have been tested in experimental studies. All of them resulting in heterogeneous effects on pain reduction before and after wound care.
Although burn injury pain has been well-described as a major clinical problem during the last two decades56–66 researchers continue to report that burn pain remains undertreated.56,67,68 Thus it becomes difficult to understand why is burn injury pain still a major problem after 20 years of research. This should be a matter of concern because unrelieved pain may be contributing to long-term sensory problems, including chronic pain, paresthesias, and dysesthesias69,70 as well as debilitating psychological conditions.71 Perhaps, the main problem is that we are focusing on the fact that burn pain is a very difficult type of pain to treat and not querying if we are doing our best to treat it. For example, reabsobable staples have been well described in literature72,73 but, to our knowledge, clinical application in burn injuries has not been mentioned again for the last 15 years. Taking into account that absorbable tacks show the advantages of metal staples for skin grafts without the associated problems in burns grafting (procedural pain during removal)73 one could think that their more widespread use should be considered. Besides, different classes of medications in combination with non pharmacologic methods (multimodal analgesia) are rarely used in Burn Units even though they appear to be very useful for pain management in the burn-injured. Therefore more research and real application of evidences is needed to be able to target specific mechanisms that contribute to the variable intensity making this type of pain such a difficult management problem.
Pressure garments and physiotherapyAfter burn wounds and grafts have acutely healed, burn patients present hypertrophic scarring risk. Custom-fit pressure garments have been used in burn patients in order to prevent or correct hypertrophic scarring. To our knowledge little scientific documentation of the effectiveness of these garments has been described. Engrav et al. published the findings of their 12-year randomized study of pressure garments to manage forearm burn scars. Special garments were used that applied pressure (17–24mmHg) for half of the garment, whereas the other half had minimal compression (<5mmHg). Authors found wounds treated with tighter compression to be thinner, softer, and show a better overall appearance, thus confirming the clinical effectiveness of pressure garments. The benefits were most evident in patients with moderate-to-severe scarring, but pressure garments did not have a significant effect in patients with mild scarring.74
Increased survival rates in patients with severe burns bring into focus the rehabilitative challenges and recovery that these patients must face. Physiotherapy rehabilitation is an integral component of burn care in order to maintain the range of motion, prevent contracture development, maximize function and promote psychological wellbeing and social integration.75
In recent years, the use of interactive video games for rehabilitation of burns has become established.76 Their ease of use, increasing affordability, interactive functionality and physically rigorous gameplay makes interactive video games ideal to motivate patients unmotivated when undergoing repetitive exercises.76,77 Recent studies utilizing the PlayStationTM EyeToy and Nintendo1 WiiTM have demonstrated that interactive video games are safe and effective in providing pain relief,78 as well as improving joint movement and functional ability among burn patients.76,79
ConclusionSignificant advancements made in the past decades have improved burn patient's care in such a way that major future improvements in survival rates seem to be much more difficult. The remarkable advances achieved by plastic and reconstructive surgery over the past century allowed a large number of techniques to substantially improve the outlook of these patients. Despite these advances, we still do not have the ability to perfectly restore patients to their preinjury state. Therefore, improvements are needed to accelerate wound closure and healing as well as upgrade psychological care to promote successful reintegration. Research in stem cells, grafting, biomarkers, inflammation control, and rehabilitation will continue to improve individualized care and create new treatment options for these patients.
Conflict of interestsThe author declares no conflict of interests.