To determine the relationship between QRS duration and dispersion and the occurrence of ventricular arrhythmias in early stages of acute myocardial infarction (AMI).
DesignA retrospective, longitudinal descriptive study was carried out.
SettingHospital General Universitario “Camilo Cienfuegos”, Sancti Spíritus, Cuba. Secondary health care.
Patients or participantsA total of 209 patients diagnosed with ST-segment elevation AMI from January 2012 to June 2014.
Main variables of interestThe duration and dispersion of the QT interval, corrected QT interval, and QRS complex were measured in the first electrocardiogram performed at the hospital. The presence of ventricular tachycardia/fibrillation was assessed during follow-up (length of hospital stay).
ResultsArrhythmias were found in 46 patients (22%); in 25 of them (15.9%), arrhythmias originated in ventricles, and were more common in those subjects with extensive anterior wall AMI, which was responsible for 81.8% of the ventricular fibrillations and more than half (57.1%) of the ventricular tachycardias. The widest QRS complexes (77.3±13.3 vs. 71.5±6.4ms; p=0.029) and their greatest dispersion (24.1±16.2 vs. 16.5±4.8ms; p=0.019) were found on those leads that explore the regions affected by ischaemia. The highest values of all measurements were found in extensive anterior wall AMI, with significant differences: QRS 92.3±18.8ms, QRS dispersion 37.9±23.9ms, corrected QT 518.5±72.2ms, and corrected QT interval dispersion 94.9±26.8ms. Patients with higher QRS dispersion values were more likely to have ventricular arrhythmias, with cutoff points at 23.5ms and 24.5ms for tachycardia and ventricular fibrillation, respectively.
ConclusionsIncreased QRS duration and dispersion implied a greater likelihood of ventricular arrhythmias in early stages of AMI than increased duration and dispersion of the corrected QT interval.
Determinar la relación entre duración y dispersión del QRS con la aparición de arritmias ventriculares en las fases iniciales del infarto agudo de miocardio (IAM).
DiseñoEstudio descriptivo retrospectivo longitudinal.
ÁmbitoHospital General Universitario «Camilo Cienfuegos» de Sancti Spíritus, Cuba. Atención secundaria.
Pacientes o participantesDoscientos nueve pacientes con diagnóstico de IAM con elevación del segmento ST entre enero de 2012 y junio de 2014.
Variables principales de interésSe midieron la duración y dispersión del QT, QTc y QRS del primer electrocardiograma hospitalario y se determinó la presencia de taquicardia/fibrilación ventricular en el seguimiento (estancia hospitalaria).
ResultadosSe detectaron arritmias en 46 pacientes (22%), en 25 (15,9%) estas fueron ventriculares; más frecuentes en el IAM anterior extenso, que fue responsable del 81,8% de las fibrilaciones ventriculares y más de la mitad (57,1%) de las taquicardias ventriculares. La duración del QRS (77,3±13,3 vs. 71,5±6,4ms; p=0,029) y su dispersión (24,1±16,2 vs. 16,5±4,8ms; p=0,019) fue superior en las derivaciones afectadas por la isquemia. Los mayores valores de todas las mediciones se presentaron, con diferencia significativa, en el IAM anterior extenso: QRS 92,3±18,8ms, dQRS 37,9±23,9ms, QTc 518,5±72,2ms y dQTc 94,9±26,8ms. Los pacientes con mayores valores de dispersión del QRS tuvieron más probabilidad de presentar arritmias ventriculares, con puntos de corte de 23,5ms para la taquicardia y de 24,5ms para la fibrilación ventricular.
ConclusionesEl incremento de la duración y dispersión del QRS mostró mayor probabilidad de aparición de arritmias ventriculares en las fases iniciales del IAM que los incrementos del intervalo QTc y su dispersión.
Ventricular arrhythmias are the main cause of death in the early stages of acute myocardial infarctions (AMI). The most recent guidelines published by the European Society of Cardiology on the management of patients with ventricular arrhythmias and prevention of cardiac sudden death state that up to 6 per cent of the patients with acute coronary syndrome (ACS) suffer from tachycardia or ventricular fibrillation (VF) in the first 48h after symptom onset, and that infarctions debuting as sudden death during those 48h are one of the main reasons of death due to AMI.1
The physiopathology of these arrhythmias in this context is complex due to the electrophysiological changes that occur in the ischaemic area. In a normal heart, the M cells located in the middle myocardium show a significantly longer action potential than the epicardium and endocardium existing an electrotonic coupling with the adjacent layers consistent with the end of the T wave of the electrocardiogram (ECG).2 Such coupling is altered after one ischaemic myocardial lesion and allows the expression of the intrinsic properties of these M cells that are shown in the surface ECG as an extension of the QT interval.3
Llois et al.4 found one positive correlation between the levels of troponin and the corrected QT interval (QTc) as an independent predictor of major clinical events after 30 day follow-up in patients with ACS; and another study5 found more serious ventricular arrhythmias during acute ischaemias and the presence of a new ACS during the follow-up of patients with greater QTc dispersion (dQTc). So there is no doubt that the greater the polarization heterogeneity expressed by the dQTc is, the higher the odds of serious ventricular arrhythmias will be; however little is known on the dispersion of the QRS complex (dQRS).
The electrophysiological changes present in the ACS are shown in the ECG as an extension of the QT interval, an increased dQTC and Tpeak-Tend dispersion.6 These alterations added to the dQRS make the ECG one diagnostic and prognostic tool of great utility for the clinical cardiologist.
Several studies have associated the dQRS with the appearance of sudden death in patients with heart failure,7–9 but no studies have assessed this parameter in the context of ACS. This is why, the main goal of this study was to determine the existing correlation between the duration and dispersion of the QRS and the appearance of ventricular arrhythmias in the early stages of the ACS, and specify its diagnostic performance, and establish cut-off points.
Patients and methodsWe conducted one retrospective, descriptive, longitudinal study with 209 patients of the 241 patients admitted in the Coronary Intensive Care Unit of the Hospital General Universitario “Camilo Cienfuegos” de Sancti Spíritus, Cuba, between January 1st, 2012 and June 30th, 2014, with a diagnosis of ST segment elevation acute coronary syndrome (STE-ACS).
In order to establish the diagnosis and topography of the STE-ACS, the third universal definition criteria of myocardial infarction10 as well as definitions established by other researchers11,12 were used.
Six (6) patients with left bundle branch block (LBBB) were precluded from the study, one of them with an accessory pathway that was evident in the ECG, 15 with insufficient clinical and lab data, 2 with a poor ECG with which the necessary measurements could not be taken, 5 with a diagnosis of atrial fibrillation prior to the STE-ACS, and 3 patients with valvular disease and hypertrophic or dilated myocardiopathy. None of the patients included in the study had been treated with antiarrhythmic drugs capable of modifying the QTc.
ECG analysisThe first 12-lead ECG was analyzed after the arrival of the patient to the hospital before any revascularizations (thrombolysis or percutaneous coronary intervention) were performed. The ECGs were obtained at a sweep speed of 25mm/s and a standard gain with one Cardiocid electrocardiogram machine (ICID, Cuba) including one bandpass filter that limits the spectrum of frequencies between 0.05 and 150Hz, and one comb filter for the electric hum at 60Hz.
With the help of one magnifying lense, two observers (authors) manually and independently measured12,13 the following parameters in all ECG leads of each and everyone of the patients:
- -
QT: It is the measured QT corresponding to the time, expressed in milliseconds (ms), elapsed from the beginning of the QRS until the end of the T wave, defined as the T wave return point to the isoelectric line, or the nadir between the T and U waves when it is present.13 It was measured in all leads and the average was calculated.
- -
QTc: The measurement following Bazett's formula14 was taken in all leads and the average was calculated. QTc intervals ≥440ms in males, and ≥460ms in females15 were considered unusual.
- -
Dispersion QT: Difference between the maximum QT and the minimum QT of the 12 lead ECG.
- -
dQTc: Difference between the maximum QTc and the minimum QTc of the 12 lead ECG.
- -
Duration of the QRS: Time, expressed in ms, elapsed between the initiation of the Q or R waves until the end of the R or S waves.
- -
dQRS: Difference between the maximum QRS and the minimum QRS of the 12 lead ECG.
Inter-observer variability measured using Cohen's kappa coefficient was scarce as we can see in its values of substantial concordance for every single parameter measured: QT: 0.82; QTc: 0.69; dispersion of QT: 0.74; dQTc: 0.67; duration of QRS: 0.79 and dQRS: 0.73.
Other variablesWhen patients were admitted at the Intensive Care Unit, their demographic data, the factors of coronary risk, and the existence of signs of left ventricular dysfunction were all collected; also patients underwent one transthoracic echocardiogram, and blood samples were extracted for further analysis followed by the management of AMI according to currently applicable clinical practice guidelines for the management of STE-ACS.16–19
Follow-upAfter 48h of clinical and electrical stability, patients were referred to the Cardiology ward until the moment of hospital discharge, which was the possible follow-up time, due to the retrospective design of the study. During the first 24h hospitalized in this ward, telemetry was used so that the monitoring time of the electrical activity of the heart could be extended until reaching a general average of 78±11h. Right from the clinical histories, further ECG traits were recorded for analysis through paper or through the digital registration of the arrhythmic event since, at times, the latter could not be recorded on paper due to its short duration or to the priority of other more decisive medical actions.
This is how the presence of ventricular tachycardia (VT) or atrial fibrillation (AF) could be determined, thanks to the established ECG criteria,1,11,12 while other arrhythmias of scarce clinical repercussion like premature contractions were disregarded.
Statistical analysisThe database created in the statistical package SPSS v.17.0 for Windows was used. The normal and homogeneous distribution of the sample (p>0.05) was checked, so that parametric tests could be conducted.
The frequence distribution of the numerical variables studied was conducted, as well as average comparisons among independent and related samples. In order to check how strong the association among qualitative variables really was, the Pearson's nonparametric Chi-Square test was used and in situations when over 20 per cent of the anticipated frequencies showed values under 5, then the Fisher's exact test was used. In order to compare the average of quantitative variables, the statistical Student's t test for independent samples was used. The statistical validation of the results from this research was nearly 95 per cent (p<0.05) for the degrees of freedom previously set for every circumstance presented.
To assess the correlation between the associated continuous variables and the occurrence of complications, mortality, or both, and isolated mortality, its capacity of discrimination was estimated through the construction of ROC curves and the assessment of the area under the curve (AUC) (“c” index). Taking these results into consideration, one cut-off point was established for the continuous variables that would be included in the univariate analysis.
As a contribution relative to establishing the risk factors, one multivariate analysis was conducted using one binary logistic regression model that resulted in a dichotomic dependent variable, in the appearance of complications, and mortality, or both, and in the presence of isolated mortality. In the multivariate analysis, factors prone to predicting those aspects contained in the variables for which Wald's test showed odds <5 per cent (p<0.05) could be identified.
Once the statistically significant variables were determined, we proceeded to build the risk score by assigning one cut-off value of 1 point for every statistically significant variable. This score was applied to the study population to later build the ROC curves, and calculate the “c” index. The calibration of the model using the Hosmer–Lemeshow method shows the capacity to predict the appearance of complications and mortality. The area under the curve, and the confidence interval using ROC curves were established in order to determine the variable that was more closely associated with the appearance of arrhythmias in the studied sample.
EthicsThis research has been approved by the Hospital Ethics Committee and Scientific Council (registration number HCC2015/178), and no identifying data from patients has been published, however the patients’ confidentiality has been observed at any time while managing the data.
ResultsA total of 209 patients were studied—87 females (41.6 per cent) and 122 males (58.4 per cent), with average ages of 69.9±11.5 and 69.7±10.8 years old, respectively.
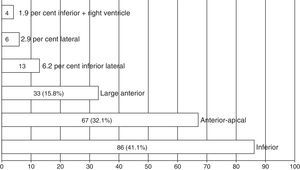
Fig. 1 shows the predominance of patients with STE-ACS of inferior (41.1 per cent) and anterior apical (32.1 per cent) topography.
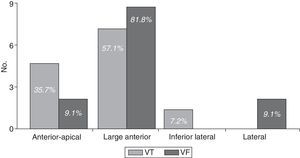
Arrythmias were diagnosed in 46 patients (22 per cent), and in 25 patients (15.9 per cent) they were ventricular arrhythmias, more common in the large anterior AMI (68.0 per cent); this location that was responsible for 81.8 per cent of all VFs and over half (57.1 per cent) of VTs (Fig. 2). No ventricular arrhythmias were diagnosed in patients with inferior isolated IAM or with right ventricle affectation.
When comparing several characteristics from the patients who had or did not have ventricular arrhythmias (Table 1) we saw that older age (p=0.013), the presence of diabetes mellitus (p=0.003), the large anterior AMI (p<0.001), the pump failure, clinical failure (Killip–Kimbal III–IV, p=0.008), or ECG failure (left ventricular ejection fractions—LVEF<0.40; p=0.004), not using any revascularization strategies (p<0.001), and longer QRS and QTc durations and dispersions, were associated, with statistically significant differences, with the appearance of VT and VF; on the other hand, the absence of ventricular dysfunction (Killip–Kimbal I–II [p=0.008], LVEF>0.50 [p=0.030]) and the appearance of thrombolysis (p=0.001) were strongly associated with a more favourable progression during the hospital stage of the AMI, with a lower incidence of VT or VF. On the other hand, the appearance of these arrhythmias was associated with mortality with a statistically significant difference (p<0.001).
Demographic, clinical, ECG, and electrocardiogram characteristics of the studied patients.
| Variables | Total (n=209) | VT/VF | p | |
|---|---|---|---|---|
| Yes (n=25) | No (n=184) | |||
| Demographic characteristics | ||||
| Sex masculine | 122 (58.4) | 13 (52.0) | 109 (59.2) | 0.64 |
| Age (years) | 69.9±11.1 | 74.6±11.4 | 68.8±10.8 | 0.013* |
| white coloured skin | 137 (65.6) | 16 (64) | 121 (65.8) | 0.96 |
| Risk factors | ||||
| Hypertension | 153 (73.2) | 17 (68) | 136 (73.9) | 0.70 |
| Diabetes mellitus | 67 (32.1) | 15 (60) | 52 (28.3) | 0.003* |
| Hyperlipidemia | 79 (37.8) | 8 (32) | 71 (38.6) | 0.68 |
| Obesity | 43 (20.6) | 5 (20) | 38 (20.6) | 0.85 |
| Smoking | 114 (54.5) | 13 (52) | 101 (54.9) | 0.95 |
| Topography of infarction | ||||
| Anterior apical | 67 (32.1) | 6 (24) | 61 (33.2) | 0.49 |
| Large anterior | 33 (15.8) | 17 (68) | 16 (8.7) | <0.001* |
| Inferior lateral | 13 (6.2) | 1 (4) | 12 (6.5) | 0.96 |
| Lateral | 6(2.9) | 1 (4) | 5 (2.7) | 0.78 |
| Other | 90 (43) | 0 (0) | 90 (48.9) | 0.001* |
| Killip–Kimbal class | ||||
| I–II | 141 (67.5) | 9 (36) | 132 (71.7) | 0.008* |
| III–IV | 68 (32.5) | 16 (64) | 52 (28.3) | 0.008* |
| LVEF | ||||
| >0.50 | 88 (42.1) | 5 (20) | 83 (45.1) | 0.030* |
| 0.50–0.40 | 59 (28.2) | 6 (24) | 53 (28.8) | 0.79 |
| <0.40 | 62 (29.7) | 14 (56) | 48 (26.1) | 0.004* |
| ECG variables | ||||
| QRS | 76.6±12.8 | 95.2±17.9 | 72.3±5.6 | <0.001* |
| dQRS | 23.2±15.5 | 42.1±24.5 | 18.9±7.8 | 0.001* |
| QTc | 461.8±68 | 511.4±72.7 | 450.5±73.9 | 0.001* |
| dQTc | 68.0±32 | 91.9±23.3 | 62.5±31.3 | <0.001* |
| Coronary reperfusion strategy | ||||
| Thrombolisis | 135 (64.6) | 7 (28) | 128 (69.6) | 0.001* |
| Percutaneous coronary intervention | 43 (20.6) | 4 (16) | 39 (21.2) | 0.73 |
| No | 31 (14.8) | 14 (56) | 17 (9.2) | <0.001* |
| Deceased | 26 (12.4) | 18 (72) | 8 (4.3) | <0.001* |
dQRS: QRS dispersion; dQTc: QTc dispersion; LVEF: left ventricular ejection fraction; VF: fventricular fibrillation; VT: ventricular tachycardia.
Data expressed number (%) or average±standard deviation.
Other clinical and lab variables shown in Table 2 (see additional material), where the largest values of glycemia and creatinine and the lowest values of systolic blood pressure, LVEF, and glomerular filtration can be seen had a statistically significant association (p<0.001) with the appearance of ventricular arrhythmias and mortality.
From the ECG point of view, the area of the AMI was the one that most commonly (183/209; 87.6 per cent) showed longer durations of the QRS in the studied patients (Table 3), while the measurements of QRS duration (77.3±13.3 vs. 71.5±6.4ms; p=0.029) and dispersion (24.1±16.2 vs. 16.5±4.8ms; p=0.019) were higher in those leads affected by ischaemia with respect to the rest of leads (Table 4).
Maximum measurement of QRS in regions affected by ischaemia based on the topography of infarction.
| Topography | Maximum QRS in affected region | Total (n=209) | |
|---|---|---|---|
| Yes (n=183) | No (n=26) | ||
| Anterior apical | 65 (35.5) | 2 (7.7) | 67 (32.1) |
| Large anterior | 30 (16.4) | 3 (11.5) | 33 (15.8) |
| Inferior+RV | 3 (1.6) | 1 (3.8) | 4 (1.9) |
| Inferior | 71 (38.8) | 15 (57.7) | 86 (41.1) |
| Inferior lateral | 10 (5.5) | 3 (11.5) | 13 (6.2) |
| Lateral | 4 (2.2) | 2 (7.7) | 6 (2.9) |
RV: right ventricle.
Chi Square Pearson test=12.148; p=0.033.
Values expressed numbers (%).
Duration and dispersion of QRS in the ECG leads with or without ischaemia.
| Variable | ECG leads | Average differences | 95 per cent CI | p | ||
|---|---|---|---|---|---|---|
| With ischaemia | Without ischaemia | Inferior | Superior | |||
| QRS, ms (average±SD) | 77.3±13.3 | 71.5±6.4 | 5.8 | 0.5 | 11.0 | 0.029 |
| dQRS, ms (average±SD) | 24.1±16.2 | 16.5±4.8 | 7.6 | 1.2 | 13.9 | 0.019 |
SD: standard deviation; dQRS: QRS dispersion; ECG: electrocardiogram; CI: confidence interval.
The highest average values on the ECG variables studied, with highly significant differences, were present in the large anterior AMI (Table 5 [see additional material]): QRS 92.3±18.8ms, dQRS 37.9±23.9ms, QTc 518.5±72.2ms, and dQTc 94.9±26.8ms. In the remaining topographical areas affected by ischaemia, the values were closer to normal. However, it was followed, in order of frequency, by the anterior apical AMI where the second widest QRS (75.7±11.5ms), the second longest QTc (476.9±69.6ms) and the third longest dQRS and dQTc were found.
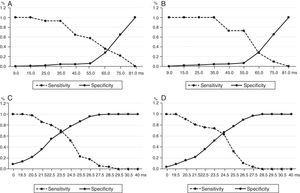
After analysing the area under the curve (AUC) (Table 6 and Fig. 3), the odds of any serious ventricular arrhythmic events occurring in the population studied was similar both for the dQRS and the dQTc (0.760 vs. 0.768), though both parameters showed statistically significant differences (p<0.001). However, when an independent analysis was conducted, there were more chances for these ventricular arrhythmias to occur in patients with higher values of dQRS with respect to dQTc: 0.942 vs. 0.660 for VT, and 0.966 vs. 0.852 for VF.
Areas under the curve and confidence intervals corresponding to the analysis of sensitivity and specificity.
| Type of arryhtmia | Variable | Area under the curve | 95 per cent CI | p | |
|---|---|---|---|---|---|
| Inferior | Superior | ||||
| Arrythmias | dQRS | 0.760 | 0.652 | 0.869 | <0.001 |
| dQTc | 0.768 | 0.703 | 0.834 | <0.001 | |
| VT | dQRS | 0.942 | 0.868 | 1.000 | <0.001 |
| dQTc | 0.660 | 0.560 | 0.760 | 0.046 | |
| VF | dQRS | 0.966 | 0.942 | 0.990 | <0.001 |
| dQTc | 0.852 | 0.777 | 0.927 | <0.001 | |
dQRS: QRS dispersion; dQTc: corrected QT dispersion; VF: ventricular fibrillation; CI: confidence interval; VT: ventricular tachycardia.
ROC curves to prognosticate the appearance of ventricular arrhythmias with the analysis of sensitivity and specificity of QRS and the QTc dispersions. (A) For any serious ventricular arrythmia. (B) Ventricular tachycardia. (C) Ventricular fibrillation. AUC: area under the curve; dQRS: QRS dispersion; dQTc: corrected QT dispersion; CI: confidence interval.
In order to establish one cut-off point that would allow the identification of patients with higher risk of VT and VF, we used independent ROC curves for each variable analyzed and found that a dQTc≥65ms was equally predicting of VT and VF (Fig. 4A and B); while in the dQRS, the cut-off value was 23.5ms for VT, with 69 and 67 per cent sensitivity and specificity, respectively (Fig. 4C); and 24.5ms for VF (Fig. 4D), with similar sensitivity and specificity.
DiscussionThe main findings of this research have been: a) that there were more arrhythmias in patients with larger ischaemic myocardial areas, b) that the duration and dispersion of the QRS were significantly higher in the AMI-associated leads, and c) that the dQRS was the ECG variable more closely associated with the appearance of VT and VF.
Several authors have explained the physiopathology of ECG alterations in acute ischaemia,6,20 that are responsible for the depolarization disorders and ventricular repolarizations detected by the aforementioned ECG measurements that explain the presence of ventricular arrhythmias during the occurrence of an AMI.21–26
Both the extension of the QTc interval and its dispersion have an elevated prognostic value in the early stages of the acute ischaemic process,27 while longer activation delays have been confirmed in dyskinetic or akinetic areas of ischaemic patients.28–30
These activation and conduction delays associated with the presence of wider QRS in the area of acute ischaemia were evident in our results. As a matter of fact, statistically significant longer durations (width) and dispersions of the QRS were found in ECG leads with ST segment supra-slope in relation to the rest of leads that explored non-ischaemic myocardial regions. Therefore, these delays of conduction in the affected regions extend the QRS proportionally to the extension of the ischaemic territory, which explains why higher values of QRS were found in the ischaemic areas of patients with large anterior AMIs followed, in order of frequency, by anterior apical AMIs, and inferior lateral AMIs.
On the other hand, since there is a sufficient local cause to extend the QRS, the dQRS affecting the electrophysiological properties of the myocardium as a whole will be more evident, and thus facilitate the appearance of potentially lethal ventricular arrhythmias. Thus, we have found the existence of a highly and more evident significant association between the dQRS and ventricular arrhythmias (with cut-off values of 23.5ms for VT and 24.5ms for VF) compared to the dQTc. This in no way negates the risk predicting capabilities of the dQTc (where we found a cut-off point of 65ms for both types of ventricular arrhythmias), since the QRS is part of this interval and the longer the QRS, the greater the QT; however, when comparing both variable independently, our results show that the dQRS was more useful for the prediction of this type of arrhythmias. This result can be the response to the QT interval being the ECG representation of the whole ventricular depolarization and repolarization process, and the QRS being the representation of the depolarization process only.31
The fact that there are more electrical complications in patients with larger ischaemic myocardial areas and the fact that the extended or dispersed QTc is a predictor of risk is nothing new; that has been perfectly proven by multiple researches5,6,12; however, the dQRS had never been studied in this context.
The dQRS was studied for the first time in the year 2000 by Anastasiou-Nana et al.7 who associated it with sudden death in patients with advanced congestive heart failure. In 2004, Yamada et al.8 considered it a powerful prognostic marker of mortality in patients with mild to moderate congestive heart failure, and that same year, Ma et al.21 define it as the most powerful independent predictor of cardiac sudden death in patients with left ventricular arrythmogenic dysplasia. Also, Chávez González et al.32 use this variable for the first time to assess the response to cardiac resynchronization.
It is important to say that no other study conducted so far has associated the duration and dispersion of the QRS with the odds of developing ventricular arrhythmias during the occurrence of an AMI. The only ones to study its duration were Kirchhof et al.31 but their results were contradictory because, paradoxically, they concluded that the increased duration of the QRS was followed by an increased dispersion of the QRS, but that this event was not related whatsoever with the presence of arrhythmic events in AMI survivors. Nevertheless, Sheng et al.33 confirmed that the presence of QRS fragmentation during the occurrence of an AMI is associated with a higher risk of developing malignant ventricular arrhythmias in the short term; and on the other hand, Bayés de Luna and Elosua34 claim that the QRS width is one of the ECG signs associated with sudden death during the occurrence of an acute myocardial infarction, while Hetland et al.6 confirmed that patients with ventricular arrhythmias 40 days after the occurrence of the AMI showed wider QRS than the group without arrhythmias (114±26 vs. 104±20ms, p=0.05).
This study shows that the odds of ventricular arrhythmias during the occurrence of an AMI are much higher when the duration and dispersion of the QRS are higher rather than when duration and dispersion of the QTc are higher; this is why it has been suggested that we are two (2) new variables capable of predicting the risk of developing these arrhythmias during the early stages of an AMI.
Limitations of the studyThe small size of the sample and the subsequent small number of patients with ventricular arrhythmias is the main limitation of this study.
ConclusionsThe duration and dispersion of the QRS were higher in the ECG leads showing acute ischaemias. The increase of these variables rather than the increase of QTc intervals and QTc dispersion proved there were greater odds of occurrence of ventricular arrhythmias in the early stages of an AMI. The risk of VT is higher starting at a dQRS of 23.5ms; while the risk of VF increases after 24.5ms.
Conflicts of interestsWe the authors declare that while conducting this paper there were no conflicts of interests linked whatsoever.
Please cite this article as: Chávez-González E, Rodríguez Jiménez AE, Moreno-Martínez FL. Duración y dispersión del QRS para predecir arritmias ventriculares en las fases iniciales del infarto agudo de miocardio. Med Intensiva. 2017;41:347–355.