Encephalitis is a potentially fatal inflammatory disorder of the central nervous system (CNS). Although the diagnosis is complex, early identification of the disorder is important, since it can condition the prognosis. In our setting, most cases of encephalitis are of viral origin, though a number of autoimmune presentations may also be observed1 that should be taken into account in order to start early treatment.
We present the case of a 40-year-old male with no disease history of relevance who reported to the emergency room repeatedly because of 15–20 days of varying neurological manifestations in the form of headache, instability or confusion starting 24h after a disabling acute headache episode. The brain CAT study revealed no alterations. In view of the variability of the clinical manifestations and the personal conflicts of the patient, the condition was taken to represent a dissociative disorder, and neuroleptic and antidepressive treatment was started. However, the patient continued to suffer disconnection from the environment and disorientation, heteroaggressive behavior, and oppositionist and behavioral disorders. The patient was admitted to the psychiatric ward with akinetic mutism. The neurological exploration revealed no alterations (though the patient was totally uncooperative), and the laboratory test results (including serological testing for syphilis and HIV infection) proved normal. On the third day his condition worsened, with no response to stimuli, the appearance of flaccid tetraparesis and a left positive Babinski reflex.
On day four the patient developed high fever, respiratory sounds suggestive of bronchoaspiration, and finally tonic-clonic seizures. Admission to our Intensive Care Unit (ICU) was thus decided. The patient was admitted under postcritical condition, with fever (38.6°C) and SatO2 75% with oxygen therapy (reservoir); intubation was decided, and mechanical ventilation was started. There were few crepitants in the right lung base, and the rest of the exploration yielded no findings of relevance. The routine laboratory test parameters were normal, with the exception of CPK 1800U/l, and the chest X-rays showed a weak right basal infiltrate. Repeat CAT showed no alterations, and lumbar puncture yielded clear cerebrospinal fluid (CSF), emerging without pressure, containing 13 cells (with 92% monocytes), no glucose depletion, and a protein concentration of 189.7mg/dl. Aciclovir and broad spectrum antibiotic treatment were prescribed.
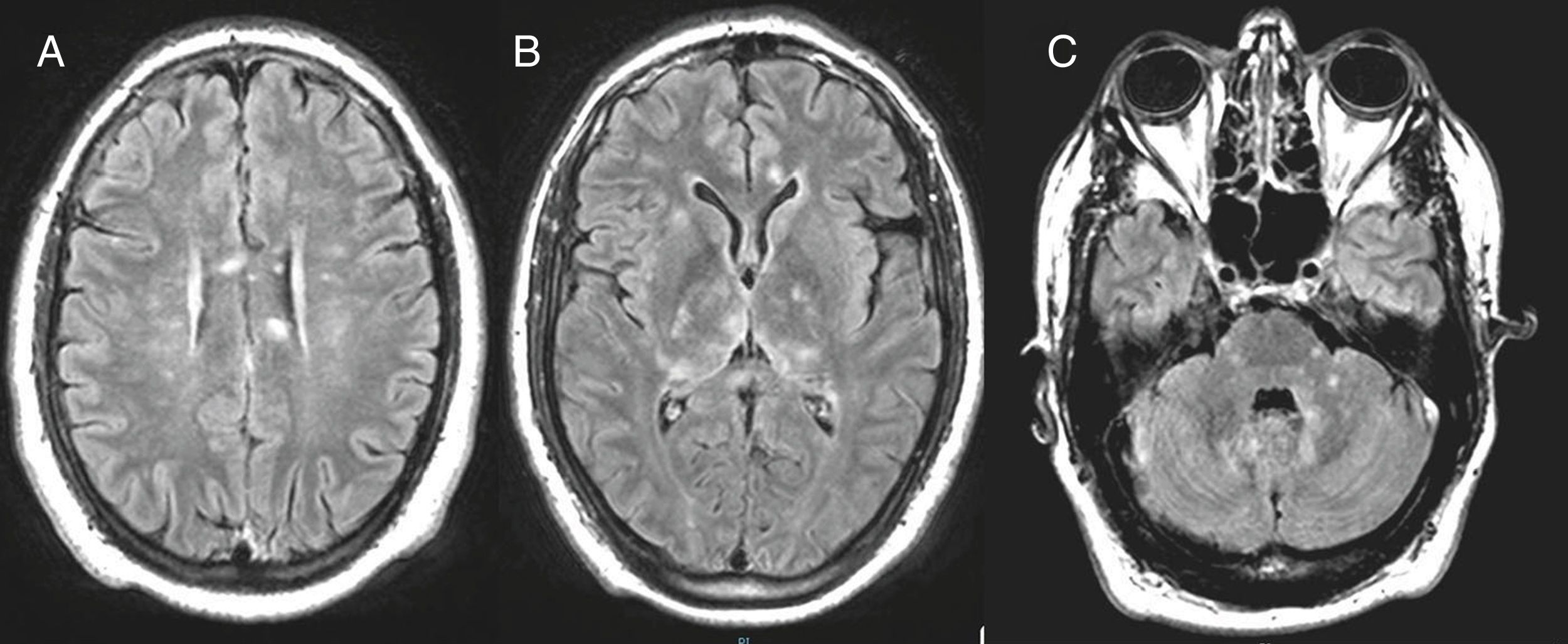
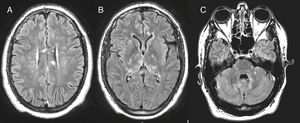
The hemodynamic and respiratory alterations quickly reverted; the blood and CSF cultures proved negative; and the usual flora was identified in the tracheal aspirate. Magnetic resonance imaging (MRI) revealed numerous foci with enhanced signal intensity in T2-FLAIR images. The lesions were of small size, tended to merge, and presented a cotton-like appearance; they were mostly restricted in size in diffusion sequencing and did not increase in intensity with contrast administration. The lesions were located bilaterally in the basal ganglia, corpus callosum (body and splenium), the medial regions of both thalami, the deep periventricular white mater, the frontal subcortical zone, the cortico-subcortical region at right parasylvian level and the left anteromedial temporal region – with involvement of the cerebellar peduncles and right hemi-mesencephalon (Fig. 1).
The epidemiological context required us to discard encephalitis caused by west Nile virus (the patient worked in an area with a strong presence of mosquitoes and where this virus had already been isolated2), with a similar MRI pattern, and encephalitis due to zika virus (recently described)3 (his partner had returned from Cuba where an epidemic outbreak had been declared at the time). Lumbar puncture was repeated, with negative PCR test results for the mentioned virus as well as for more common varicella-zoster virus and enterovirus, and we requested CSF and serum testing for onconeural antibodies, anti-Ma (Ma1, Ma2/Ta), anti-NMDA, anti-LGI1, anti-voltage gated potassium channel antibodies and anti-GAG. All proved negative.
A guided interview revealed that the patient (a musician) had suffered marked hearing loss in the left ear as well as vision problems, since his partner noticed that he failed to see the signals she usually showed him on stage. The triad of encephalitis, hearing impairment and loss of visual acuity secondary to retinal arterial obstruction, together with the MRI findings, were indicative of Susac's syndrome (SS)4 – a form of autoimmune encephalitis. Evaluation of the ocular fundus confirmed the diagnosis, with the right eye presenting sectorial macular edema and a pale retina in the area of the inferior cilioretinal artery suggestive of arterial obstruction. The left eye in turn presented a small ischemic area in the region of the inferior temporal arch with sectorial vasculitis but no involvement of the foveal region.
Corticosteroid pulses were quickly prescribed (1g/day for 3 days), followed by 100mg/day, and immunoglobulin (2g/kg in 5 days). The patient started to awaken after 72h, being able to follow simple instructions, and extubation was decided on day 6 of admission. He was finally discharged to the ward awake, though with slowed mental processing, disorientation in time and space, flaccid tetraparesis and a bilateral positive Babinski reflex. Audiometry diagnosed hearing deficiency referred to acute tones in the left ear. Clinical progression in the ward was interrupted by another psychotic outbreak coinciding with a lowering of the corticosteroid dose. Ten months after the episode, the patient has fully recovered, except for mild frontal symptoms.
In recent years there has been an increase in reported encephalitis of non-infectious origin, including cases published in our setting.5 Most of these cases have been of autoimmune origin, associated to the presence of antineuronal antibodies, with a clinical picture that may simulate an infectious origin or manifest as neurological and/or psychiatric disorders, sometimes without fever or CSF pleocytosis. This group of disorders comprises limbic encephalitis, anti-NMDA receptor encephalitis, Bickerstaff encephalitis and disseminated acute encephalitis – including SS. The latter is a rare disease (with about 350 cases described to date), involving vasculitic alterations of the brain, retina and cochlea. The condition characteristically starts with psychiatric symptoms, and admission to the psychiatric ward (as in our patient) is common.4,6,7 The diagnostic criteria of SS have been established recently.8 The syndrome is three-fold more common in women than in men, and the clinical triad is observed in only 13% of the cases (more often in males). We consider that our case illustrates the importance of the differential diagnosis with viral encephalitis (our case moreover involving two unusual viruses) and the fundamental role played by early MRI study, which in addition to discarding a herpetic origin of the disorder allows the differentiation of disease patterns characterizing each type of autoimmune encephalitis.1 Involvement of the corpus callosum, for example, is considered to be pathognomonic of SS.9 An early diagnosis allows the prompt start of treatment, which is associated to an improve patient response10 with fewer sequelae in the form of persistent cognitive impairment.
Please cite this article as: Mora López D, Tristancho Garzon A, Guzmam Llorente M, Jiménez Conde C, Montero Urbina A, Oliva Fernandez P. Síndrome de Susac. A propósito de las encefalitis autoinmunes. Med Intensiva. 2019;43:182–184.