A comparison was made between invasive mechanical ventilation (IMV) and noninvasive positive pressure ventilation (NPPV) in hematological patients with acute respiratory failure.
DesignA retrospective observational study was made from 2001 to December 2011.
SettingA clinical–surgical intensive care unit (ICU) in a tertiary hospital.
PatientsPatients with hematological malignancies suffering acute respiratory failure (ARF) and requiring mechanical ventilation in the form of either IMV or NPPV.
Variables of interestAnalysis of infection and organ failure rates, duration of mechanical ventilation and ICU and hospital stays, as well as ICU, hospital and mortality after 90 days. The same variables were analyzed in the comparison between NPPV success and failure.
ResultsForty-one patients were included, of which 35 required IMV and 6 NPPV. ICU mortality was higher in the IMV group (100% vs 37% in NPPV, p=.006). The intubation rate in NPPV was 40%. Compared with successful NPPV, failure in the NPPV group involved more complications, a longer duration of mechanical ventilation and ICU stay, and greater ICU and hospital mortality. Multivariate analysis of mortality in the NPPV group identified NPPV failure (OR 13 [95% CI 1.33–77.96], p=.008) and progression to acute respiratory distress syndrome (OR 10 [95% CI 1.95–89.22], p=.03) as prognostic factors.
ConclusionThe use of NPPV reduced mortality compared with IMV. NPPV failure was associated with more complications.
Comparación de la ventilación mecánica invasiva (VMI) frente a la no invasiva (VMNI) en pacientes hematológicos que desarrollaron insuficiencia respiratoria aguda (IRA).
DiseñoEstudio observacional retrospectivo desde 2001 y hasta diciembre de 2011.
ÁmbitoUnidad de cuidados intensivos (UCI) médico-quirúrgica de un hospital de tercer nivel.
PacientesAquellos con patología hematológica con IRA y que precisaron ventilación mecánica (VM), tanto VMI como VMNI.
Variables de interésNúmero de infeccciones y fracaso de órganos, duración de VM y estancias en la unidad y hospitalaria, así como mortalidad en UCI, hospitalaria y a los 90 días. En el subgrupo de VMNI se comparó éxito y fracaso en cuanto a las variables mencionadas.
ResultadosSe incluyeron 41 pacientes que precisaron VM, 35 con VMNI y 6 con VMI. La mortalidad en UCI fue superior en VMI (100 vs 37% en VMNI, p=0,006). El porcentaje de intubación en VMNI fue del 40%. El grupo fracaso de VMNI presentó mayor tasa de complicaciones, mayor duración de la VM, mayor estancia en UCI, así como de mortalidad en UCI y hospitalaria que el grupo que no precisó intubación. El análisis multivariante de mortalidad en el grupo VMNI estaba relacionada con el fracaso de la VMNI (OR 13 [IC 95% 1,33–77,96], p=0,008) y el desarrollo de síndrome de distrés respiratorio del adulto (OR 10 [IC 95% 1,95–89,22], p=0,03).
ConclusiónLa utilización de VMNI redujo la mortalidad en comparación con la VMI. El fracaso de la VMNI llevó aparejada una mayor tasa de complicaciones.
The prognosis of malignant hematological disease has improved over the last few decades,1,2 though patients requiring admission to the intensive care unit (ICU) continue to present a high mortality rate.1–5 The main cause of admission to the ICU is the development of acute respiratory failure (ARF), sometimes requiring ventilatory support,1–7 due to the appearance of both infectious and non-infectious pulmonary infiltrates.8 On the other hand, mechanical ventilation (MV), and particularly invasive mechanical ventilation (IMV), has been shown to be one of the risk factors of mortality in the ICU.1,2
The development of noninvasive positive pressure ventilation (NPPV) as a ventilatory support technique has represented a great step forward in the ventilatory care of critically ill patients. NPPV offers a series of benefits,9,10 since it lessens the need for orotracheal intubation thanks to resting of the muscle burden generated by the respiratory disease underlying ARF; improves oxygenation; and facilitates the elimination of carbon dioxide. As a direct consequence of this, NPPV is able to reduce the incidence of ventilator associated pneumonia (VAP) and shorten patient stay in the ICU and in hospital, especially in immune depressed individuals. Nevertheless, despite these reported benefits and the existence of studies supporting the success of the technique,6,10–13 NPPV is still less widely used than expected, and IMV remains the gold standard for the ventilation support of these patients.5
The present study analyzes the incidence of ARF in hematological patients admitted to the ICU and requiring mechanical ventilation (invasive and noninvasive), with the purpose of determining whether NPPV is superior to IMV in terms of the development of infections, organ failure, the duration of mechanical ventilation, the duration of stay in the ICU and in hospital, and mortality in the ICU, in hospital, and after 90 days. Likewise, an analysis was made of the incidence of NPPV failure, comparing the same variables between the success and failure groups, and of the factors related to mortality in the NPPV group.
Materials and methodsA retrospective observational was carried out, following approval by the hospital Clinical Research Ethics Committee. We included all hematological patients with ARF admitted to the ICU between January 2001 and December 2011, and who required ventilatory support. The hematological diseases mainly comprised acute leukemia (lymphoblastic or myelocytic), non-Hodgkin lymphoma, multiple myeloma, chronic leukemia and Hodgkin's disease. The patients had received chemotherapy, corticosteroid therapy or treatment in the form of hematopoietic precursor cell transplantation. Neutropenia was defined as a leukocyte count of under 1000cells/mm3.
Acute respiratory failure was defined as a respiratory frequency (RF) of >30rpm, a partial oxygen pressure (PaO2) of <60mmHg or a partial carbon dioxide pressure (PaCO2) of >45mmHg. Community-acquired pneumonia (CAP) was considered as a lower airway infection characterized by opacifications on the chest X-rays, signs and symptoms of respiratory infection such as fever, cough, pleuritic pain, leukocytosis or leukopenia, and the presence or absence of secretions.14 Hypoxemic conditions were classified as acute lung injury (ALI) or adult respiratory distress syndrome (ARDS), based on the following criteria15: bilateral infiltration, pulmonary wedge pressure<18mmHg, PaO2/FiO2<300 (ALI) or PaO2/FiO2<200 (ARDS). For the microbiological study we determined soluble antigens in urine, and peripheral blood samples were obtained for blood culture and pneumonia serological testing. Lastly, where possible, we obtained sputum for culture and gram staining. Nasopharyngeal aspiration in turn was performed for determination of the new H1N1 influenza virus – this test being carried out on a routine basis since 2009 in all cases of pneumonia exhibiting an interstitial radiological pattern. Non-bronchoscopic invasive samples (bronchoalveolar lavage and bronchial aspirate), were collected once the patients were intubated. The criteria for sepsis and septic shock were established according to the literature.16
Monitorization and study variablesUpon patient admission, invasive hemodynamic monitorization was carried out, with arterial catheterization and a central venous line. Respiratory monitorization in turn was carried out by recording transcutaneous oxygen saturation (SatcO2) with an Oxisensor Nellcor II D-25 pulsioxymeter (Nellcor® Puritan Bennet Inc., Decasanton, CA, USA), and arterial blood samples were obtained for blood gas determinations using an ABL560 cooxymeter (Radiometer Medical A/S®, Copenhagen, Denmark).
Upon patient admission and during the stay in the ICU, we collected personal data and information referred to the diagnosis, severity based on the Simplified Acute Physiology Score (SAPS) 2 and organ failure based on the Sequential Organ Failure Assessment (SOFA). We also recorded the corresponding hemodynamic, respiratory, blood gas and biochemical variables. The duration of stay in the ICU and in hospital was registered, along with the duration of mechanical ventilation. In turn, the complications occurring during stay in the Unit were documented, such as orotracheal intubation, barotrauma, nosocomial infections, the need for tracheotomy and mortality (in the ICU, in hospital, and 90 days after admission). The organ dysfunction rate was assessed based on the Marshall scale,17 which contemplates acute renal failure (with or without hemofiltration) and cardiovascular, hematological, neurological and hepatic failure.
Noninvasive ventilatory supportUse was made of the BiPAP Vision respirator9 (Respironics Inc.®, PA, USA) connected to an orofacial or Total face® mask (Respironics Inc.®, PA, USA) with an MR850 active humidification system (Fischer and Payckel Healthcare Ltd., New Zealand). After explaining the technique to the patient, the mask was fitted and we progressively increased the positive end-expiratory pressure (PEEP) and the support pressure to above the PEEP (SP), until achieving a tidal volume (Vt) of 10–15ml/kg and a RF of 25–28rpm, thereby ensuring a minimum SP of 10–15cmH2O and a PEEP of 5–6cmH2O in the first hour of ventilatory support. The oxygen concentration was adjusted until reaching SatcO2 >94%. Once the clinical and/or blood gas condition of the patient improved, gradual ventilator withdrawal was carried out until complete disconnection of NPPV. The changes in FiO2 and SP/PEEP levels were made according to the criterion of the supervising physician. NPPV failure was considered in the presence of any of the following criteria9: persistence of respiratory effort or hypoxemia, cognitive impairment, or asynchrony with the respirator.
Invasive ventilatory supportPatient sedation was carried out with midazolam or propofol associated to morphine, followed by orotracheal intubation and connection to the respirator. Initial parameters: volume control/assist ventilation (CMV/a), Vt: 6–8ml/kg, flow 60l/min, RF: 12–14rpm, FiO2 to achieve SatcO2 94–96%, and minimum PEEP 5cmH2O. Progression of the respiratory process to ARDS required modification of the ventilatory parameters18: Vt<6ml/kg, plateau pressure<35cmH2O, progressive PEEP and FiO2 as low as possible with the aim of achieving SactO2>94%. After recovery, weaning was started, followed by extubation with the spontaneous breathing test. The modifications of the ventilatory parameters and weaning were carried out by the supervising physician. The patient was considered to have passed the breathing test if there was no hemodynamic or respiratory worsening during 2h. In such cases extubation was considered indicated, always conditioned to medical criterion.
The duration of mechanical ventilation included the time (in days) of mechanical ventilation and the weaning time. Hospital stay in turn was documented as the total stay of the patient in the ICU and in hospital. The application of IMV or NPPV, along with the rest of supportive measures (vasoactive drugs, antibiotherapy, renal replacement therapy, blood product transfusion, nutritional support), was regarded as the responsibility of the attending physician at the time of admission, in collaboration with the Department of Hematology.
Given the characteristics of the study, informed consent from the patient and/or family was not considered necessary.
Statistical analysisThe SPSS version 18.0 statistical package was used for analysis of the results. Quantitative variables were analyzed using parametric (Student t-test) or nonparametric tests (Mann–Whitney U-test), according to the results of the Kolmogorov–Smirnov test for the assessment of normal distributions. Qualitative variables in turn were analyzed using the chi-squared test, with the Fisher exact test (2-tailed) when the number of cases was under 5. Statistical significance was considered for p<0.05. Multivariate analysis was performed based on a logistic regression model to identify factors related to mortality in the NPPV group. We decided not to include the IMV group in the analysis, in order to avoid bias resulting from the inclusion of a group of patients who upon admission showed significant differences with respect to the NPPV group. The variables were included in the model using the enter method with a cutoff point of 0.1. The predictive capacity of the model was established from the Hosmer–Lemeshow test, the positive predictive value, the negative predictive value, diagnostic accuracy, and analysis of the area under the receiver operating characteristic (ROC) curve.
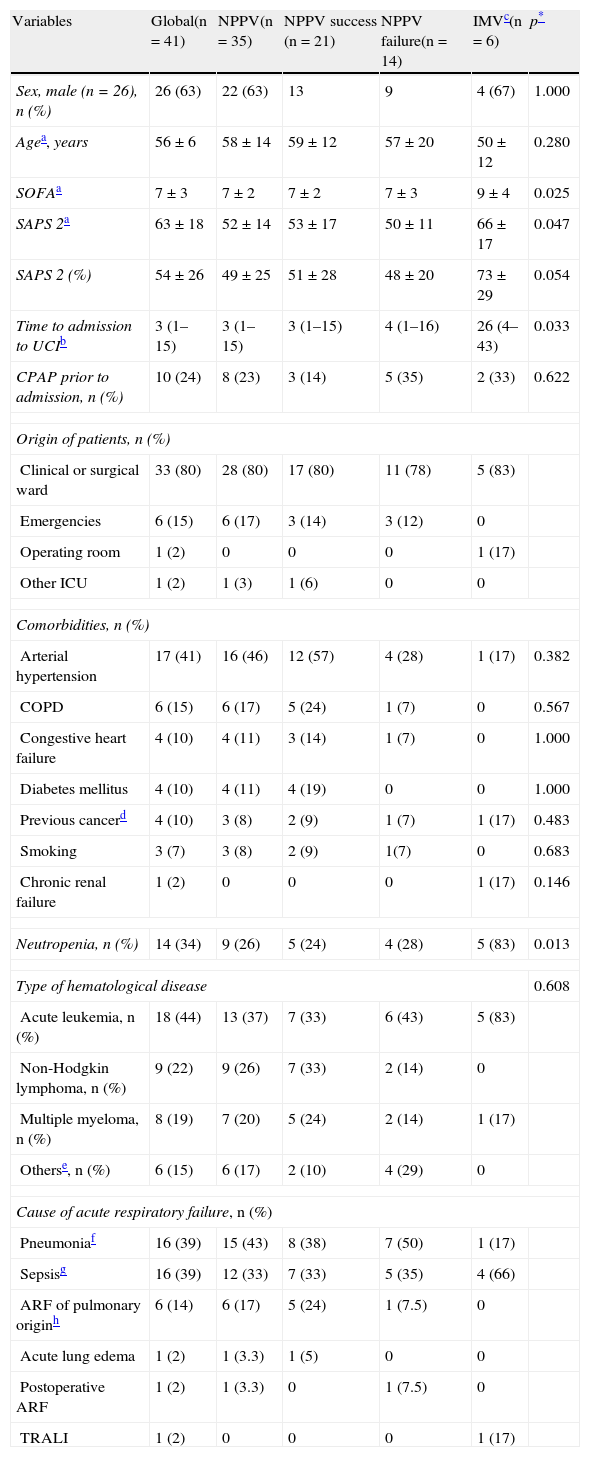
ResultsDuring the study period, a total of 132 patients with hematological disease were admitted to the ICU, out of a total of 11,501 hospitalized patients. Of the mentioned 132 patients, 41 required ventilatory support (31%): NPPV in 35 cases (85%) and IMV in 6 (15%). The patient sample (Table 1) consisted mainly of males (n=26; 63%), with a mean age of 56±6 years, a SOFA score of 7±3, and a SAPS 2 score of 63±18. Comparison of the two groups showed that upon admission, the IMV group had a greater incidence of organ failure as assessed by the SOFA (9±4 vs 7±2 in NPPV, p=0.025), and a poorer prognosis as determined by the SAPS 2 (66±17 vs 52±14 in NPPV, p=0.047). In contrast, the time to admission to the ICU was shorter in the NPPV group (3 [1–15] vs 26 [4–43] in IMV, p=0.033). Most of the patients came from hospital wards, where in some cases continuous positive airway pressure (CPAP) had been applied as a step prior to admission to the ICU. The most frequent comorbid condition in both groups was arterial hypertension, the predominant disease was acute leukemia, and the cause of ARF was pneumonia, without significant differences. The percentage of neutropenia was greater in the IMV group (83% vs 26% in NPPV, p=0.018). Comparison of the physiological parameters at baseline showed the IMV group to have more metabolic acidosis secondary to base excess (−9±7 vs −2±6 in NPPV, p=0.013), thrombopenia (14,500 [11,000–21,000] vs 96,000 [29,000–239,000] in NPPV, p=0.001), leukopenia (35 [20–100] vs 7210 [670–14,445] in NPPV, p=0.003), coagulopathy as determined by the Quick index (51 [21–65] vs 70 [52–81] in NPPV, p=0.05) and liver failure as estimated by bilirubin concentration (5 [3.12–5.00] vs 1 [0.79–1.55] in NPPV, p=0.022).
Demographic characteristics, comorbidities and cause of acute respiratory failure.
| Variables | Global(n=41) | NPPV(n=35) | NPPV success (n=21) | NPPV failure(n=14) | IMVc(n=6) | p* |
| Sex, male (n=26), n (%) | 26 (63) | 22 (63) | 13 | 9 | 4 (67) | 1.000 |
| Agea, years | 56±6 | 58±14 | 59±12 | 57±20 | 50±12 | 0.280 |
| SOFAa | 7±3 | 7±2 | 7±2 | 7±3 | 9±4 | 0.025 |
| SAPS 2a | 63±18 | 52±14 | 53±17 | 50±11 | 66±17 | 0.047 |
| SAPS 2 (%) | 54±26 | 49±25 | 51±28 | 48±20 | 73±29 | 0.054 |
| Time to admission to UCIb | 3 (1–15) | 3 (1–15) | 3 (1–15) | 4 (1–16) | 26 (4–43) | 0.033 |
| CPAP prior to admission, n (%) | 10 (24) | 8 (23) | 3 (14) | 5 (35) | 2 (33) | 0.622 |
| Origin of patients, n (%) | ||||||
| Clinical or surgical ward | 33 (80) | 28 (80) | 17 (80) | 11 (78) | 5 (83) | |
| Emergencies | 6 (15) | 6 (17) | 3 (14) | 3 (12) | 0 | |
| Operating room | 1 (2) | 0 | 0 | 0 | 1 (17) | |
| Other ICU | 1 (2) | 1 (3) | 1 (6) | 0 | 0 | |
| Comorbidities, n (%) | ||||||
| Arterial hypertension | 17 (41) | 16 (46) | 12 (57) | 4 (28) | 1 (17) | 0.382 |
| COPD | 6 (15) | 6 (17) | 5 (24) | 1 (7) | 0 | 0.567 |
| Congestive heart failure | 4 (10) | 4 (11) | 3 (14) | 1 (7) | 0 | 1.000 |
| Diabetes mellitus | 4 (10) | 4 (11) | 4 (19) | 0 | 0 | 1.000 |
| Previous cancerd | 4 (10) | 3 (8) | 2 (9) | 1 (7) | 1 (17) | 0.483 |
| Smoking | 3 (7) | 3 (8) | 2 (9) | 1(7) | 0 | 0.683 |
| Chronic renal failure | 1 (2) | 0 | 0 | 0 | 1 (17) | 0.146 |
| Neutropenia, n (%) | 14 (34) | 9 (26) | 5 (24) | 4 (28) | 5 (83) | 0.013 |
| Type of hematological disease | 0.608 | |||||
| Acute leukemia, n (%) | 18 (44) | 13 (37) | 7 (33) | 6 (43) | 5 (83) | |
| Non-Hodgkin lymphoma, n (%) | 9 (22) | 9 (26) | 7 (33) | 2 (14) | 0 | |
| Multiple myeloma, n (%) | 8 (19) | 7 (20) | 5 (24) | 2 (14) | 1 (17) | |
| Otherse, n (%) | 6 (15) | 6 (17) | 2 (10) | 4 (29) | 0 | |
| Cause of acute respiratory failure, n (%) | ||||||
| Pneumoniaf | 16 (39) | 15 (43) | 8 (38) | 7 (50) | 1 (17) | |
| Sepsisg | 16 (39) | 12 (33) | 7 (33) | 5 (35) | 4 (66) | |
| ARF of pulmonary originh | 6 (14) | 6 (17) | 5 (24) | 1 (7.5) | 0 | |
| Acute lung edema | 1 (2) | 1 (3.3) | 1 (5) | 0 | 0 | |
| Postoperative ARF | 1 (2) | 1 (3.3) | 0 | 1 (7.5) | 0 | |
| TRALI | 1 (2) | 0 | 0 | 0 | 1 (17) | |
CPAP, continuous positive airway pressure; COPD, chronic obstructive pulmonary disease; ARF, acute respiratory failure; SAPS 2, Simplified Acute Physiology Score (range 0–56); SOFA, Sequential Organ Failure Assessment; TRALI, transfusion-related acute lung injury; ICU, Intensive Care Unit; IMV, invasive mechanical ventilation; NPPV, noninvasive positive pressure ventilation.
Causes of intubation: urgent surgery (2 cases), urgent intubation in ward due to respiratory fatigue (2 cases), severe hypoxemia (1 case) and diminished consciousness (1 case).
Bone marrow aplasia (1 case), hyaline-vascular type Castleman's disease (1 case), chronic leukemia (2 cases), Hodgkin's disease (2 cases).
Causes of sepsis: bacteremia (5 cases in NPPV and 1 case in IMV), unknown focus (4 cases in NPPV), urological (1 case in NPPV), infections from blood products (1 case in NPPV), soft tissues (2 cases in IMV), ear, nose and throat (1 case in IMV), abdominal (1 case in NPPV).
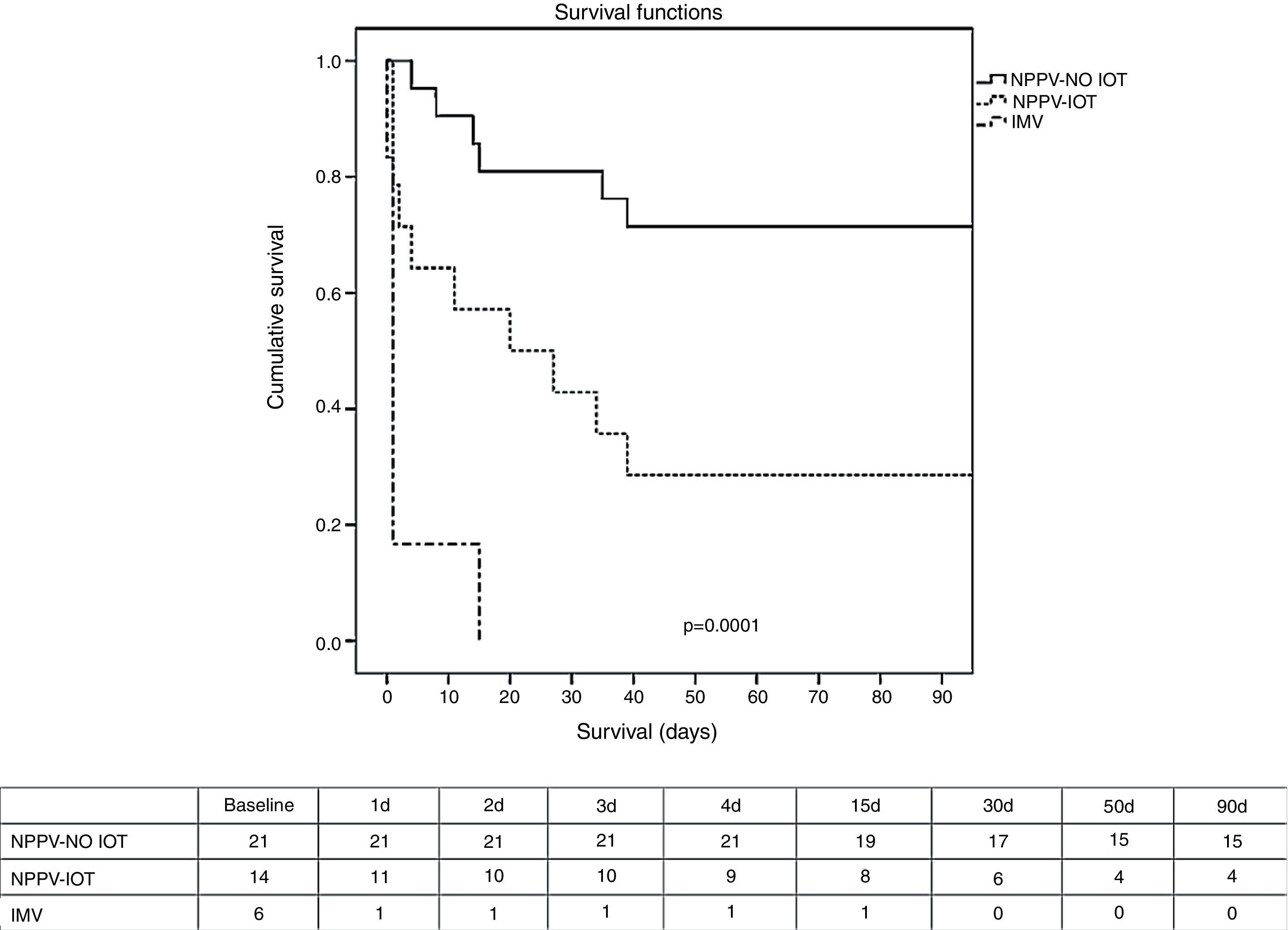
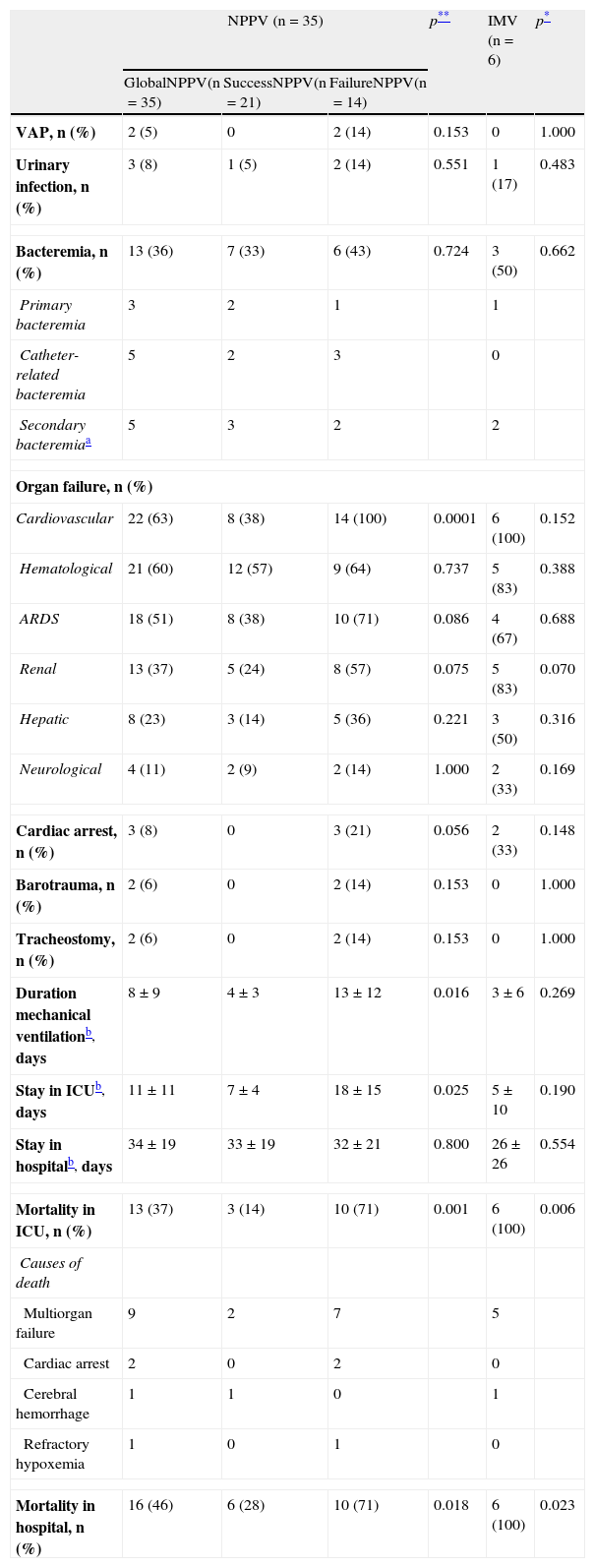
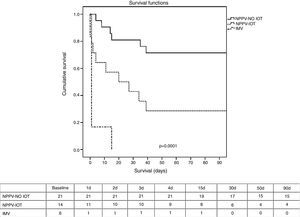
Regarding the clinical course (Table 2), there were no significant differences between IMV and NPPV regarding the percentage of infections, organ failure rate, duration of mechanical ventilation, or stay in the ICU and in hospital. The mortality rate both in the ICU and in hospital (Table 2 and Fig. 1) was significantly higher in the IMV group (100% vs 37% in NPPV, p=0.006, in the ICU; and 100% vs 46% in NPPV, p=0.023, in hospital).
Comparison of complication rates, duration of ventilation and duration of stay between NPPV (both success and failure) and IMV.
| NPPV (n=35) | p** | IMV (n=6) | p* | |||
| GlobalNPPV(n=35) | SuccessNPPV(n=21) | FailureNPPV(n=14) | ||||
| VAP, n (%) | 2 (5) | 0 | 2 (14) | 0.153 | 0 | 1.000 |
| Urinary infection, n (%) | 3 (8) | 1 (5) | 2 (14) | 0.551 | 1 (17) | 0.483 |
| Bacteremia, n (%) | 13 (36) | 7 (33) | 6 (43) | 0.724 | 3 (50) | 0.662 |
| Primary bacteremia | 3 | 2 | 1 | 1 | ||
| Catheter-related bacteremia | 5 | 2 | 3 | 0 | ||
| Secondary bacteremiaa | 5 | 3 | 2 | 2 | ||
| Organ failure, n (%) | ||||||
| Cardiovascular | 22 (63) | 8 (38) | 14 (100) | 0.0001 | 6 (100) | 0.152 |
| Hematological | 21 (60) | 12 (57) | 9 (64) | 0.737 | 5 (83) | 0.388 |
| ARDS | 18 (51) | 8 (38) | 10 (71) | 0.086 | 4 (67) | 0.688 |
| Renal | 13 (37) | 5 (24) | 8 (57) | 0.075 | 5 (83) | 0.070 |
| Hepatic | 8 (23) | 3 (14) | 5 (36) | 0.221 | 3 (50) | 0.316 |
| Neurological | 4 (11) | 2 (9) | 2 (14) | 1.000 | 2 (33) | 0.169 |
| Cardiac arrest, n (%) | 3 (8) | 0 | 3 (21) | 0.056 | 2 (33) | 0.148 |
| Barotrauma, n (%) | 2 (6) | 0 | 2 (14) | 0.153 | 0 | 1.000 |
| Tracheostomy, n (%) | 2 (6) | 0 | 2 (14) | 0.153 | 0 | 1.000 |
| Duration mechanical ventilationb, days | 8±9 | 4±3 | 13±12 | 0.016 | 3±6 | 0.269 |
| Stay in ICUb, days | 11±11 | 7±4 | 18±15 | 0.025 | 5±10 | 0.190 |
| Stay in hospitalb, days | 34±19 | 33±19 | 32±21 | 0.800 | 26±26 | 0.554 |
| Mortality in ICU, n (%) | 13 (37) | 3 (14) | 10 (71) | 0.001 | 6 (100) | 0.006 |
| Causes of death | ||||||
| Multiorgan failure | 9 | 2 | 7 | 5 | ||
| Cardiac arrest | 2 | 0 | 2 | 0 | ||
| Cerebral hemorrhage | 1 | 1 | 0 | 1 | ||
| Refractory hypoxemia | 1 | 0 | 1 | 0 | ||
| Mortality in hospital, n (%) | 16 (46) | 6 (28) | 10 (71) | 0.018 | 6 (100) | 0.023 |
ARDS, adult respiratory distress syndrome; ICU, Intensive Care Unit; IMV, invasive mechanical ventilation; NPPV, noninvasive positive pressure ventilation; VAP, ventilator associated pneumonia.
Kaplan–Meier survival analysis (log-rank test) between the NPPV group (success and failure) and the IMV group after 90 days. Table: number of patients alive during this period of time. IMV, invasive mechanical ventilation; NPPV, noninvasive positive pressure ventilation; NPPV OTI, intubated noninvasive ventilation; NPPV-NO OTI, non-intubated noninvasive ventilation.
Within the NPPV group, on comparing success versus failure of the technique, no significant differences were observed in relation to the demographic characteristics, comorbidities or cause of respiratory failure (Table 1). Comparison of the physiological parameters showed differences between the success and failure of NPPV in terms of the level of PaO2 (82 [59–126] vs 58 [41–76], p<0.005), bicarbonate (24±6 vs 19±5, p<0.005), excess base deficit (0±6 vs −5±4, p<0.005), and blood hemoglobin (9±1 vs 10±2, p<0.005). The intubation rate was 40% (Table 2), which implies a greater organ dysfunction rate in this group, particularly as regards cardiovascular failure with the need for vasoactive support (100% vs 38% in the patients with successful NPPV, p=0.0001). There were no significant differences in infection rate, though a shorter duration of mechanical ventilation, a shorter stay in the ICU, and a lesser mortality rate both in the ICU and in hospital were recorded in the group of patients with successful NPPV. The mortality rate after 90 days was significantly lower (p=0.001) in the case of successful NPPV versus either failed NPPV or the IMV group (Fig. 1).
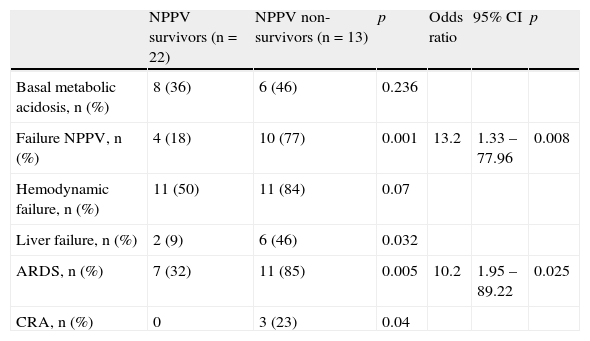
The multivariate analysis of all the variables significantly related to mortality in the NPPV group (Table 3) found the failure of NPPV (OR 13 [95% CI 1.95–89.22], p=0.008) and the development of ARDS (OR 10 [95% CI 1.33–77.9], p=0.03) to be related to mortality in the NPPV group. The positive predictive value of the model was 100%, with a negative predictive value of 59%, and a diagnostic accuracy of 74%. The area under the ROC curve was 0.88 (95% CI 0.773–0.993). The Hosmer–Lemeshow test with 4 degrees of freedom (d.f.) was not significant (p=0.525).
Multivariate analysis of factors related to mortality in the NPPV group.
| NPPV survivors (n=22) | NPPV non-survivors (n=13) | p | Odds ratio | 95% CI | p | |
| Basal metabolic acidosis, n (%) | 8 (36) | 6 (46) | 0.236 | |||
| Failure NPPV, n (%) | 4 (18) | 10 (77) | 0.001 | 13.2 | 1.33 – 77.96 | 0.008 |
| Hemodynamic failure, n (%) | 11 (50) | 11 (84) | 0.07 | |||
| Liver failure, n (%) | 2 (9) | 6 (46) | 0.032 | |||
| ARDS, n (%) | 7 (32) | 11 (85) | 0.005 | 10.2 | 1.95 – 89.22 | 0.025 |
| CRA, n (%) | 0 | 3 (23) | 0.04 |
95% CI, 95% confidence interval; CRA, cardiorespiratory arrest; ARDS, adult respiratory distress syndrome; NPPV, noninvasive positive pressure ventilation.
Our retrospective study found hematological patients admitted to the ICU and subjected to mechanical ventilation due to ARF to have lesser mortality than in other series,3,5,7 and the use of NPPV constituted a key element in the supportive measures, since it influenced the decrease in mortality among such patients.
Of note in our series is the use of NPPV as ventilatory support measure; in this sense, it is common practice in our Unit to make an attempt with this technique before considering IMV. Such practice has also been extended to the hospitalization ward, where a percentage of admitted patients receive ventilatory support with CPAP as a step prior to admission to the ICU. A randomized study19 showed that the use of CPAP versus oxygen therapy in the Hematology ward in patients with ARF reduced progression toward ARDS, the need for admission to the ICU, and the need for invasive ventilatory support. Despite these results,19 the application of CPAP in our small sample was greater in the group in which NPPV failed, and did not result in benefit of any kind. In our series, intubation from the start was limited to patients with multiorgan dysfunction as assessed by the SOFA score, which was higher than in the NPPV group–though not all the values reached statistical significance. This increased severity could account for the poor results obtained in the IMV group, since all the patients died in the ICU during the first hours, and mostly under conditions of multiorgan failure–this proportion being greater than expected from the SAPS 2 score. Different authors5,7 have underscored the predictive value of multiorgan failure in relation to mortality, though it should be taken into account that most patients upon admission, at least in our series, already presented dysfunction of several organs. In this sense, our data coincide with the findings of a Spanish multicenter study20 that analyzed the ventilatory support measures in hematological patients, and in which the SOFA score was higher in those patients directly subjected to IMV versus the NPPV group, and the initial respiratory SOFA score was similar in the IMV and NPPV (success and failure) groups–though in the NPPV failure group the score worsened significantly with respect to the rest of the patients over time.
In our opinion, NPPV has been a determinant factor in the reduction of mortality, since as we have seen, the number of complications and infections was not significantly greater in the IMV group (mainly due to the brief stay in the ICU caused by early mortality)–though all of them considered jointly, together with the organ dysfunction already present at the time of admission, probably influenced the final results obtained. These results therefore reaffirm our opinion that whenever possible, NPPV should be used for initial ventilatory support, in concordance with the observations of most studies published to date,2,20–22 in which utilization of the technique has been associated with a high level of evidence.23 Nevertheless, recent studies5,20,22 continue to describe a greater use of IMV versus NPPV, though these same publications20,22 have shown NPPV to afford a substantial decrease in mortality compared with IMV. Another reason why our results are consistent with those obtained by other authors6,11–13,22,24 could be the close collaboration between the Department of Hematology and our Department of Intensive Care Medicine, thereby allowing earlier management of the many complications which these patients tend to present, and which are difficult to deal with in a hospitalization ward. The main element conditioning such close collaboration between our Departments was the introduction of NPPV in our range of therapeutic options. The high mortality associated with the need for IMV1,2,6 raised some doubts about admitting such patients to our Unit, in view of the important care burden involved (respiratory support, vasoactive and renal therapy in many cases, and the adoption of isolation measures), and the ominous outcomes. But the introduction of NPPV and the publication of studies6,11–13 warranting the use of this technique in immune depressed patients led to a substantial change in admission policy. Another consideration was the rapid development and severity of organ dysfunction in these patients, making it futile to admit such cases of established multiorgan failure to the ICU. Early intervention with early admission thus proved essential. In this same line, some studies25 point to the benefits in terms of lessened mortality of admitting patients with hematological malignancies to the ICU based on a less restrictive admission policy. In this sense, consideration is also required of the fact that a delay in admission to the ICU is directly correlated to mortality–thus advocating early patient admission.4
In this scenario favorable to NPPV, doubts remain as to why the technique is still underused. The reason could be the high incidence of ARDS upon admission or during patient stay in the ICU, and the controversial indication of NPPV in ARDS.10,26,27 Different studies28,29 have shown NPPV in hypoxemic patients to be more effective than oxygen therapy, with particular emphasis upon ALI or ARDS30–32–registering an NPPV failure rate of between 4.8%32 and 70% in ALI,31 and between 46%29 and 51% in patients with ARDS.32 The multivariate analysis28,33 found the development of ARDS to be a predictor of NPPV failure–this possibly being the reason why there is no clear recommendation on the use of NPPV in the context of ARDS,9,10,25 and why the technique is little used in hematological patients with severe hypoxemia. In contrast, however, the relationship between IMV and mortality in this patient population has been well established.3,5,20–22 In this sense, a multicenter observational study22 involving a series of 1302 hematological patients with ARF showed the use of NPPV to be less widespread than that of IMV (21% vs 79%). The noninvasive group presented more neutropenia (16.8% vs 10%, p<0.002) and hypoxemia than the invasive ventilation group. In contrast, the patients in the IMV group were in more serious condition as established by the APACHE score, and had a poorer level of consciousness. This may have justified the initial use of IMV. A significant difference was also noted in the use of NPPV versus IMV in patients with ALI (21% vs 11%, p=0.0001). In the case of patients with ARDS, the difference failed to reach statistical significance. The results showed the NPPV group to have a shorter duration of mechanical ventilation and stay in the ICU, as well as lesser mortality both in the ICU and in hospital, but these results were not reproduced in the subgroup of patients with ALI or ARDS. Another observational study6 found mortality after 30 days in the NPPV group to be significantly lower than in the IMV group (43.7% vs 70.8%, p=0.008).
On analyzing the NPPV group, failure of the technique was seen to be associated with a greater complications rate. These results coincide with those obtained in other studies,20,22,24,30 where the failure of NPPV markedly increased the percentage of complications, the duration of stay, and mortality. In coincidence with other authors,20,22,27,28 we found mortality to be associated with failure of NPPV and development of ARDS, along with other variables20,22 such as age, septic shock, coma, coagulation disorders or a high SAPS 2 score, which were not analyzed in our series. Given the strong negative influence of NPPV failure upon mortality, it seems logical to explore the factors that influence such failure. In this context, different studies24,28 have identified delayed introduction of ventilatory support, the development of ARDS, and the need for vasoactive and renal support as predictors of NPPV failure. Another cohort study of patients with ARDS30 found severity as determined by a SAPS 2 score of >34, and the absence of improvement in oxygenation (PaO2/FiO2<175) 60min after starting NPPV, to be predictors of failure. From the above it can be concluded that NPPV will probably fail if the start of the technique is delayed in a hypoxemic patient with scant clinical and blood gas response after 1h–intubation being required in such cases.
The limitations of our study are represented by its retrospective nature, and the fact that it was carried out in a single center where NPPV moreover is routine practice in patients with hypoxemic ARF.
Despite the poor results obtained in the IMV group, we do not intend to discard the use of the technique. Rather, we wish to underscore the benefits of NPPV in extremely ill patients, with a poor prognosis and with multiple organ dysfunction. The routine use of NPPV, and the risk of intubation particularly in these patients, implies that the few individuals directly included in the IMV group were in a condition in which NPPV was literally contraindicated. The conduction of a prospective study comparing NPPV versus IMV with a literature basis3,5,7 demonstrating the mortality associated to IMV, along with studies6,22 showing the good results obtained with NPPV, therefore would be very questionable. Consequently, on the basis of our results, we could recommend the use of NPPV as a first ventilatory support measure in hematological patients with ARF, without considering the classical NPPV indication criteria,10,26,27 and without regarding ARDS or multiorgan dysfunction as exclusion criteria–since at the time of admission to the ICU, most of these patients already suffer dysfunction of one or more organ systems.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Belenguer-Muncharaz A, Albert-Rodrigo L, Ferrandiz-Sellés A, Cebrián-Graullera G. Evolución de 10 años de aplicación de la ventilación mecánica en la insuficiencia respiratoria aguda del paciente hematológico ingresado en la unidad de cuidados intensivos. Med Intensiva. 2013;37:452–460.