Despite low mortality in patients undergoing major abdominal surgery, the number of high-risk patients is increasing and has become a health problem. At present, surgery is understood as a continuous process, in which numerous guidelines added to less invasive techniques offering a lesser physiological impact upon patients with serious comorbidities are responsible for the final outcome. The prevention, identification and early treatment of complications prove as important as the preoperative or surgical technique. The introduction of ERAS (Enhanced Recovery After Surgery) protocols is the cornerstone for the management of these patients, and is advocated by most surgical societies for reducing mortality, length of hospital stay and hospital costs. The postoperative management of these patients in postsurgery Intensive Care Units guarantees effectiveness and efficiency in maintaining optimum patient care.
A pesar de una baja mortalidad en los pacientes sometidos a procedimientos de cirugía mayor abdominal, el número de pacientes con alto riesgo aumenta cada día, convirtiendo a esta en un problema de salud. En la actualidad la cirugía se entiende como un proceso continuo en el que el resultado final depende de numerosas pautas sumadas a técnicas menos invasivas que ofrezcan menor impacto fisiológico sobre pacientes con graves comorbilidades. La prevención, el reconocimiento y el tratamiento precoz de las complicaciones se hacen tan importantes como el preoperatorio o la técnica quirúrgica. La instauración de los protocolos Enhaced Recovery After Surgery es la piedra angular para el tratamiento de estos pacientes, dado que la mayoría de las sociedades quirúrgicas reconocen que reduce la mortalidad, la duración del ingreso y los costes hospitalarios. El manejo postoperatorio de estos pacientes en las unidades de cuidados intensivos garantiza la efectividad y la eficiencia para contribuir al mantenimiento de la prestación de servicios de salud.
Postoperative complications are an important health problem that cause 3–12 million deaths each year,1 thus representing one of the leading causes of patient mortality.2 Measures designed to prevent, promptly identify and treat any potentially fatal complication that may arise during the postoperative period are essential. Accordingly, the admission to the Intensive Care Unit (ICU) of high-risk surgical patients historically has been regarded as vital in order to reduce the perioperative mortality risk. In Spain, 2.1 million people undergo surgery each year.3 In most cases the surgical risk is low, though the evidence increasingly suggests that complications following surgery are an important cause of mortality.1 Approximately 10% of all patients subjected to surgery are at a high risk of suffering complications – a circumstance that accounts for 80% of all postoperative deaths.4 The identification of patients at increased risk, and who could benefit from admission to the ICU, remains a major challenge that is associated to an unacceptable imbalance in the distribution of the available resources.5,6
The utility of routine admission to the ICU after elective surgery has recently been questioned, in view of the costs involved and the lack of solid backing evidence. In this regard, a recent article published by Kahan7 has described the results of a secondary analysis of the database of the International Surgical Outcomes Study (ISOS). In a cohort of 44,000 patients from 474 hospitals in 27 countries, no association was found between postoperative mortality and admission to the ICU. However, this was not a randomized study, and the potential impact of confounding factors upon the analysis of the results was not taken into account. Likewise, there was no clear criterion regarding routine admissions, electively planned admissions of high-risk patients, or emergency admissions secondary to major complications after surgery (e.g., severe bleeding). Furthermore, the simplistic definition of the ICU could limit external validity (human and technological resources during the 24h of the day).6
Ghaferi8 evaluated the variations in postoperative hospital morbidity–mortality in patients undergoing major surgery. The authors conducted a rigorous, risk-adjusted prospective data analysis of more than 80,000 patients from over 150 hospitals, under the auspices of the national program for the improvement of surgical quality. As expected, the mortality rates varied among hospitals, within a range of 3.5–6.9%. The surprising conclusion was that in those hospitals with the highest complication rates, mortality was twice as high. This implies that importance must be placed not only on the type of surgery but also on the effective and early detection of complications.9
The decision to admit a patient to the ICU after surgery is fundamented on a series of factors, and includes reasons that are not easily reflected in the administrative data, such as unexpected perioperative events, concerns on the part of the clinical team, and the availability of beds in the ICU.10 It seems clear that in patients subjected to low-risk surgery without organ dysfunction and involving scant morbidity, admission to the ICU does not result in differences referred to mortality,11 and such individuals may be managed in areas such as intermediate care. The existence of monitoring systems outside the ICU and of rapid response teams may be adequate for the detection of complications and can result in the rescue of patients with early problems of this kind – some of which are not directly associated to the surgical procedure but to the patient comorbidities. High-risk operations, such as esophagectomy with previous radiotherapy, extensive hepatectomy particularly after aggressive chemotherapy cycles, duodenopancreatectomy, cytoreductive surgery combined with intraperitoneal hyperthermic chemotherapy, or open abdominal aneurysm surgery are some of the procedures requiring “precision” management in the administration of fluids and hemodynamic optimization, the early detection of infectious complications, pain, bleeding and coagulation control, as well as the prevention of venous thromboembolism (VTE) and postoperative respiratory failure. At present, there is no clear consensus regarding the benefit of patient admission to the ICU. Relating ICU admission to mortality is a complex issue, unless more specific risk classification systems are established, and we moreover must take equally important aspects such as safety, quality and precision into account.
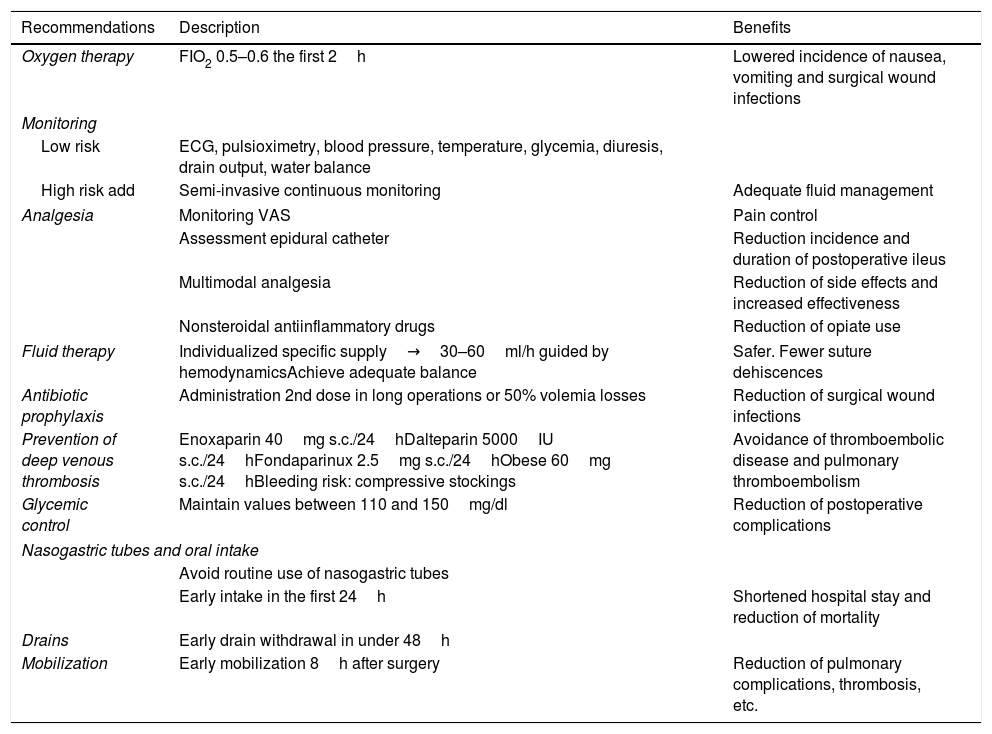
Enhanced Recovery After Surgery (Table 1)Enhanced Recovery After Surgery comprises a specific multimodal approach to the entire surgery process in which the postoperative measures seek to attenuate the catabolic response to surgical stress, accelerating patient recovery. It was originally proposed for colorectal cancer surgery,12 but has since been extended to the postoperative setting of procedures such as duodenopancreatectomy,13 hepatectomy,14 esophagogastric surgery,15 radical cystoprostatectomy,16 etc. The four key elements of this approach are: comprehensive preoperative evaluation and preparation of the patient, optimum anesthesia and minimally invasive surgery to reduce the patient response to surgical stress, adequate postoperative management of the symptoms such as pain with early mobilization, and prompt reintroduction of a normal diet.
Enhanced Recovery After Surgery (ERAS) protocol.
| Recommendations | Description | Benefits |
|---|---|---|
| Oxygen therapy | FIO2 0.5–0.6 the first 2h | Lowered incidence of nausea, vomiting and surgical wound infections |
| Monitoring | ||
| Low risk | ECG, pulsioximetry, blood pressure, temperature, glycemia, diuresis, drain output, water balance | |
| High risk add | Semi-invasive continuous monitoring | Adequate fluid management |
| Analgesia | Monitoring VAS | Pain control |
| Assessment epidural catheter | Reduction incidence and duration of postoperative ileus | |
| Multimodal analgesia | Reduction of side effects and increased effectiveness | |
| Nonsteroidal antiinflammatory drugs | Reduction of opiate use | |
| Fluid therapy | Individualized specific supply→30–60ml/h guided by hemodynamicsAchieve adequate balance | Safer. Fewer suture dehiscences |
| Antibiotic prophylaxis | Administration 2nd dose in long operations or 50% volemia losses | Reduction of surgical wound infections |
| Prevention of deep venous thrombosis | Enoxaparin 40mg s.c./24hDalteparin 5000IU s.c./24hFondaparinux 2.5mg s.c./24hObese 60mg s.c./24hBleeding risk: compressive stockings | Avoidance of thromboembolic disease and pulmonary thromboembolism |
| Glycemic control | Maintain values between 110 and 150mg/dl | Reduction of postoperative complications |
| Nasogastric tubes and oral intake | ||
| Avoid routine use of nasogastric tubes | ||
| Early intake in the first 24h | Shortened hospital stay and reduction of mortality | |
| Drains | Early drain withdrawal in under 48h | |
| Mobilization | Early mobilization 8h after surgery | Reduction of pulmonary complications, thrombosis, etc. |
ECG: electrocardiogram; VAS: visual analog scale; FiO2: fraction of inspired oxygen.
Some authors associate a high fraction of inspired oxygen (FiO2 80%) during the first 6h and antibiotic prophylaxis to a decrease in surgical wound infection rate; however, the existing evidence does not allow firm recommendations in this regard.17,18
MonitoringIn previously extubated patients without comorbidities (ASA score I, II), monitoring should include vital signs: heart rate, pulsioximetry, noninvasive blood pressure values, core temperature, glycemia, urine output, drainage measures (quality and quantity) and fluid administration (partial balances). At present we are able to obtain noninvasive continuous hemoglobin measurements (SpHb), as well as record variations in plethysmographic index and oxygen reserve.
In high-risk patients with cardiovascular/respiratory comorbidities, due assessment should be made of the use of semi-invasive monitoring procedures involving devices based on pulse wave analysis, variations in systolic volume, transthoracic bioimpedance or esophageal Doppler ultrasound.19–21
AnalgesiaAnalgesia is one of the cornerstones of the Enhanced Recovery After Surgery strategy. Measures such as early mobilization require maximum pain control. In this respect, control of pain sensation is crucial, based on the use of validated scales in patients either able (visual analog scale [VAS] and verbal numerical scale) or not able to communicate (Behavioral Pain Scale and Critical Care Observation Tool), together with pain indicating behavioral scales.
In open abdominal surgery, thoracic epidural analgesia has been shown to be effective in reducing the incidence and duration of postoperative ileus.22 This is due both to sympathetic block produced by the epidural local anesthetic and to the avoidance of systemic opiates. A combination of local anesthetic opiates affords good pain control. Administration using infusion pumps is preferable to elastomer systems. The physiological effects of epidural anesthesia may serve as a justification for improving the respiratory and cardiovascular outcomes after general, urological and vascular surgeries, as evidenced by meta-analyses and randomized controlled trials.23
The administration of nonsteroidal antiinflammatory drugs as coadjuvant therapy contributes to reduce the use of opiates. In this regard, analgesic strategies without opiates are the predominant tendency.
Fluid therapyTissue injury produces the release of catabolic hormones and inflammatory mediators that facilitate salt and water retention to preserve the circulating volume, maintain blood pressure and vasoconstriction, and provide gluconeogenic substrates for metabolism and cell function. Body temperature decreases to minimize oxygen consumption, and the blood is deviated from “non-vital” organs such as the intestine, skin and muscles in order to maintain the perfusion of vital organs such as the heart, brain and kidneys.
It has been shown that salt and water overload affect anastomotic integrity. Furthermore, ileus and an increase in postoperative complications leading to the prolongation of hospital stay have been reported when a patient fluid balance of close to zero is not maintained.24,25 In general, postoperative complications have been found to increase when the body weight increment in the postoperative period exceeds 2.5kg (indicative of a cumulative fluid overload of 2.5l).
At present, a protocol including moderately liberal fluid administration is accepted to be safer than a restrictive regimen.26 There is a replacement period intended to achieve normovolemia, followed by a maintenance period, and then fluid therapy should be lowered as soon as the patient resumes oral salt intake. Each patient requires a specific supply, depending on the type of surgery, the blood losses, comorbidities, etc. In general, 30–60ml/h of balanced crystalloids is accepted, though algorithms driven by mean blood pressure (MBP), cardiac index (CI), systolic (stroke) volume variation (SVV) or pulse wave variation (PWV), and Doppler ultrasound, together with vasopressors and inotropic drugs, will help to ensure adequate perfusion.27 The objective is to maintain body weight and a zero fluid balance.
Antibiotic prophylaxisEffective surgical antibiotic prophylaxis should secure antimicrobial drug concentrations in serum and tissues in excess of the minimum inhibitory concentration (MIC) of the most likely microorganisms found in the surgical site 30–60min before the start of surgery. A second dose is required in cases of prolonged surgery or in the event of volemia losses of 50%.28,29
Prevention of venous thromboembolic diseaseVenous thromboembolism (VTE) and pulmonary thromboembolism (PTE) are a frequent and serious complication in patients subjected to different abdominopelvic surgical procedures.30 In the absence of prophylaxis, the risk of silent deep venous thrombosis is 25% in general surgery, 19% in abdominal vascular surgery and 15% in peripheral vascular surgery. The reported prevalence of PTE is 1.6% and that of fatal PTE 0.8%.31 Knowing the clinical risk factors, patients can be classified as being at high, moderate or low risk of developing VTE. However, the lack of prophylaxis in most patients is a consequence of the fear of bleeding. The use of compressive stockings to secure sufficient levels of safety has been shown to be effective. Low-dose unfractionated low molecular weight heparin (LMWH) and fondaparinux have been seen to significantly reduce the risk of VTE. The administration of both enoxaparin 40mg s.c./24h32 and dalteparin 5000IU s.c./24h and fondaparinux 2.5mg s.c./24h has been recommended. In patients with morbid obesity, the dose needs to be incremented.
Glycemic controlThere is abundant evidence in the literature of a clear association between perioperative hyperglycemia and adverse clinical outcomes.33–36 The risk of postoperative complications and increased mortality is related to both the long-term control of blood glucose and to the severity of hyperglycemia upon admission and during hospital stay. It is advisable to keep the glycemia values between 110 and 150mg/dl.
Nasogastric tube and oral intakeThe postoperative management of patients subjected to abdominal surgery traditionally has demanded the use of nasogastric tubes in order to avoid oral liquid and nutrient intake until postoperative ileus has resolved. This notion has come under questioning in recent years, however.37 Experimental and clinical studies have shown that the traditional restrictions referred to oral intake are not based on scientific evidence. At present, there is agreement in avoiding the routine use of nasogastric tubes.38 Effective techniques have been developed to reduce postoperative ileus, and the early resumption of oral nutrition after surgery may improve the postoperative outcomes.
The current recommendation is early intake in the first 24h (tolerance testing can be made after 6h), with the objective of having suspended intravenous fluid therapy by day three.39
DrainsEarly drain withdrawal is advised. Drains are often associated to precarious sutures; they are bothersome for the patient, and afford no clinical advantages beyond 48h.40 They are useful in pelvic surgery in the first 24h.41
MobilizationImmobilization, very much in favor in the past, currently poses greater inconveniences. It increases insulin resistance, generates a greater risk of thrombosis, and medullary regenerative capacity decreases. Early mobilization of the patient in the absence of anemia and with hemodynamic stability contributes to lessen pulmonary complications, thrombosis,42–44 etc. However, good pain control is required. Patient mobilization is advised 8h after surgery.
Major complications in major abdominal surgeryDespite low mortality, the number of patients undergoing elective major abdominal surgery defines that latter as a health problem.45 Most fatalities occur in patients at high risk due to old age, comorbidities or the complexity of the surgical procedure. Approximately 15% of the patients that undergo major surgery are at a high risk of complications. This group represents 80% of all deaths occurring during the postoperative period.46
At present, emphasis on safety has contributed to minimize the adverse events attributable to shortcomings in the surgical technique or anesthesia, despite the large number of operations made. However, many patients develop some degree of postoperative morbidity as a consequence of the physiological, endocrine and inflammatory changes associated with surgical trauma. The relatively minor consequences, such as temporary pain and immobility, are common, though serious or even fatal complications may also occur. The magnitude, duration and consequences of postoperative morbidity are conditioned to the complex interactions among the indication of surgery, the resulting tissue injury, and patient factors such as age and comorbidities. The chronic conditions that often affect the postoperative outcomes include diabetes, heart failure and chronic obstructive pulmonary disease (COPD).47,48
Foreseeable common adverse events in the postoperative period of major abdominal surgerySurgical wound infectionInfections associated to healthcare are the most common adverse events affecting patient safety worldwide. Of all nosocomial infections, surgical wound infections49 are the most common problem in developing countries, and the second most common problem in the industrialized world. Depending on their location, these infections are classified as superficial, deep or organ-space infections. Superficial infections affect the skin and cellular subcutaneous tissue in the region of the surgical incision. Deep infections in turn affect the fascias and muscle layers, while organ-space infections are found in any body location other than the skin, subcutaneous tissues, fascias or muscle layers, and that has been opened or manipulated during the surgical procedure.50 The estimated prevalence is 1.21–26%49 with a mortality rate of 14%.
Dehiscence of the abdominal wallThe incidence of abdominal wall dehiscence is relatively low (0.4–3.5%), but the associated mortality rate can reach 45%. This problem requires repeat surgery and a prolonged patient stay.50
Anastomotic leakageAnastomotic leakage is one of the most important major complications of colorectal surgery, with an incidence of 3–19%51 and a historical mortality rate of 6–22% – though this figure has now been reduced to 10%52 thanks to technical advances in surgery. In esophageal surgery, the incidence of anastomotic leakage of the neck sutures has been as high as 40%, though with a mortality rate of less than 5%,53 in contrast to intrathoracic anastomoses, which present a lesser incidence of 5% but with a higher mortality rate. At present, the use of self-expanding stents constitutes an effective alternative to repeat surgery.54
Gastrointestinal surgery is responsible for 75% of all enterocutaneous fistulas.55 Most of them originate in the small bowel, and in many cases they are of an iatrogenic nature,56,57 and usually resolve spontaneously or with surgery. The mortality rate in recent years is close to 10%.58
Pancreatic fistulization is observed in 30% of all cases of cephalic duodenopancreatectomy59 but in only 5% of all distal pancreatectomies.60 It is associated to increased morbidity and a prolongation of hospital stay, though in a center with a large number of pancreatic surgeries the mortality rate is in the order of 5%.61
Cardiovascular complicationsMyocardial infarction occurs in 5% of all patients subjected to non-cardiac surgery, and of these subjects, 74.1% suffer infarction in the first 48 postoperative hours. Most of the affected patients (65.3%) present no clinical signs; troponin determination is therefore required in individuals at high risk and/or with a history of ischemic events. The mortality rate at 30 days is 11%.62 Arterial hypertension is to be avoided, balancing the chronic medication of the patient, and pain and hypotension likewise are to be avoided. Atrial fibrillation occurs in 8% of all non-cardiac surgery patients in the postoperative period. The triggering factors are not always clear, but catecholamine stress secondary to tissue trauma and pain, hypovolemia or atrial stretch, hypoxia (which causes pulmonary vasoconstriction) and electrolyte disturbances may be implicated. The hemodynamic effects are often subtle, but atrial filling loss reduces the stroke volume by 25%, pulmonary artery pressure increases, and tachycardia can cause myocardial ischemia secondary to the decrease in diastolic filling time and increased myocardial work. This is often well tolerated, and most patients with postoperative atrial fibrillation episodes are asymptomatic; however, individuals with pre-existing arterial hypertension or diastolic dysfunction can often become hemodynamically unstable.63 Postoperative heart failure may be a consequence of an acute ischemic cardiac event. Other causes include inadequate fluid management (in terms of volume and quality), acute kidney injury, sepsis, acute lung injury and/or volume overload related to blood product transfusions, diastolic dysfunction,64 etc.
Respiratory complicationsRespiratory complications are observed in 10–40% of all patients subjected to major abdominal surgery, though in acute respiratory distress syndrome (ARDS) the incidence of required invasive ventilation is only 3.1%.65
Acute kidney injurySurgery is considered to be a risk factor for the development of acute renal failure.66 It manifests in 7% of all patients in the postoperative period, and of these, 6% require renal replacement measures.67 The mortality rate among patients with acute renal failure who require such replacement measures is 50–70%.67
Delirium and cognitive impairmentDelirium is common in hospitalized patients, with an incidence of 36.8% among patients admitted to the ICU after surgery.68 However, the difficulty of diagnosing the condition without the use of adequate instruments means that the incidence may be underestimated.69,70 Delirium is currently known to be related to increased mortality, a greater need for re-hospitalization, and a prolongation of stay.71
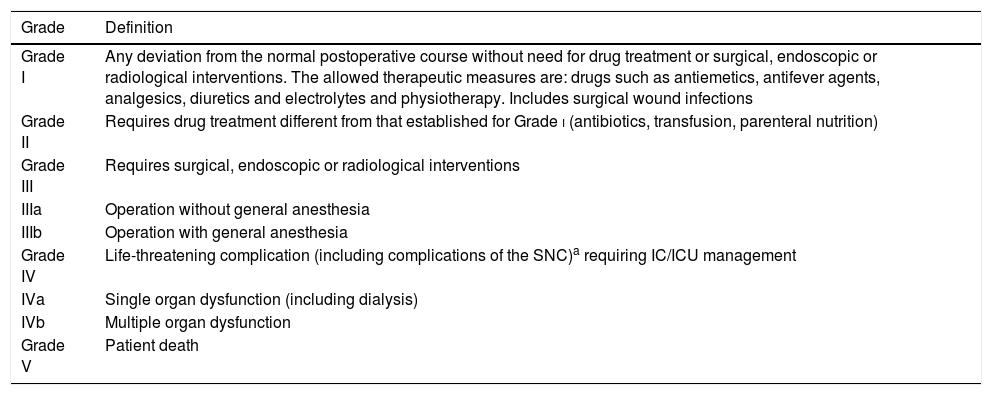
Classification of the complications. Severity (Table 2)The analysis of morbidity–mortality is a basic tool in the assessment of surgical quality, though the lack of consensus regarding the description of complications occurring after a surgical procedure has impeded objective comparison of the outcomes.
Clavien–Dindo classification. Postoperative complications.
| Grade | Definition |
|---|---|
| Grade I | Any deviation from the normal postoperative course without need for drug treatment or surgical, endoscopic or radiological interventions. The allowed therapeutic measures are: drugs such as antiemetics, antifever agents, analgesics, diuretics and electrolytes and physiotherapy. Includes surgical wound infections |
| Grade II | Requires drug treatment different from that established for Grade i (antibiotics, transfusion, parenteral nutrition) |
| Grade III | Requires surgical, endoscopic or radiological interventions |
| IIIa | Operation without general anesthesia |
| IIIb | Operation with general anesthesia |
| Grade IV | Life-threatening complication (including complications of the SNC)a requiring IC/ICU management |
| IVa | Single organ dysfunction (including dialysis) |
| IVb | Multiple organ dysfunction |
| Grade V | Patient death |
IC: intermediate care; CNS: central nervous system; ICU: Intensive Care Unit.
The most widely accepted classification of complications is that developed by Clavien72 (Table 2), which considers severity and clinical course over time.
ConclusionsIn order to secure improved outcomes in surgical patients, Departments of Intensive Care Medicine must offer care throughout the process. The creation of rapid response teams and ICUs without walls (teamwork involving different professionals, and automated severity detection integrating clinical and laboratory test parameters) improves the outcomes and moreover avoids unnecessary admissions. Innovation in management, through the use of tools adapted from industry, such as the Lean techniques (based on the reduction of process variability and the suppression of elements that do not offer added value), and coordinated and multidisciplinary work make it possible to improve critical patient care, as well as the outcomes, efficiency, patient safety and professional satisfaction. These experiences have been shown to reduce delays in discharge from the ICU to the hospital ward, and this may lower the number of elective surgeries that are canceled because of a lack of ICU beds and unforeseen discharges with increased patient risk. Lastly, Departments of Intensive Care Medicine can contribute value to the surgical process in chronic critical patients through follow-up in other hospital areas after discharge.73
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Puppo Moreno AM, Abella Alvarez A, Morales Conde S, Pérez Flecha M, García Ureña MÁ. La unidad de cuidados intensivos en el postoperatorio de cirugía mayor abdominal. Med Intensiva. 2019;43:569–577.