Patients in the vegetative state (VS) generally remain in the same clinical situation, though some may evolve toward a minimally conscious state (MCS).1,2 It is very important to differentiate these two conditions, since the associated clinical decisions may be very distinct and pose different ethical dilemmas. In the case of permanent VS, characterized by irreversible damage, limitation of life-sustaining treatment may be decided. In comparison, patients presenting MCS are unable to maintain functional communication, but are occasionally able to follow instructions, utter some words, visually follow moving objects or people, and show emotions such as smiling, laughing or even crying. Neurological exploration in such cases is extremely difficult, since the patient movements may be very small, inconsistent and easily exhausted–thus giving rise to erroneous diagnostic interpretations.3 It has been estimated that 40% of all cases of VS actually constitute MCS4–a circumstance that reflects the need to complement clinical exploration with other diagnostic methods.
Positron emission tomography–computed tomography (PET/CT) is an emerging neuroimaging technique that can differentiate between VS and MCS. Its availability is still limited, however, and interpretation of the findings is sometimes difficult5. PET/CT with 18F-2-fluoro-2-deoxy-d-glucose (18F-FDG)–a radiolabeled glucose analog–can be used to study brain activity. 18F-FDG uptake is related to cell transport, and thus assesses regional cerebral carbohydrate metabolism, representing an in vivo measurement of neuronal integrity.6 In recent years, several studies have correlated specific metabolic patterns to different levels of consciousness. In this regard, patients presenting VS show marked global frontal and parietal cortical hypometabolism, while MCS is characterized by preserved metabolism of these cortical areas.7–11 One of the limitations of PET/CT is that it requires the patient to be moved out of the Intensive Care Unit (ICU), and the exploration moreover takes about 1h to complete. Specifically, the time from injection of the radiotracer to adequate distribution of the radiodrug is about 40–45min, and another 10–15min are needed for image acquisition.
We present a clinical case in which PET/CT was able to discard VS. The patient was a 15-year-old male presenting cardiorespiratory arrest with atrial fibrillation secondary to electrocution. After 10min of basic cardiopulmonary resuscitation (CPR), he was attended by the out-hospital emergency service, which performed advanced CPR during 15min, requiring 6 defibrillatory discharges and intravenous amiodarone. Following admission to the ICU, therapeutic hypothermia was carried out for 36h. The initial bispectral index (BIS) score was <15, and the brain CT findings proved normal. Sedation was suspended after 72h, and the patient was able to follow simple instructions, with a Glasgow coma score of 10. On day 4 of admission he suffered an important worsening of consciousness and dystonic episodes, with right deviation of the gaze and reactive mydriasis. Antiseizure medication was prescribed. The electroencephalogram (EEG), median nerve somatosensory evoked potentials (SEPs) and brainstem auditory evoked potentials were normal. Repeat CT discarded ischemic/hemorrhagic damage, and the brain magnetic resonance imaging (MRI) study revealed bilateral basal ganglia and hippocampal cortical signal alterations consistent with severe anoxic encephalopathy. Following the suspension of sedation, status dystonicus, opisthotonus and diencephalic alterations were observed with paroxysmal sympathetic hyperactivity, arterial hypertension, perspiration and tachycardia. SPECT with 99mTc-HMPAO revealed occipital cortical and basal ganglia hyperperfusion. In the course of admission, antiseizure treatment was administered in the form of levetiracetam, valproic acid, lacosamide, phenobarbital and clonazepam, with the unsuccessful evaluation of a number of combinations and dosing schemes. Treatment with clonidine, propranolol, morphine and baclofen partially controlled the sympathetic activity. In view of the extreme status dystonicus that made patient handling and care impossible, barbiturate-induced coma with thiopental sodium was decided. After suspending thiopental, the crises returned with the same frequency and intensity, with the maintenance of status dystonicus. During the brief intercrisis intervals, some observers had the impression that the patient seemed to follow instructions, though the explorations were not reproducible, and there were many doubts regarding his level of consciousness.
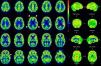
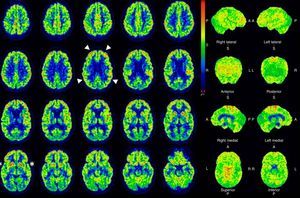
In view of the magnitude and severity of the crises, manifesting continuously 24h a day and requiring deep sedoanalgesia and relaxation, we were unable to assess the level of consciousness of the patient. In this context, and in order to progress in the clinical decision making process, we performed PET/CT with 18F-FDG (Fig. 1). The exploration revealed marked hypometabolism in both posterior putamina, coinciding with the signal alteration described in the previous MRI scan, together with preserved carbohydrate metabolism in the caudate nuclei consistent with the SPECT hyperperfusion findings. Metabolism of the frontoparietal cortex was within normal limits. Consequently, based on the literature,5 the findings were not suggestive of a metabolic pattern consistent with VS. Since the glucose-consuming cortical activity was normal, we concluded that the lack of patient contact with the environment was related to the status dystonicus and sedation used to control the condition.
Given the refractory clinical condition, and upon recommendation from experts in movement disorders and epilepsy, we tried new antiseizure treatment schemes, infiltration with botulinum toxin, and the use of different drugs and drug combinations (bromocriptine, gabapentin, dantrolene, piracetam, biperiden, dronabinol plus cannabidiol, intrathecal baclofen, olanzapine, chloral hydrate, trihexyphenidyl, clorazepate, perampanel, tetrabenazine, tizanidine, fluoxetine, paroxetine, benzodiazepines and cisatracurium)–though without achieving dystonia control.
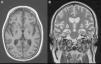
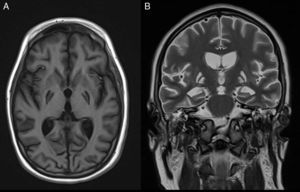
The control CT and MRI studies (Fig. 2) in the second month of admission revealed almost complete destruction of the basal ganglia as a result of the electrocution and anoxic damage. Repeat PET/CT performed 6 months after the first exploration confirmed destruction of the basal ganglia and the preservation of frontoparietal cortical metabolism. In order to control the status dystonicus, two neurostimulators were implanted in both cerebral thalami. Although this measure partially improved the clinical manifestations, the crises again could not be fully controlled. An intrathecal morphine pump allowed gradual reduction of the cisatracurium and benzodiazepine perfusion doses to the point of suspension of the medication–the patient at present receiving only a 5mg diazepam dose every 8h via the enteral route. Status dystonicus has a very poor prognosis, since all the imaging studies show catastrophic structural damage, with almost complete disappearance of the basal ganglia. However, at present, the neurostimulators, morphine pump, periodic botulinum toxin infiltrations, rehabilitation and drug treatment with diazepam and baclofen afford an acceptable degree of control of the dystonias.
The patient was discharged from the ICU after 11 months of admission fully conscious, oriented and cooperative. He preserves higher mental functions that allow him to play chess and to express and manifest complex emotions. He also has a degree of motor coordination that even allows him to play with a ball and take some steps with help. Within the limitations posed by the tracheostomy cannula, he shows acceptable communication with his family and the health professionals. The patient is currently fed through a percutaneous gastrostomy tube. Phoniatric evaluation is pending for decannulation and the start of an oral diet if swallowing function is adequate.
In conclusion, PET/CT with 18F-2-fluoro-2-deoxy-d-glucose can be used to explore brain metabolism and cortical glucose consumption, discarding or confirming VS–this being very important in deciding the limitation of life-sustaining treatment. Positron emission tomography–computed tomography appears to be a very useful tool for establishing the functional outcome of critical patients with anoxic damage–such assessment being crucial in daily clinical practice.
Financial supportThe authors have received no financial support for carrying out this study.
Please cite this article as: Astola I, Escudero D, Forcelledo L, Viña L, Vigil C, González F. Utilidad del estudio metabólico cerebral con PET/TC 18F-fluorodeoxiglucosa para descartar estado vegetativo. Med Intensiva. 2017;41:127–129.