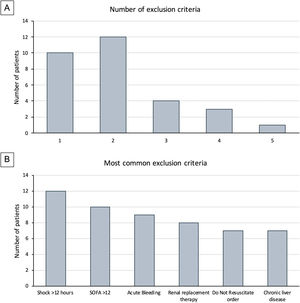
Clinical researchers face numerous challenges, while receiving <2% of the National Institutes of Health funding in previous years.1–5 A search for the terms “critically ill” and “ICU” and “randomized” in PubMed between 2010 and 2021 rendered 2,585 publications; only 3% of those included “cancer.” We conducted a single-center, randomized controlled clinical trial, and included adult cancer patients who met the Sepsis-3 septic shock definition within 12h of ICU admission. Patients were randomized to either standard of care or Early Metabolic Resuscitation (dextrose 50%, amino acids, and micronutrients). The sample size (112 patients, 56 per group) provided 90% power to detect a 30% absolute reduction in 28-day mortality. The complete methodology is available at ClinicalTrials.gov (NCT03895853). During the available enrollment month, 56/194 cancer patients had sepsis and shock. Among them, 32 met inclusion criteria, but 94% had at least one exclusion criteria (Figs. 1A, B and 2); only two patients were enrolled/randomized to standard of care. On May 4, 2020, the study was terminated due to overwhelming obstacles (Fig. 2).
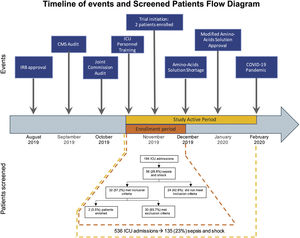
The timeline shows the sequence of events faced by our trial. The study active period went from November 2019 to February 2020. During this period (yellow), 586 patients were admitted to the ICU; of them, 135 patients had sepsis and shock. However, we were able to enroll patients only during the first month of study activity (orange) due to shortage of the amino-acid solution, which was part of the intervention. The flow diagram corresponds to this one-month period.
Despite significant planning, we report the failure of the trial associated with a combination of unforeseen events. Using a previously described matrix, we organized the discussion of these obstacles in six groups2:
- A)
External factors. The study start was delayed for over three months because of two unexpected and consecutive institution-wide audits conducted by the Centers for Medicare and Medicaid Services and the Joint Commission. The audits distracted dozens of teams from clinical research to address the additional workload caused by the regulatory activities.
- B)
Specific unit needs. The complex and intensively involved protocol demanded scrupulous re-education of the participating healthcare personnel due to the delays in starting the trial caused by the audits.
- C)
Study-related factors. Previous studies have identified the difficulty of defining diseases in the intensive care environment.3 Defining sepsis and septic shock has been a critical point of discussion for the experts in the field that has evolved over time. In our study, almost half of the patients did not meet the Sepsis-3 criteria because 43% had normal lactate despite shock and vasopressors. Moreover, between the Sepsis-3 definition and the exclusion criteria, most of the screened patients (96.4%) became ineligible, thus impacting enrollment and potential generalizability of the trial.4
- D)
Study population. Our institution is a referral comprehensive cancer center. We manage high volumes of complex cancer patients that are admitted to our unit, many of whom arrive in multi-organ failure with SOFA scores above 12 and Do-Not-Resuscitate (DNR) orders (21.9%) at the time of diagnosis. Both the SOFA and DNR status are frequent reasons for the exclusion of critically ill cancer patients in clinical trials.
- E)
Resources, including an unexpected national shortage of the branched-chain amino acid solution after enrolling two patients, forced us to stop enrollment. Once the protocol was ready to restart enrollment, another external and unexpected factor surfaced. The COVID-19 pandemic was declared, and all clinical research activities in the hospital were paused. As the study prolonged, our funding could no longer sustain the 4-member team, which was created to recruit and enroll patients 24/7, as Francois et al. suggested.6
- F)
Clinicians. A sudden deterioration of the health condition of the primary investigator led to his leave of absence during the pandemic.
From the above factors, the study-related category encompasses a challenge that should be thoroughly addressed when planning a randomized controlled trial. The difficulty of finding eligible patients is not a phenomenon unique to our trial.7 Therefore, we suggest that anticipating the number of potential participants is essential. Additionally, we encourage the investigators to perform an exhaustive run-through screening which could illustrate the times of the day when patients become eligible and the most common reasons for exclusion. As specific unit transfer dynamics differ widely, knowing the best times for recruitment before starting the trial would allow planning to facilitate the enrollment process. This will guide the investigators in determining whether 24/7 coverage is necessary. Additionally, we recommend examining the reasons for exclusions of the simulated screening, which can also facilitate discussions on whether the exclusion criteria could be loosened up to increase enrollment rate without compromising safety.
Among the obstacles described, the main reason for the termination of the trial was the COVID-19 crisis. The pandemic has had a devastating effect on the research community as a whole. Both basic science and clinical research have been disrupted by the reasonable efforts taken to protect all the stakeholders and prevent the spread of the virus. However, stopping operations of clinical trials that were addressing vital and relevant clinical problems is an issue by itself,8 particularly the studies involving vulnerable populations. By February 2021, over 1200 clinical trials had reported a disruption of their operations, most of them even suspending the enrollment altogether.9 The pandemic has shown the multiple ways that the designing, conducting, and reporting of clinical trials can improve.8 Furthermore, as the research community has been adapting to the pandemic, the number of clinical trials that have resumed enrollment has started to rise. The lessons learned will make researchers embark in modern ways to streamline processes that could result in a more efficient and resilient community in the future.
The complexity and prohibitive costs of clinical trials have increased exponentially in the last few years. Our tale should be a cautionary message for future study designs in this population. In addition to the increasing internal and external regulatory hurdles or unforeseeable chain of unfortunate events, enrolling critically ill cancer patients in clinical trials is extremely challenging with rates as low as 2–5% reported in previous studies.1 Some of these events are rare, and robust mitigation plans are not cost-effective or feasible; therefore, only limited precautions can be put in place. Prior feasibility studies, far-sighted planning of study implementation, enrollment forecasting, and allocation of the needed resources have never been more important. In fact, all of them are essential if we want to prevent continuously excluding this population from future trials.
Authors’ contributionsDoctors Nates and de Villalobos had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data. Concept and design: de Villalobos, Nates. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: All authors. Critical revision of the manuscript for important intellectual content: de Villalobos, Nates. Obtained funding: de Villalobos, Nates. Supervision: de Villalobos, Nates.
FundingThis work was supported by The University of Texas MD Anderson Cancer Center Grant Resources, and the NIH/NCI award number P30CA016672. The funders had no role in the design and conduct of the study, preparation of the manuscript, and the decision to submit the manuscript for publication.
Conflict of interestsThe authors declare that there is no conflict of interest.
The authors want to thank the language assistance of Mrs. Mary Ann Oler, BA, MPA; Education Program Coordinator for the Department of Critical Care Medicine at The University of Texas MD Anderson Cancer Center.