Severe head injuries have a great socioeconomic and public health impact. Despite progress in diagnosis and treatment, no sufficiently reliable predictive models have been established for developing clinical trials and promoting effective therapeutic strategies capable of improving the prognosis. In the last decades, several brain damage biomarkers have been studied as potential diagnostic and prognostic tools in traumatic brain injury. However, all of them have limitations that preclude their universalized application. The properties of the known biomarkers – both those traditionally shown to correlate with severity and prognosis, and those recently announced as promising options – should be analyzed. New studies are needed to define their properties, both isolatedly and in combined use.
El traumatismo craneoencefálico grave es una entidad clínica con gran repercusión en términos socioeconómicos y de salud pública. Pese a los avances obtenidos en el ámbito del diagnóstico y tratamiento, no se han consolidado modelos predictivos suficientemente fiables que permitan desarrollar ensayos clínicos e impulsen estrategias terapéuticas efectivas que mejoren su pronóstico. En este sentido, durante las últimas décadas se han estudiado diversos biomarcadores de lesión cerebral con el fin de establecerlos como herramientas diagnósticas y pronósticas de la lesión traumática cerebral. Sin embargo, todos ellos presentan alguna limitación que impide su aplicación universalizada. Es necesario analizar las propiedades de los biomarcadores conocidos hasta la fecha, tanto los que tradicionalmente han demostrado correlación con la gravedad y pronóstico como aquellos que recientemente se anuncian prometedores. Para ello, convendría diseñar nuevos estudios que definan sus propiedades de forma aislada y que diluciden el papel de su uso combinado.
Severe traumatic brain injury (TBI) remains an important public health problem, due to the large percentage of unfavorable outcomes involved (death and disabling sequelae) and the great associated treatment costs, compensations, disability pensions and years of work lost in affected individuals fundamentally belonging to the active population.1,2
Although TBI is an extremely complex condition,3 there have been many advances in recent years in relation to the diagnosis, monitoring and treatment of the affected patients.4,5 However, given the heterogeneity of severe TBI, there are still important shortcomings in our knowledge of the physiopathology of TBI and the development of reliable predictive models capable of offering an early orientation as to the patient outcome, with the purpose of improving the diagnostic and therapeutic strategies on an individualized basis. Likewise, we need valid predictive models in severe TBI in order to define efficacy endpoints in the evaluation of new drugs or treatment strategies–since the usual primary endpoints (death and disability) are widely recognized as being inadequate and could explain the discouraging results obtained with certain promising drugs.6
Considering the above, and in the same way as in other disease processes, such as ischemic heart disease, research is carried out to identify biological markers that could offer a more precise indication of the extent and severity of TBI, independently of the prior biological substrate and of other circumstances that accompany severe TBI–thereby contributing to homogeneously define different patient categories. Such markers would not only facilitate individualization of the intensity and timing of patient management but could also contribute to the development of strategies for preventing the consolidation of injury and enhancing neuroprotective effects capable of avoiding or minimizing secondary damage.
The present study offers a critical review of the main brain damage biomarkers studied till date.
Brain damage biomarkersA biomarker is defined as a quantifiable biological indicator specific of a given physiological or pathological condition. Vos concluded that the use of biomarkers contributes to improve knowledge of the physiopathology of brain damage, affording essential complementary information for the diagnosis and for predicting the outcome of these patients.7 However, the definition of a brain damage marker must establish differentiations with respect to other alterations, since the central nervous system (CNS) is very complex and can present a range of different lesions, which in turn can affect different target cells with variable degrees of severity. Furthermore, the existence of the blood–brain barrier (BBB) conditions the structural characteristics of these biomarkers, which must be able to cross the mentioned barrier in order to reach the bloodstream.
Over 20 years ago, the ideal TBI biomarker was defined as an indicator with high specificity and sensitivity for the brain tissue, with release occurring only after irreversible brain tissue damage, and with rapid appearance in both cerebrospinal fluid (CSF) and blood after damage. The marker moreover must reflect the extent and severity of the damage, following a known time course. In turn, the marker variations between age and gender groups must be minimal. On the other hand, the tools for analysis and immediate detection of the marker must be available and reproducible. Lastly, and most importantly, determination of the marker must be clinically relevant. It should be underscored that biomarkers are dynamic elements that experience changes in response to different inflammatory states, tissue necrosis phenomena and damage caused by oxidative stress.3 Serial measurements rather than isolated or point determinations are thus required in order for the collected data to be of practical significance.
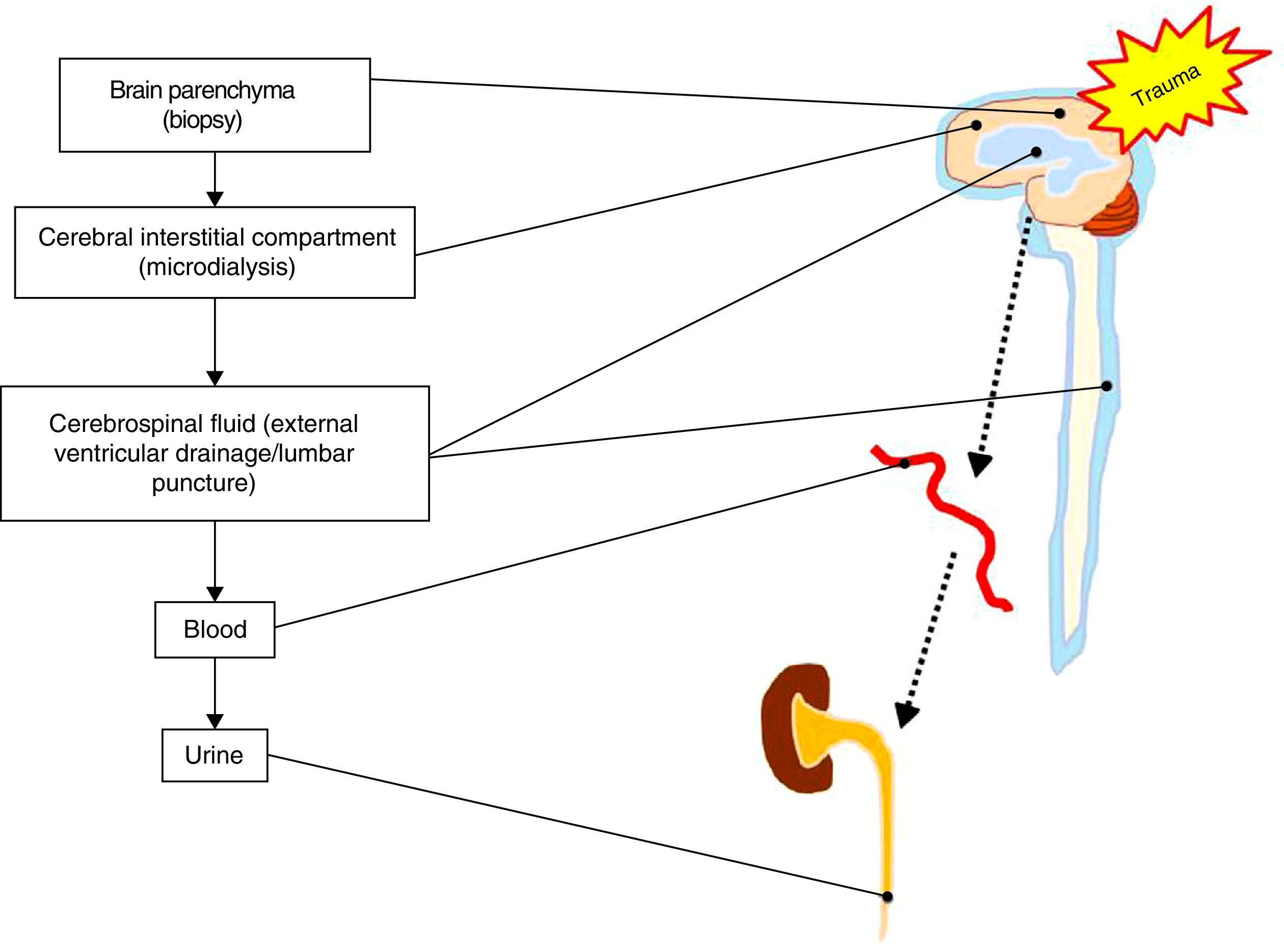
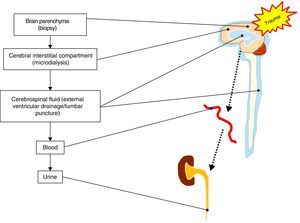
On the other hand, there is some controversy regarding the type of biological fluid that should be analyzed. Direct sampling of the damaged brain tissue or of brain tissue at risk is not plausible, though it would be the only source of biomarkers affording unequivocal and direct information on the changes occurring after severe TBI. The rest of the determinations are conditioned to the mechanism underlying biomarker release (passive or active), the crossing of membranes and barriers (cell membrane, BBB, etc.), and dilution phenomena once the systemic compartment has been reached.8 In this regard, techniques such as microdialysis can be used to determine metabolites and biomarkers corresponding to the cerebral interstitial compartment or space.9 On the other hand, the CSF compartment is located closer to the damage site; measurements at this level are therefore not conditioned by integrity of the BBB. However, the collection of CSF samples involves problems in terms of accessibility and availability, with the need for invasive maneuvers which are often contraindicated in patients with severe TBI. As a result, most biomarkers are studied in peripheral blood, since the technique in this case is simple, accessible and reproducible. In view of the above, a brain damage biomarker that is released into the bloodstream is the most appropriate option for performing simple and minimally invasive serial measurements. In turn, it is also interesting to study the determination of biomarkers in fluids that serve as vehicles for their clearance, for example urine10 (see Fig. 1).
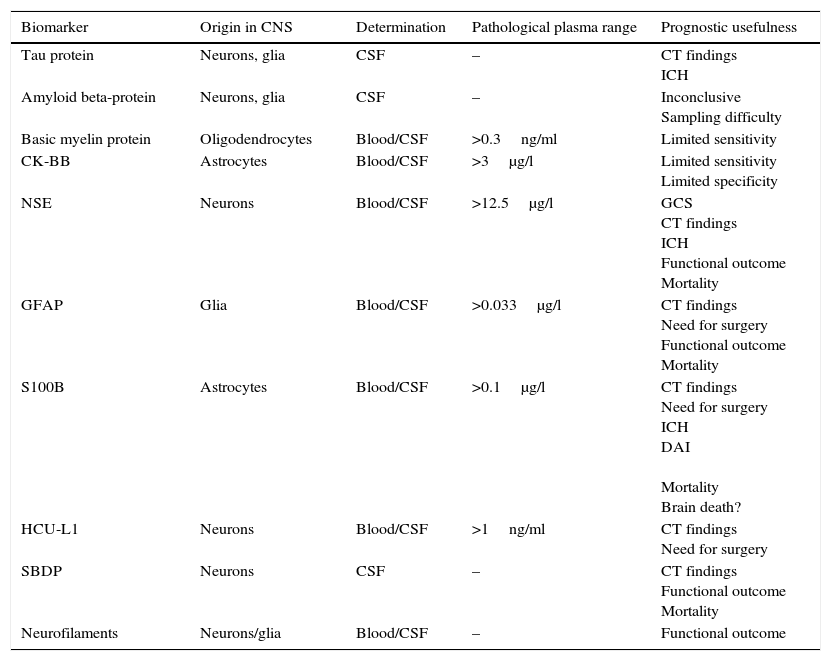
A description is provided below of the main brain damage biomarkers, the tissues in which they originate, the compartment in which samples are collected to determine them, their pathological serum concentrations, and their main prognostic features (Table 1).
Principal biomarkers of central nervous system damage.
| Biomarker | Origin in CNS | Determination | Pathological plasma range | Prognostic usefulness |
|---|---|---|---|---|
| Tau protein | Neurons, glia | CSF | – | CT findings ICH |
| Amyloid beta-protein | Neurons, glia | CSF | – | Inconclusive Sampling difficulty |
| Basic myelin protein | Oligodendrocytes | Blood/CSF | >0.3ng/ml | Limited sensitivity |
| CK-BB | Astrocytes | Blood/CSF | >3μg/l | Limited sensitivity Limited specificity |
| NSE | Neurons | Blood/CSF | >12.5μg/l | GCS CT findings ICH Functional outcome Mortality |
| GFAP | Glia | Blood/CSF | >0.033μg/l | CT findings Need for surgery Functional outcome Mortality |
| S100B | Astrocytes | Blood/CSF | >0.1μg/l | CT findings Need for surgery ICH DAI Mortality Brain death? |
| HCU-L1 | Neurons | Blood/CSF | >1ng/ml | CT findings Need for surgery |
| SBDP | Neurons | CSF | – | CT findings Functional outcome Mortality |
| Neurofilaments | Neurons/glia | Blood/CSF | – | Functional outcome |
CK-BB: cerebral creatine kinase isoenzyme; GCS: Glasgow Coma Score; GFAP: glial fibrillary acidic protein; HCU-L1: ubiquitin carboxy-terminal hydrolase L1; ICH: intracranial hypertension; DAI: diffuse axonal injury; CSF: cerebrospinal fluid; NSE: neurospecific enolase; SBDP: spectrin degradation products; CNS: central nervous system; S100B: S100 beta-protein; CT: computed tomography.
Tau protein is an axonal cytoskeleton-stabilizing protein that conforms tubular junctions, structural elements of the cytoskeleton and elements that are crucial for neuronal protein flow. There are 6 different tau protein isomers, and the action of proteases induced as a result of trauma results in the release of tau protein fragments into the serum and into the CSF in cleaved tau (c-tau) form, which has a molecular weight of 30–50kDa.11 Studies in humans have shown the c-tau levels in CSF to increase in the first 24h after severe TBI. Zemlan et al. correlated c-tau to the development of intracranial hypertension (ICH) and to patient functional outcome over the short term.12 Other studies have associated the c-tau concentration in CSF to the initial radiological findings and to patient functional outcome after one year (Glasgow Outcome Scale-extended).13 However, the practical role of this molecule has not been fully established, since the significant results have been obtained from measurements made in CSF samples–with the logistic difficulties its involves.
Amyloid beta-proteinAmyloid precursor protein takes part in cell adhesion processes. High concentrations of this protein are therefore found at neuronal synaptic junction level. Its precursor form is the target of a certain type of caspase that breaks it down into a series of products. The latter in turn accumulate on cell bodies (somas) and axons as part of a phenomenon that intervenes in certain neurodegenerative disorders such as Alzheimer's disease, Creutzfeldt-Jakob disease or amyotrophic lateral sclerosis.14 A study of 29 patients with severe TBI revealed low levels of this protein in CSF, and concluded that this could be explained by reabsorption of the protein in the form of amyloid plaques.15 In contraposition to the above, Emmerling et al. recorded increased levels in CSF after trauma, and suggested that this could be a result of secondary axonal damage or loss of integrity of the BBB.16 These contradictory findings, which may be related to the different CSF sampling methods used (lumbar, ventricular, etc.), have caused amyloid beta-protein to be regarded as a scantly reproducible biomarker, and its potential role is not clear.
Basic myelin proteinWith a molecular weight of 18.5kDa, basic myelin protein is found at oligodendroglial level, and is a myelin-specific protein that is released into the bloodstream in situations of brain damage and demyelinating diseases.17 With regard to its usefulness in TBI, some studies have correlated the serum levels of this protein to patient severity and outcome. However, the existing publications in this respect are few and old, with small sample sizes, and with results that reflect high specificity but limited sensitivity.18
Cerebral creatine kinase isoenzymeThere are three creatine kinase isoforms: muscular, cardiac and brain tissue-specific creatine kinase. The latter, with a molecular weight of 40–53kDa, is found in astrocytes, though it has also been identified in intestinal, prostatic, uterine and vascular tissues. A peak in serum cerebral creatine kinase concentration is observed in the first few hours after severe TBI. The levels then gradually decrease, except in individuals with potentially serious injuries, where the marker remains high for days.19 Serum cerebral creatine kinase has low sensitivity and specificity–this being the main factor limiting its routine use as a biomarker.17
Neurospecific enolaseNeurospecific enolase (NSE) is a glycolytic enzyme with a molecular weight of 78kDa and a half-life of 48h.20 Its γ–γ isoform is specific of neuronal tissue, while the α–γ isoform is found in endocrine cells, erythrocytes and platelets.21 The normal concentration in blood is <10ng/ml.17,22 Increased levels of NSE have been recorded in the serum of patients following non-traumatic brain damage such as ischemic events, intracerebral hemorrhage or cardiopulmonary resuscitation maneuvering with secondary cerebral hypoxia.23 Experimental models of trauma have correlated serum NSE to the severity of damage in TBI.24 We developed a murine model of mass-type injury in which a linear release of NSE was observed after the controlled induction of intracranial hypertension.25 In relation to clinical studies, an inverse correlation has been described between the plasma NSE concentration and the Glasgow Coma Score (GCS). On the other hand, NSE has been associated to the initial radiological findings, and has been proposed as a potent predictor of poor outcome and mortality.26,27 Some studies have related this biomarker to the development of diffuse axonal injury (DAI), though its behavior in humans in the presence of intracranial hypertension has not been clearly established.28
Neurospecific enolase was initially suggested to be a very promising TBI severity marker, since it is more specific of neuronal tissue than of glial cells. However, the results published till date have been contradictory–some studies having reported no correlation to the clinical prognosis of patients with severe TBI.29 On the other hand, the long half-life of NSE questions its applicability in monitoring the time course of progressing brain injuries, as well as its usefulness in validating treatment efficacy. Although initially considered to be specific of the nervous system, its extracranial origin has subsequently been demonstrated in processes such as hemorrhagic shock, long bone fracture, hemolysis, heart surgery, ischemia–reperfusion injury and malignant lung tumors. This situation implies limitations in interpreting the levels of the marker.30 At present, NSE has become established as a neoplastic disease biomarker in the clinical setting.31
Glial fibrillary acidic proteinGlial fibrillary acidic protein is derived from astrocytic tissue, and is characterized by brain-specific expression and release. This defines it as an exclusive biomarker of brain damage in different situations: trauma, ischemic events and in certain neurodegenerative processes.32 It is a monomeric protein with a molecular weight of 52kDa, and enters the bloodstream after crossing the BBB following brain injury–exhibiting an early plasma peak on the first day.33 The blood levels then decrease gradually over the first week, from day three post-injury. Plasma concentrations >0.033μg/l are regarded as pathological.33 Missler et al. were the first to propose the possible use of glial fibrillary acidic protein as an identifier of brain damage in serial serum measurements.34 Later studies confirmed that the serum concentration of this protein is not conditioned by extracranial injuries.35 Glial fibrillary acidic protein has been correlated to a poor outcome in the acute phase of severe TBI, radiological lesions evidenced by tomographic scans, the need for urgent neurosurgery, long-term functional outcome, and patient mortality.36,37 All these features suggest that glial fibrillary acidic protein might be an ideal biomarker of brain damage–though multicenter studies are needed to confirm this possibility.
S100 beta-proteinS100 beta-protein (S100B) is the most widely studied brain damage biomarker. It is a low molecular weight (20kDa) calcium binding protein, with three subtypes derived from dimeric combinations of the α and β chains. The beta-subtype comprises the β chain in αβ heterodimer or ββ homodimer combination, with a molecular weight of 10–12kDa, and is synthesized in the astrocytes of the central nervous system and in the Schwann cells of the peripheral nerves.38 The biological function of this protein has not been fully established till date, though it is known to participate in neurogenesis, astrocytosis, axonal elongation, signal transduction through the inhibition of phosphorylation proteins, the regulation of enzyme activity, calcium homeostasis, interaction with cytoskeletal elements (regulating cell morphology), and in protecting mechanisms against cell oxidation damage.3,39 At low concentrations in the extracellular compartment, S100 beta-protein favors cell differentiation and growth, while at high concentrations it induces apoptotic phenomena.39 On the other hand, the molecule can be produced and found outside the central nervous system, e.g., in adipocytes, chondrocytes, melanocytes and hematopoietic cells.40 Metabolization takes place in the kidneys, followed by excretion in urine, with an approximate half-life of 30–113min, and is not affected by hemolytic phenomena.41 The maximum serum concentration is reached 20min after brain damage, i.e., the marker is detectable practically from the time of patient admission to hospital.42 The upper limit of normal for this protein in relation to the detection of intracranial damage was defined as 0.1μg/l by Biberthaler et al., based on a multicenter study in patients with mild TBI.43 On examining the information from other studies, plasma levels of over 0.13–0.20μg/l can be regarded as pathological.44 The measurement of S100 beta-protein can be influenced by patient age and gender in CSF samples but not in serum samples–a fact that confers an added advantage to the latter type of sample.45
Experimental models have demonstrated the usefulness of S100 beta-protein as a marker of brain damage.25,46 With regard to its clinical usefulness, its behavior has been described in acute neurological processes such as ischemic stroke and hemorrhagic stroke, as well as in other situations where secondary brain involvement can be observed, such as during heart surgery or spontaneous aneurysmal rupture.47
In relation to severe TBI, the serum levels of the protein have been associated to clinical severity, radiological severity, and an unfavorable outcome.6,44 Some studies have determined its usefulness as a predictor of mortality, establishing orientative serum cutoff points for predicting a course leading to death or an unfavorable outcome.48 On the other hand, S100 beta-protein has been correlated to the presence of secondary lesions, the extent of diffuse brain damage, and to modifications in intracranial pressure following different release patterns.49,50 Another possible application of this protein refers to its time course according to the severity of the patient condition. In this respect, a number of studies have documented persistently elevated serum levels in patients who do not survive, while the plasma levels have been seen to decrease after 36h among survivors.36 Likewise, the appearance of secondary serum S100 beta-protein peaks after the initial peak has been described in patients with a negative outcome.36 In relation to the above, there is some controversy regarding the role of the BBB, since changes in barrier permeability secondary to TBI could explain protein release patterns according to the time course; accordingly, traumatic loss of BBB integrity is not the only implicated physiopathological mechanism.8 On the other hand, S100 beta-protein has been suggested as a tool for monitoring management efficacy, since it has been seen that the blood concentrations of the protein decrease after effective neurosurgical treatment.48 Other studies have obtained interesting results in terms of the long-term functional outcome.29,51
In our experience, S100 beta-protein has demonstrated its usefulness in identifying patients with intracerebral lesions on the computed tomography scan and good initial neurological grading,44 and has afforded relevant results in terms of the long-term prognosis of patients with severe TBI.52 In a recent study we established a plasma cutoff point in the first 6h after brain damage capable of predicting mortality with a sensitivity of 90%. In addition, we defined a cutoff point in urine samples, though of lesser significance than in the case of blood samples.10 Lastly, we have obtained new data in patients with severe TBI that evolved toward brain death,53 in a study that may represent an important preliminary step toward future lines of research designed to develop early and effective management strategies, as well as to screen for potential organ donors.
Despite its high sensitivity and negative predictive value, S100 beta-protein is not a specific marker of the central nervous system. In this regard, it must be mentioned that polytraumatized patients without TBI can present S100 beta-protein elevations in blood, though the concentrations return to normal within 6h after trauma.54 On the other hand, patients with brain damage and associated extracranial injuries often present a series of physiopathological circumstances upon admission (hypotension, hypothermia, coagulopathy, inotropic drugs, sedatives, corticosteroids, etc.) that can alter the early assessment of S100 beta-protein, since secondary brain damage also would be progressing. Therefore, early determination of this protein is to be avoided in patients with extracranial injuries associated to TBI.53
New biomarkers of interestUbiquitin carboxy-terminal hydrolase L1Ubiquitin carboxy-terminal hydrolase L1 is a protein of neuronal origin representing approximately 5% of all the soluble brain proteins. It is implicated in the elimination of degraded and denaturalized proteins following oxidative phenomena. At present, some studies point to it as a promising brain damage biomarker, since there are data indicating that it is able to predict the presence of lesions on the computed tomography scan, the need for neurosurgery, and the outcome of patients with TBI.55 Mondello et al. have obtained interesting results regarding its possible capacity to distinguish between local and diffuse brain damage.56 Furthermore, ubiquitin carboxy-terminal hydrolase L1 can be detected in blood, with early increases in its serum concentration following brain injury. The marker can be determined during approximately 168h, though in cases of increased severity or the development of secondary lesions the levels remain elevated for longer periods of time.55 For the time being, most of the available information comes from a single research group, and considering the relevance of the results, further investigations are needed to corroborate the properties of this protein as a biomarker of brain damage.
Spectrin degradation productsSpectrin is a structural protein and a principal component of the cortical cytoskeleton of red blood cells. It is composed of two α and two β chains. Spectrin can undergo proteolysis mediated by calpain and caspase-3, which trigger necrotic and apoptotic processes, respectively.57 The presence of degradation products of spectrin has been described n the central nervous system in axons and presynaptic neuronal endings. Different authors have demonstrated an increase in spectrin degradation products determined in CSF in the context of TBI–underscoring its role as a predictor of severity and mortality in patients of this kind.58
NeurofilamentsThe light neurofilaments (68kDa) form part of the axonal cytoskeleton. In the case of axon damage, these proteins are released into both CSF and the bloodstream. A number of investigational studies have underscored the role of neurofilaments as biomarkers of axon damage and neurological disability in neurodegenerative and neuroinflammatory diseases.59 With regard to their role in TBI, an increase in the concentration of these proteins has been described as a consequence of axon damage, and has been correlated to patient outcome.60
ConclusionsSince all brain damage biomarkers have some limitation precluding their universal application in the management of severe TBI, they do not yet form part of routine clinical practice. Some markers, such as NSE and S100 beta-protein, have shown good correlations to clinical severity, the extent of brain damage, response to treatment, and patient outcome. However, the limitations associated to clinical yield of the molecule or invasiveness of the technique required to obtain the sample have not allowed their generalized use in this patient population.
On the other hand, further studies are needed to understand the role of these proteins in the physiology of the central nervous system and in the physiopathology of severe TBI, as well as to clarify the usefulness of those biomarkers that appear to be promising in this field. In this respect, mention must be made of nervous tissue-specific glial fibrillary acidic protein, as well as of other biomarkers that are currently the focus of interest, such as ubiquitin carboxy-terminal hydrolase L1, the light neurofilaments and spectrin degradation products. Since these molecules offer isolated information on some of the many elements implicated in the physiopathology of TBI, we believe that the best strategy is to analyze them in combination. Rather than seeking a biomarker exclusive of brain damage, this approach would allow us to define a panel of biomarkers which jointly – and considering the characteristics inherent to each of them–could offer information referred to severity, the potential benefits of management, and the evolutive course of patients following severe TBI. Only in this way can we hope to complement the traditional methods with a tool that is simple, noninvasive, reproducible and extraordinarily useful for addressing and managing severe TBI.
Financial supportNone.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Gordillo-Escobar E, Egea-Guerrero JJ, Rodríguez-Rodríguez A, Murillo-Cabezas F. Utilidad de los biomarcadores en el pronóstico del traumatismo craneoencefálico grave. Med Intensiva. 2016;40:105–112.