To evaluate the usefulness of procalcitonin (PCT) for diagnosing infection in patients with liver cirrhosis admitted to an Intensive Care Unit.
DesignA retrospective study was carried out.
ScopeIntensive Care Unit. Versatile, twenty-four beds.
ParticipantsPatients with liver cirrhosis admitted to our Intensive Care Unit in the last 4 years with suspected infection and measurement of PCT.
ResultsAmong the 255 patients with cirrhosis admitted to our unit, PCT was determined for the differential diagnosis of infection in 69 cases (27%). Three patients were excluded from analysis due to a lack of clinical data. The average stay was 10.6±9.2 days, with a mortality rate of 65%. The origin of cirrhosis was mainly viral (57%) or alcoholic (37%). The Child–Pugh and MELD scores were 9.5±2 and 23±8, respectively. Infection was diagnosed in 54 patients (82%). The most common infection was pneumonia (72%), followed by intraabdominal infections (18%) and bacteremia (5%). In patients without infection, the median PCT concentration was 0.57ng/ml (range 0.28–1.14) versus 2.99 (1.31–9.4) in those with infection (p<.001). Diagnostic capacity was maintained in patients with intraabdominal infections. The diagnostic cutoff point was set at 0.8ng/ml (sensitivity 83%, specificity 75%, AUC 0.82 [0.702–0.93]).
ConclusionsIn patients with liver cirrhosis, PCT is useful for identifying bacterial infections, including intraabdominal processes.
Analizar la utilidad de la procalcitonina (PCT) para el diagnóstico de infección en pacientes con cirrosis hepática ingresados en una unidad de cuidados intensivos.
DiseñoEstudio observacional retrospectivo.
ÁmbitoUnidad de Cuidados intensivos. Polivalente, 24 camas.
ParticipantesPacientes con cirrosis hepática ingresados en nuestra unidad de cuidados intensivos en los últimos 4 años con diagnóstico de sospecha de infección y determinación de PCT.
ResultadosEntre los 255 pacientes con cirrosis ingresados en nuestra unidad; se determinó la PCT para el diagnóstico diferencial de infección en 69 casos (27%). Tres pacientes fueron excluidos del análisis por falta de datos clínicos. La estancia media fue de 10,6±9,2 días y la mortalidad del 65%. El origen de la cirrosis fue vírico (57%) o enólico (37%), con una puntuación de 9,5±2 en la escala de Child-Pugh y 23±8 en la escala de MELD. En 54 pacientes (82%) se estableció el diagnóstico de infección. La infección más frecuente fue la neumonía (72%), seguida de la infección intraabdominal (18%), y la bacteriemia (5%). En los pacientes sin infección la mediana de PCT fue de 0,57ng/ml (0,28-1,14) frente a 2,99 (1,31-9,4) p<0,001 en aquellos con infección. La capacidad diagnóstica se mantuvo en los pacientes con infección intraabdominal. El punto de corte diagnóstico se estableció en 0,8ng/ml (sensibilidad 83%, especificidad 75%, AUC 0,82 [0,702-0,93]).
ConclusionesEn los pacientes con cirrosis hepática la PCT es útil para identificar la presencia de infecciones bacterianas incluyendo las intraabdominales.
Bacterial infections are among the most frequent and serious complications in patients with liver cirrhosis.1 In these individuals the presence of iatrogenic factors (diagnostic and therapeutic), and the alteration of different immune mechanisms, favor the development of infections.1,2 On the other hand, the diminished intestinal motility that characterizes these patients, together with hypochlorhydria and lowered intestinal IgA levels, facilitate intestinal bacterial overgrowth.2 This situation, together with disruption of the intestinal epithelium secondary to the increase in nitric oxide resulting from portal hypertension, allow bacterial translocation and ultimately bacteremia (or endotoxinemia) and spontaneous bacterial peritonitis.3–5
In patients with liver cirrhosis, infection is often accompanied by organ dysfunction (in many cases secondary to decompensation of cirrhosis itself), which increases the risk of a fatal outcome.
It is therefore very important to establish an early and firm diagnosis of bacterial infection in cirrhotic individuals. However, the signs and symptoms inherent to the infection are often missing or are difficult to identify in these subjects.6,7 The use of infection biomarkers in the diagnostic algorithm of patients with cirrhosis is therefore particularly interesting.
C-reactive protein (CRP) is synthesized in the liver; as a result, its usefulness in patients of this kind is uncertain.8,9 In contrast, procalcitonin (PCT) is produced in many tissues10–13 and moreover appears to have a greater diagnostic capacity than CRP.14 However, since PCT requires the existence of a systemic inflammatory response, it might prove ineffective in diagnosing localized infections such as spontaneous bacterial peritonitis.
With the purpose of determining the usefulness of PCT in diagnosing bacterial infections in patients with liver cirrhosis, we decided to review the information from all the patients with liver cirrhosis and PCT determinations that had been admitted to our Intensive Care Unit (ICU).
Material and methodsType of study: A retrospective, consecutive cases study was carried out.
Inclusion criteria: Patients over 18 years of age with confirmed liver cirrhosis admitted to the ICU over the last four years, and who in the course of admission had undergone PCT determination within the first 12h following the suspicion of infection. We excluded those patients in which the data contained in the case history could not firmly establish whether there had been infection during admission to the ICU or not.
Study variables: Demographic data were compiled, along with the medical history, information on the liver disease (etiology, Child–Pugh score, MELD score, treatments provided), other organ dysfunction, APACHE II score and SOFA score, and data related to infection (temperature, leukocyte count, neutrophil count, PCT).
Diagnosis of liver cirrhosis: The diagnosis was established by specialists in liver disease based on an algorithm including liver Doppler ultrasound and laboratory test parameters for determining the degree of liver dysfunction and its cause.
Diagnosis of infection: The patient medical histories were examined to detect the diagnostic criteria for infection established by the Centers for Disease Control (CDC) and Prevention (Atlanta, USA) in chronological association to the determination of PCT.15
Spontaneous bacterial peritonitis (SBP) was defined as infection of the abdominal cavity with no apparent cause and meeting the following criteria: (1) over 250 polymorphonuclear cells/mm3 of ascitic fluid; and (2) positive ascitic fluid culture findings.15
The patients were divided into two groups according to the presence or absence of infection.
Determination of procalcitonin: Serum PCT was determined within the first 12h following the suspicion of infection, using TRACE (Time-Resolved Amplified Cryptate Emission) in a Kryptor analyzer (Brahms Diagnostica, Berlin, Germany).
Data collection and statistical analysis. The data were processed using the SPSS version 17.0 statistical package. Calculations were made of the mean, median, standard deviation, and maximum and minimum values for continuous variables, and of the absolute and relative frequencies for categorical variables. Categorical variables were compared using the chi-squared test and fisher exact test, as applicable. Comparison of numerical and categorical variables was made using the Mann–Whitney U-test in the event the second variable was dichotomic in nature, and the Kruskal–Wallis test in the event the mentioned variable had more than two categories. Statistical significance was considered for p<0.05. The sensitivity and specificity of PCT were determined by comparing the patients with infection according to the criteria of the CDC versus those without infection. The optimum cutoff point was calculated from the receiver operating characteristic (ROC) curves.
ResultsGeneral characteristicsDuring the study period, a total of 255 patients diagnosed with liver cirrhosis were admitted to the ICU of our hospital. In 73% of the cases admission was related to decompensation of the liver disease. In 69 cases (27%) at least one PCT measurement was made in the context of the differential diagnosis of infection. Three patients in which the data contained in the case history could not firmly establish whether there had been infection during admission to the ICU or not were excluded.
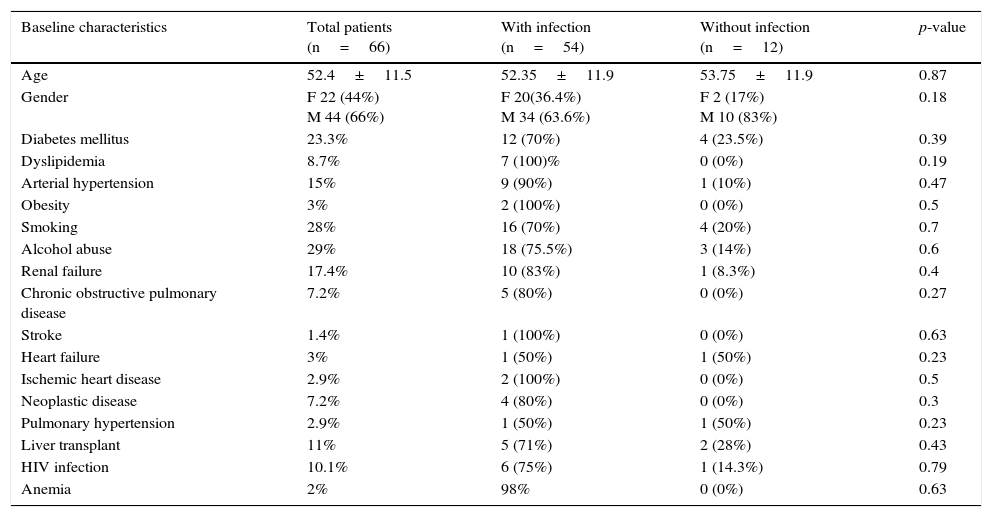
The characteristics of the patients included in the study are shown in Table 1. Most were males, with a relatively low mean age (52.4±11.5 years). The most common disease antecedent was diabetes mellitus (23.3%), with a notable persistence of toxic habits such as alcohol abuse (29%) or smoking (28%). Eleven percent of the patients (n=7) had undergone liver transplantation and suffered liver cirrhosis relapse affecting the graft.
General characteristics of the study population.
| Baseline characteristics | Total patients (n=66) | With infection (n=54) | Without infection (n=12) | p-value |
|---|---|---|---|---|
| Age | 52.4±11.5 | 52.35±11.9 | 53.75±11.9 | 0.87 |
| Gender | F 22 (44%) M 44 (66%) | F 20(36.4%) M 34 (63.6%) | F 2 (17%) M 10 (83%) | 0.18 |
| Diabetes mellitus | 23.3% | 12 (70%) | 4 (23.5%) | 0.39 |
| Dyslipidemia | 8.7% | 7 (100)% | 0 (0%) | 0.19 |
| Arterial hypertension | 15% | 9 (90%) | 1 (10%) | 0.47 |
| Obesity | 3% | 2 (100%) | 0 (0%) | 0.5 |
| Smoking | 28% | 16 (70%) | 4 (20%) | 0.7 |
| Alcohol abuse | 29% | 18 (75.5%) | 3 (14%) | 0.6 |
| Renal failure | 17.4% | 10 (83%) | 1 (8.3%) | 0.4 |
| Chronic obstructive pulmonary disease | 7.2% | 5 (80%) | 0 (0%) | 0.27 |
| Stroke | 1.4% | 1 (100%) | 0 (0%) | 0.63 |
| Heart failure | 3% | 1 (50%) | 1 (50%) | 0.23 |
| Ischemic heart disease | 2.9% | 2 (100%) | 0 (0%) | 0.5 |
| Neoplastic disease | 7.2% | 4 (80%) | 0 (0%) | 0.3 |
| Pulmonary hypertension | 2.9% | 1 (50%) | 1 (50%) | 0.23 |
| Liver transplant | 11% | 5 (71%) | 2 (28%) | 0.43 |
| HIV infection | 10.1% | 6 (75%) | 1 (14.3%) | 0.79 |
| Anemia | 2% | 98% | 0 (0%) | 0.63 |
Cirrhosis was of viral origin (HCV or HBV) in 57% of the cases, alcoholic in 37%, autoimmune in 3%, and involved other causes (including Budd-Chiari syndrome) in 3% of the patients. With regard to the grade of cirrhosis, the mean Child–Pugh score was 9.5±2, with a mean MELD score of 23±8 (Table 2).
The mortality rate was high (62.9%, n=43), and most of the deaths (87%, n=40) occurred during admission to the ICU. The remaining 13% (n=6) of fatal outcomes occurred during the same hospital admission, though outside the ICU. Two patients underwent liver transplantation but died in the resuscitation unit.
Diagnosis of infectionOf the 66 patients included in our study, 82% (n=54) met the criteria of infection. According to the established criteria, 74.5% of these patients (n=41) suffered severe sepsis or septic shock.16
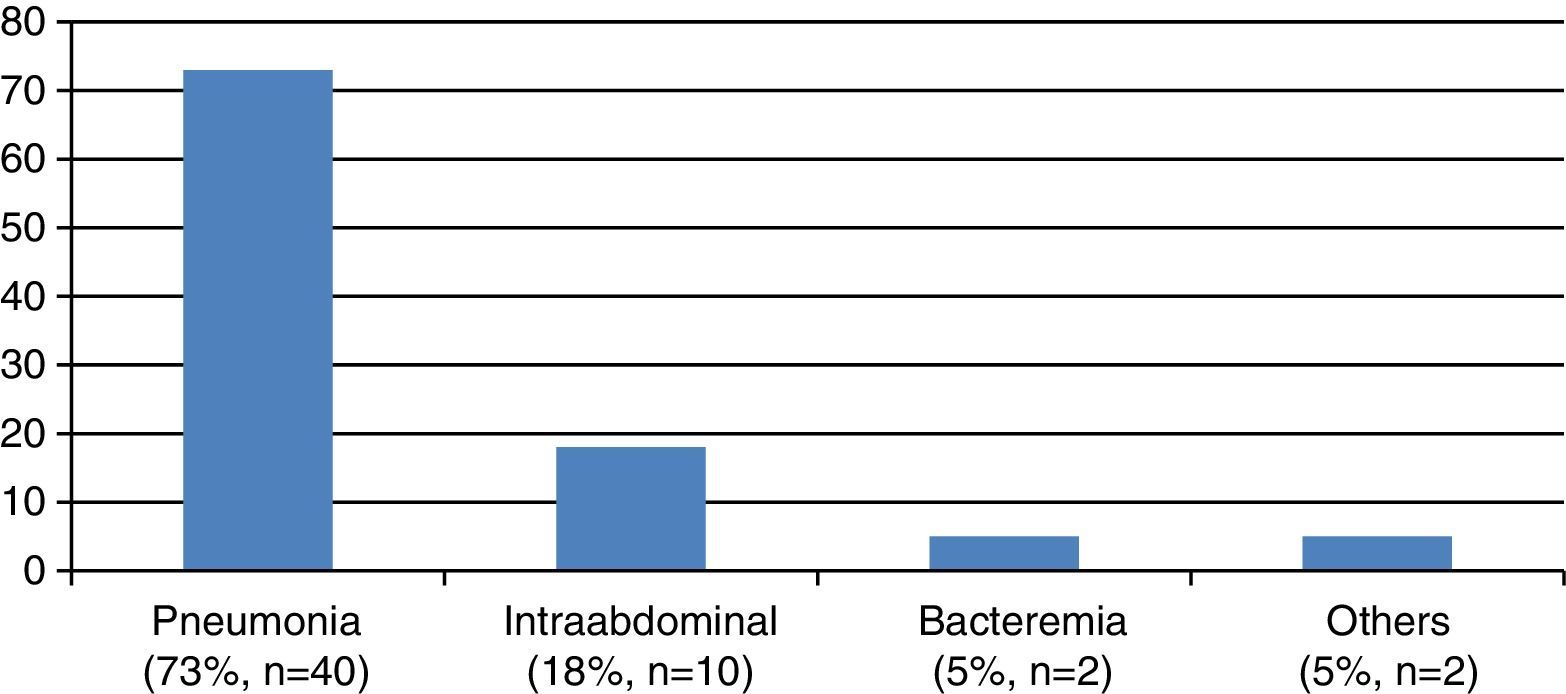
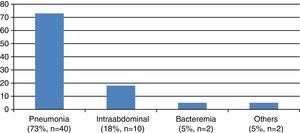
Nosocomial pneumonia was the predominant infectious process (74%), and an etiological diagnosis could be established in 62.5% of the patients with pneumonia. The most common microorganisms were Acinetobacter baumannii (40%), Pseudomonas aeruginosa (16%) and methicillin-resistant Staphylococcus aureus (MRSA)(12%). Intraabdominal infection was the second most frequent type of infection (18%). This category was taken to include infectious colitis (n=1; 11%), abscessified hematoma (n=1; 11%), liver abscess (n=1; 11%), surgical wound infection (n=1; 11%) and spontaneous bacterial peritonitis (n=5; 44%). In these cases the most frequently isolated microorganisms included Escherichia coli and Sphingobacterium multivorum. The third most common infectious process was primary or catheter-related bacteremia (3.5%). The microorganisms isolated in these cases were S. aureus and E. coli. Finally, 5% of the patients (n=2) were diagnosed with other types of infections such as skin and soft tissue infection, and sinusitis (Fig. 1).
Etiology of the infections. Nosocomial pneumonia was the predominant infection, followed in order of frequency by intraabdominal infection (spontaneous bacterial peritonitis was included in this category), primary or catheter-related bacteremia, and finally other infections such as infection of the skin and soft tissues, or sinusitis.
The mean Child–Pugh and MELD scores in the patients with and without infection showed no statistically significant differences (Table 2).
The mortality rate among the patients without infection (75%) was higher than in the patients with infection (62.9%), though the difference failed to reach statistical significance (p=0.74) (Table 3).
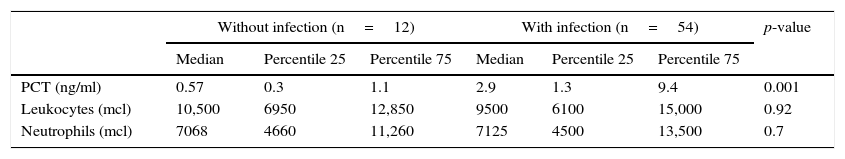
Infection biomarkersNeither the total leukocyte count nor the neutrophil count was able to distinguish between patients with and without infection (Table 4). However, the PCT concentration in the patients without infection (0.57ng/ml [0.3–1.1]) clearly differed from that in the patients with infection (2.99ng/ml [1,3,4,4,5,6,7,8,9]) (p<0.001). The ROC curve for assessing the diagnostic capacity of PCT yielded an area under the curve (AUC) of 0.82 [0.702–0.93] (Fig. 2); the best cutoff point in terms of sensitivity and specificity was 0.8ng/ml (sensitivity 83%, specificity 75%).
Biomarker characteristics in the patients with and without infection.
| Without infection (n=12) | With infection (n=54) | p-value | |||||
|---|---|---|---|---|---|---|---|
| Median | Percentile 25 | Percentile 75 | Median | Percentile 25 | Percentile 75 | ||
| PCT (ng/ml) | 0.57 | 0.3 | 1.1 | 2.9 | 1.3 | 9.4 | 0.001 |
| Leukocytes (mcl) | 10,500 | 6950 | 12,850 | 9500 | 6100 | 15,000 | 0.92 |
| Neutrophils (mcl) | 7068 | 4660 | 11,260 | 7125 | 4500 | 13,500 | 0.7 |
After excluding the transplanted patients (n=7), the above results were seen to persist (p=0.004). On exclusively analyzing the transplanted patients, the PCT levels were found to be higher in the patients with infection than in those without (4.20 [1.4–10.2] versus 0.16 [0.1–0.23])–though statistically significant differences were not reached (p=0.53).
A situation of severe sepsis or septic shock was associated to higher PCT concentrations (3.62 [1.74–9.6] versus 1.23 [0.78–3.1]; p=0.042).
The behavior of PCT proved similar in the different types of infection (p=0.17). A comparison was made of the PCT profile between the patients with intraabdominal infection and the patients without infection. The median PCT level in the patients with intraabdominal infection was 3ng/ml [1,3,4,5,6], while in the patients without infection the concentration was found to be 0.57ng/ml [0.3–1.1]. The difference was statistically significant (p=0.007). The data referred to PCT were likewise significant on comparing the patients with spontaneous bacterial peritonitis versus the patients without infection (3.6ng/ml [1,3,4,4] versus 0.57ng/ml [0.3–1.1]; p=0.01).
DiscussionThe main findings of our study can be described as follows: (1) PCT is a useful biomarker for diagnosing infection in patients with liver cirrhosis; (2) the behavior of PCT is similar in the different infectious processes diagnosed in our population; and (3) intraabdominal infections in patients with liver cirrhosis, including spontaneous bacterial peritonitis, are also characterized by a significant increase in serum PCT concentration.
The incidence and consequences of bacterial infections in patients with liver cirrhosis are very important in the clinical context; the definition of effective diagnostic tools is therefore of great interest.2,17
Since the liver is the main source of CRP, patients with cirrhosis or even liver dysfunction produce lesser amounts of this molecule compared with individuals having normal liver function.18,19 As a result, this inflammation biomarker has been relatively ignored in relation to liver cirrhosis. In contrast, PCT is produced by a range of tissues, and severe liver dysfunction therefore should not interfere with its serum levels.20 Nevertheless, an experimental study showed an absence of PCT in response to endotoxin administration in an anhepatic monkey versus two healthy controls–this situation being scantly extendable to the clinical setting.21 On the other hand, different observational studies in patients with liver cirrhosis seen in emergency care or in the hospital ward have shown PCT to be effective in diagnosing infection.22–26
The only study to date involving critical patients included individuals with confirmed infection that were analyzed according to the presence or absence of liver cirrhosis. The authors recorded lower serum CRP and PCT concentrations in the presence of liver dysfunction, though statistically significant differences were not observed.27 Our cohort of critical patients has yielded results similar to those obtained in other patient populations–thus supporting the diagnostic usefulness of PCT in patients with liver cirrhosis admitted to the ICU.24–26
Different studies have questioned the usefulness of PCT in diagnosing localized infection. Intraabdominal infection–and particularly spontaneous bacterial peritonitis (SBP)–may have few systemic repercussions, including the absence or a deficit of serum inflammatory response (biomarker variations). Spahr et al. carried out a prospective case-control study in cirrhotic patients with and without SBP, and analyzed the behavior of serum PCT, IL-6 and CRP. Both serum PCT and CRP were seen to be higher in patients with SBP.28,29 Our study also showed the usefulness of PCT in identifying critical patients with SBP. Another factor that can affect the diagnostic capacity of inflammation biomarkers is the severity of the infectious process. In coincidence with other authors, our study showed patients with severe sepsis or septic shock to have higher PCT levels.16
Our study has important limitations. Firstly, it is a retrospective study; consequently, although the case histories afforded detailed information on the infection, it is not possible to discard possible shortcomings in terms of the accuracy of the reported data. Secondly, the study only analyzed PCT; as a result, we cannot assess the behavior of other biomarkers such as CRP. Although liver biopsy is the most specific and sensitive technique for diagnosing liver cirrhosis, the diagnosis is currently established using noninvasive methods, including particularly abdominal ultrasound, which offers high specificity that can be improved upon thanks to use of the Doppler technique. Liver biopsy is mainly reserved for evaluating the degree of liver involvement and for determining the etiology of the process.30,31
ConclusionsThe results of our study show PCT to be an effective tool for diagnosing infection in patients with liver cirrhosis, including intraabdominal infection/SBP. It is therefore an excellent aid in the differential diagnosis of infectious disease in this patient population.
Conflict of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Villarreal E, Vacacela K, Gordon M, Calabuig C, Alonso R, Ruiz J, et al. Utilidad de la procalcitonina para el diagnóstico de infección en el paciente crítico con cirrosis hepática. Med Intensiva. 2016;40:84–89.



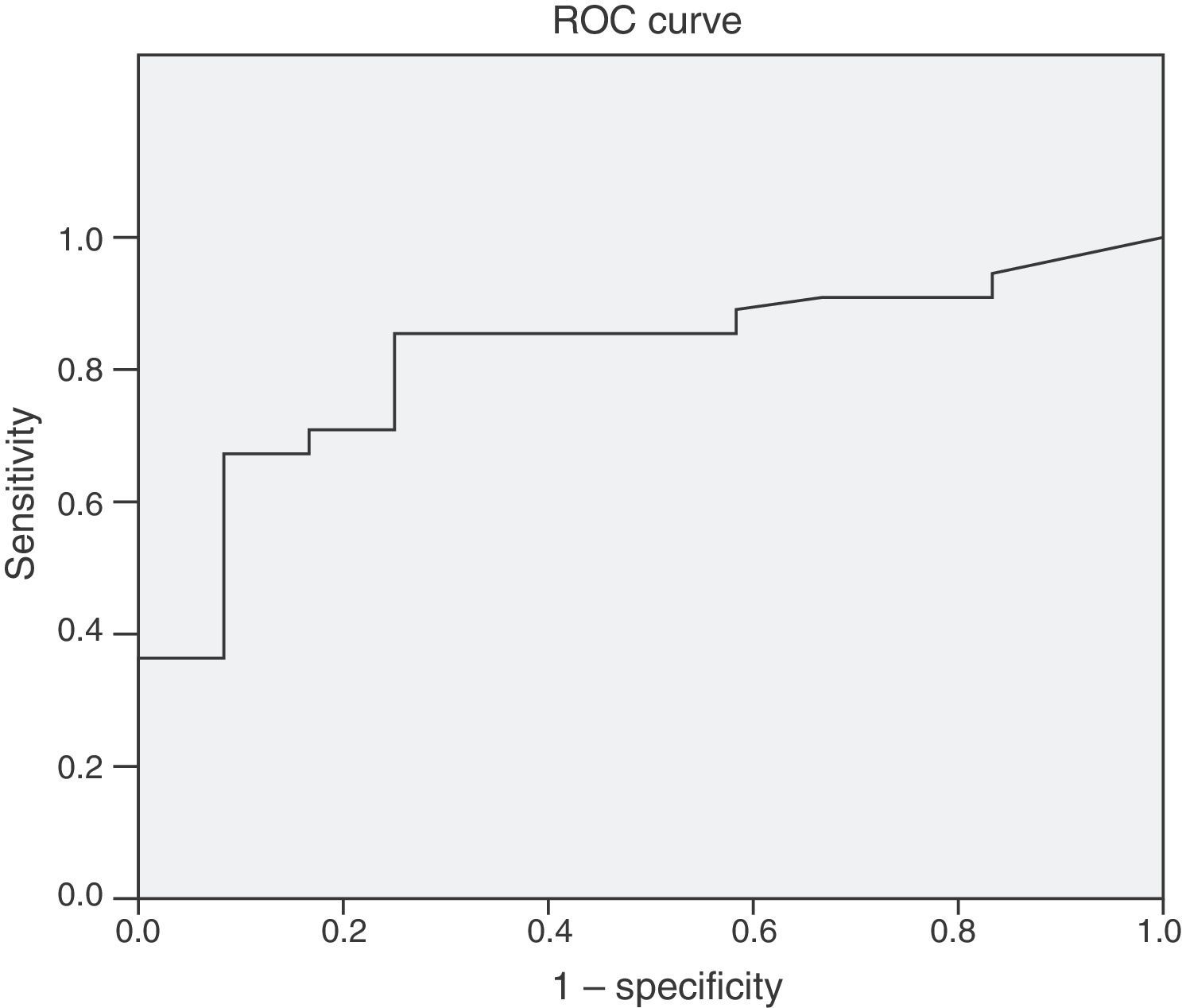


![Receiver operating characteristic (ROC) curve of PCT, with an area under the curve (AUC) of 0.82 [0.702–0.93]; the best cutoff point in relation to sensitivity and specificity was 0.8ng/ml (sensitivity 83%, specificity 75%). Receiver operating characteristic (ROC) curve of PCT, with an area under the curve (AUC) of 0.82 [0.702–0.93]; the best cutoff point in relation to sensitivity and specificity was 0.8ng/ml (sensitivity 83%, specificity 75%).](https://static.elsevier.es/multimedia/21735727/0000004000000002/v1_201603080056/S2173572716000072/v1_201603080056/en/main.assets/thumbnail/gr2.jpeg?xkr=1dZuESKpnCAWr3yCSGZ24A==)


