Most patients who require mechanical ventilation for longer than 24h, and who improve the condition leading to the indication of ventilatory support, can be weaned after passing a first spontaneous breathing test. The challenge is to improve the weaning of patients who fail that first test. We have methods that can be referred to as traditional, such as the T-tube, pressure support or synchronized intermittent mandatory ventilation (SIMV). In recent years, however, new applications of usual techniques as noninvasive ventilation, new ventilation methods such as automatic tube compensation (ATC), mandatory minute ventilation (MMV), adaptive support ventilation or automatic weaning systems based on pressure support have been described. Their possible role in weaning from mechanical ventilation among patients with difficult or prolonged weaning remains to be established.
La mayoría de los pacientes que requieren ventilación mecánica durante más de 24 h y que mejoran de la causa que motivó el soporte ventilatorio pueden ser extubados tras una primera prueba de respiración espontánea. El reto es cómo mejorar la desconexión de los pacientes que fracasan en esa prueba. Hasta el momento disponíamos de los métodos, que podemos denominar tradicionales, como son el tubo en T, la presión de soporte o la ventilación mandatoria intermitente sincronizada (SIMV). En los últimos años se han descrito nuevas aplicaciones de técnicas habituales como la ventilación no invasiva, nuevos métodos de ventilación como la compensación automática del tubo (ATC), la ventilación minuto mandatoria (MMV), la ventilación adaptativa de soporte (ASV) o estrategias automáticas de desconexión de la ventilación mecánica basadas en la presión de soporte. Queda por definir su posible papel en la desconexión de la ventilación mecánica en los enfermos con desconexión difícil o prolongada.
Weaning from mechanical ventilation is one of the most frequent procedures in intensive care units (ICUs). International epidemiological studies1–3 have shown that a little over one-half of all patients requiring mechanical ventilation are extubated after a weaning process. Of these, 57% are extubated after a first spontaneous breathing test, while the remaining 43% require a median of three days for extubation. Globally, this process accounts for 40% of the total ventilatory support time.2,4
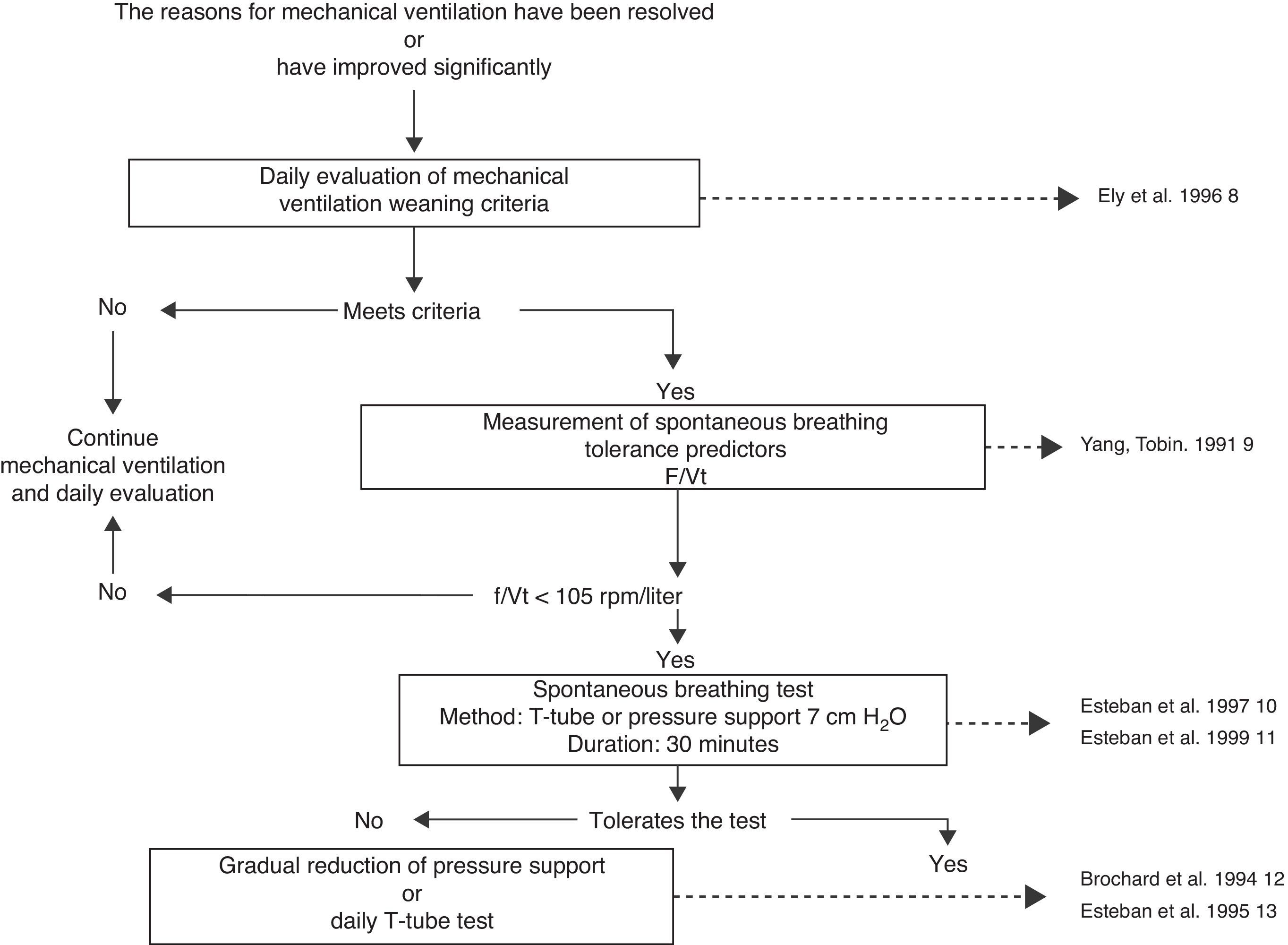
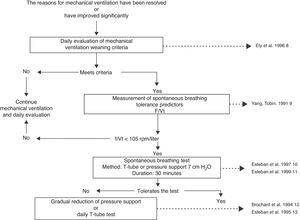
The fact that it is a common procedure and consumes a significant amount of time in the routine activity of physicians and nurses may be the reason why weaning from mechanical ventilation is one of the most widely evaluated procedures and one of the techniques most solidly based on scientific evidence.5,6 Briefly, weaning from mechanical ventilation starts with the daily identification of those patients amenable to performing a spontaneous breathing test, and is followed by three consecutive diagnostic procedures7: measurement of spontaneous breathing test tolerance predictors, a spontaneous breathing test, and an extubation test (Fig. 1). With this strategy, a group of patients can be extubated on the first spontaneous breathing attempt a situation known as simple or easy weaning6–but approximately 45% of the patients14 will need progressive weaning from mechanical ventilation.14–16 Such progressive weaning can be performed using traditional methods: T-tube, continuous positive airway pressure (CPAP), synchronized intermittent mandatory ventilation (SIMV), or pressure support (PS). These methods have been subjected to comparative evaluations in different studies,12,13,17,18 which in turn have been analyzed in a systematic review.19 Differences in protocol design, in the way of reducing ventilatory support with SIMV and pressure support, and in the extubation criteria used, have prevented this review from identifying a superior technique among the three contrasted modes. Nevertheless, it does seem clear that SIMV can prolong weaning longer than the T-tube or the gradual reduction of pressure support (Table 1).
Mechanical ventilation weaning algorithm.8–11
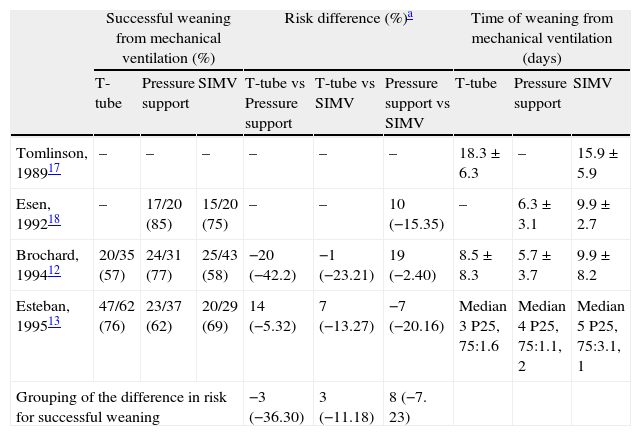
Comparison of the results of the clinical trials contrasting the traditional mechanical ventilation weaning modes.
| Successful weaning from mechanical ventilation (%) | Risk difference (%)a | Time of weaning from mechanical ventilation (days) | |||||||
| T-tube | Pressure support | SIMV | T-tube vs Pressure support | T-tube vs SIMV | Pressure support vs SIMV | T-tube | Pressure support | SIMV | |
| Tomlinson, 198917 | – | – | – | – | – | – | 18.3±6.3 | – | 15.9±5.9 |
| Esen, 199218 | – | 17/20 (85) | 15/20 (75) | – | – | 10 (−15.35) | – | 6.3±3.1 | 9.9±2.7 |
| Brochard, 199412 | 20/35 (57) | 24/31 (77) | 25/43 (58) | −20 (−42.2) | −1 (−23.21) | 19 (−2.40) | 8.5±8.3 | 5.7±3.7 | 9.9±8.2 |
| Esteban, 199513 | 47/62 (76) | 23/37 (62) | 20/29 (69) | 14 (−5.32) | 7 (−13.27) | −7 (−20.16) | Median 3 P25, 75:1.6 | Median 4 P25, 75:1.1, 2 | Median 5 P25, 75:3.1, 1 |
| Grouping of the difference in risk for successful weaning | −3 (−36.30) | 3 (−11.18) | 8 (−7. 23) | ||||||
Adapted from19. SIMV, synchronized intermittent mandatory ventilation.
The differences in risk are expressed as the difference in the percentages of successfully weaned patients among the different modes. The negative numbers indicate a lesser success rate, while the positive numbers indicate a greater success rate. The 95% confidence interval (95% CI) appears in parentheses.
In recent years, new applications of common techniques, and new ventilation methods that may play a role in weaning from mechanical ventilation in patients with difficult or prolonged weaning have been described.
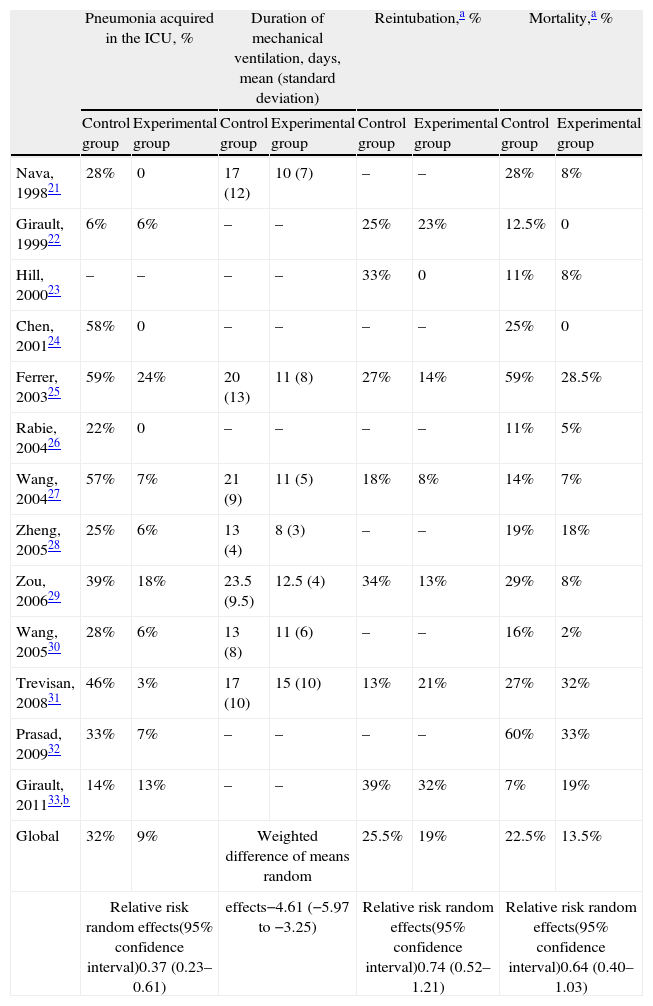
Alternative modes and new modes for weaning from mechanical ventilationNoninvasive ventilationIn an attempt to reduce the complications associated to prolonged mechanical ventilation, some authors have explored the role of noninvasive ventilation in weaning from mechanical ventilation. In essence, those patients who fail a spontaneous breathing test are extubated and immediately connected to noninvasive ventilation. Since the first experience described by Udwadia et al. in 1992,20 a number of clinical trials21–33 have evaluated this technique as a mechanical ventilation weaning mode. There are several limitations to the applicability and generalization of these studies, however. Firstly, almost all of them involve small sample sizes. Secondly, many of the published studies predominantly22,25 or exclusively21,24,26,31–33 involve patients with chronic lung disease – this being a population in which noninvasive ventilation is particularly indicated due to its capacity to lessen respiratory fatigue, increase the tidal volume, and lessen the intrinsic positive end-expiratory pressure (PEEP). The question of whether other causes of respiratory failure could benefit from weaning with noninvasive ventilation remains to be established. Thirdly, there has been great heterogeneity in the weaning strategies used both in the invasive weaning group and in the noninvasive weaning group. The patients assigned to invasive weaning were preferentially disconnected with pressure support, but with different strategies referred to its gradual reduction: two daily observations with an optional spontaneous breathing test22; a reduction of 2cmH2O every 4h according to clinical tolerance32; a daily reduction of 2–4cmH2O26; or gradual reduction of pressure support to 7cmH2O.33 Most of the studies have interpositioned or alternated spontaneous breathing tests with the T-tube during different periods of time.21–26,31,33 Likewise, in the patients assigned to weaning with noninvasive ventilation, application of the latter and the criteria for total weaning in noninvasive ventilation were heterogeneous. Following extubation, the application of noninvasive ventilation was continuous in 5 studies,21,23–25,32 intermittent in one study,22 performed over at least 2h in another study,29 and over 20–22h (depending on tolerance), with resting periods for meals, in another study.26 The level of support and the duration of the ventilation periods were gradually reduced, but this also involved different protocols: reduction of pressure support by 2cmH2O every 4h,32 or a reduction of 2–4cmH2O on a daily basis26 according to patient tolerance to an inspiratory pressure of 8cmH2O and an expiratory pressure of 4cmH2O,32 or an inspiratory pressure of less than 10cmH2O,28,29 or until the difference between inspiratory pressure and expiratory pressure was less than 5cmH2O.32 Lastly, the results reported (Tables 2 and 3) in the control groups of some of the studies do not reflect routine clinical practice.34 Taking these limitations into account, the conclusion of the studies is that noninvasive ventilation may be a promising technique for weaning from mechanical ventilation, but further studies are needed in order to fully evaluate the clinical benefits and risks associated to extubation failure.35
Studies that have evaluated noninvasive ventilation as mechanical ventilation weaning method. Compared populations and strategies.
| Study population | Compared strategies | |||
| Control group | Experimental group | |||
| Nava, 199821 | Patients with exacerbated chronic obstructive pulmonary disease | No.=25Invasive pressure supportSpontaneous breathing test with T-tube or CPAP<5cmH2O | No.=25Noninvasive pressure support applied through face mask | |
| Girault, 199922 | Patients with exacerbated chronic pulmonary disease (obstructive, restrictive or mixed) | No.=16Invasive pressure support | No.=17Noninvasive pressure support applied through face or nasal mask | |
| Hill, 200023a | Patients with acute respiratory failure | No.=9Invasive pressure supportSpontaneous breathing test with T-tube | No.=12Noninvasive ventilation using a specific ventilator (VPAP®) in ST-A mode | |
| Chen, 200124 | Patients with exacerbated chronic obstructive pulmonary disease | No.=12Invasive pressure supportSpontaneous breathing test with T-tube | No.=12BiPAP | |
| Ferrer, 200325 | Patients with acute respiratory failure that fail 3 spontaneous breathing tests(77% patients with chronic pulmonary disease) | No.=22Invasive pressure supportSpontaneous breathing test with T-tube or CPAP<5cmH2O | No.=21Noninvasive ventilation in S/T mode applied through face or nasal mask | |
| Rabie, 200426a | Patients with exacerbated chronic obstructive pulmonary disease | No.=18Invasive pressure support | No.=19Proportional assist ventilation/timed mode through face or nasal mask | |
| Wang, 200427 | Patients with chronic obstructive pulmonary disease and bronchopulmonary infection | No.=14SIMV+PSSpontaneous breathing test with T-tube | No.=14Noninvasive pressure support applied through non-specified mask | |
| Zheng, 200528 | Patients with chronic obstructive pulmonary disease and bronchopulmonary infection | No.=16Invasive pressure support | No.=17BiPAP applied through face or nasal mask | |
| Zou, 200629 | Patients with chronic obstructive pulmonary disease and bronchopulmonary infection | No.=38SIMV+PS | No.=38BIPAP in mode ST applied through face or oronasal mask | |
| Wang, 200530 | Patients with chronic obstructive pulmonary disease and bronchopulmonary infection | No.=43SIMV+PS | No.=47BiPAP | |
| Trevisan, 200831 | Patients with mechanical ventilation>48h (35% patients with chronic pulmonary disease) | No.=37Invasive pressure supportSpontaneous breathing test with T-tube | No.=28BiPAP through face mask | |
| Prasad, 200932 | Patients with exacerbated chronic obstructive pulmonary disease | No.=15Invasive pressure support | No.=15BiPAP through face mask | |
| Girault, 201133 | Patients with exacerbated chronic pulmonary disease (obstructive, restrictive or mixed) | No.=69Invasive pressure supportSpontaneous breathing test with T-tube or pressure support | No.=70Oxygen therapy without ventilatory support | No.=70Noninvasive pressure support through face mask |
Abbreviations: BiPAP, bilevel positive airway pressure; SIMV, synchronized intermittent mandatory ventilation; PS, pressure support.
Studied outcomes.
| Pneumonia acquired in the ICU, % | Duration of mechanical ventilation, days, mean (standard deviation) | Reintubation,a % | Mortality,a % | |||||
| Control group | Experimental group | Control group | Experimental group | Control group | Experimental group | Control group | Experimental group | |
| Nava, 199821 | 28% | 0 | 17 (12) | 10 (7) | – | – | 28% | 8% |
| Girault, 199922 | 6% | 6% | – | – | 25% | 23% | 12.5% | 0 |
| Hill, 200023 | – | – | – | – | 33% | 0 | 11% | 8% |
| Chen, 200124 | 58% | 0 | – | – | – | – | 25% | 0 |
| Ferrer, 200325 | 59% | 24% | 20 (13) | 11 (8) | 27% | 14% | 59% | 28.5% |
| Rabie, 200426 | 22% | 0 | – | – | – | – | 11% | 5% |
| Wang, 200427 | 57% | 7% | 21 (9) | 11 (5) | 18% | 8% | 14% | 7% |
| Zheng, 200528 | 25% | 6% | 13 (4) | 8 (3) | – | – | 19% | 18% |
| Zou, 200629 | 39% | 18% | 23.5 (9.5) | 12.5 (4) | 34% | 13% | 29% | 8% |
| Wang, 200530 | 28% | 6% | 13 (8) | 11 (6) | – | – | 16% | 2% |
| Trevisan, 200831 | 46% | 3% | 17 (10) | 15 (10) | 13% | 21% | 27% | 32% |
| Prasad, 200932 | 33% | 7% | – | – | – | – | 60% | 33% |
| Girault, 201133,b | 14% | 13% | – | – | 39% | 32% | 7% | 19% |
| Global | 32% | 9% | Weighted difference of means random | 25.5% | 19% | 22.5% | 13.5% | |
| Relative risk random effects(95% confidence interval)0.37 (0.23–0.61) | effects−4.61 (−5.97 to −3.25) | Relative risk random effects(95% confidence interval)0.74 (0.52–1.21) | Relative risk random effects(95% confidence interval)0.64 (0.40–1.03) | |||||
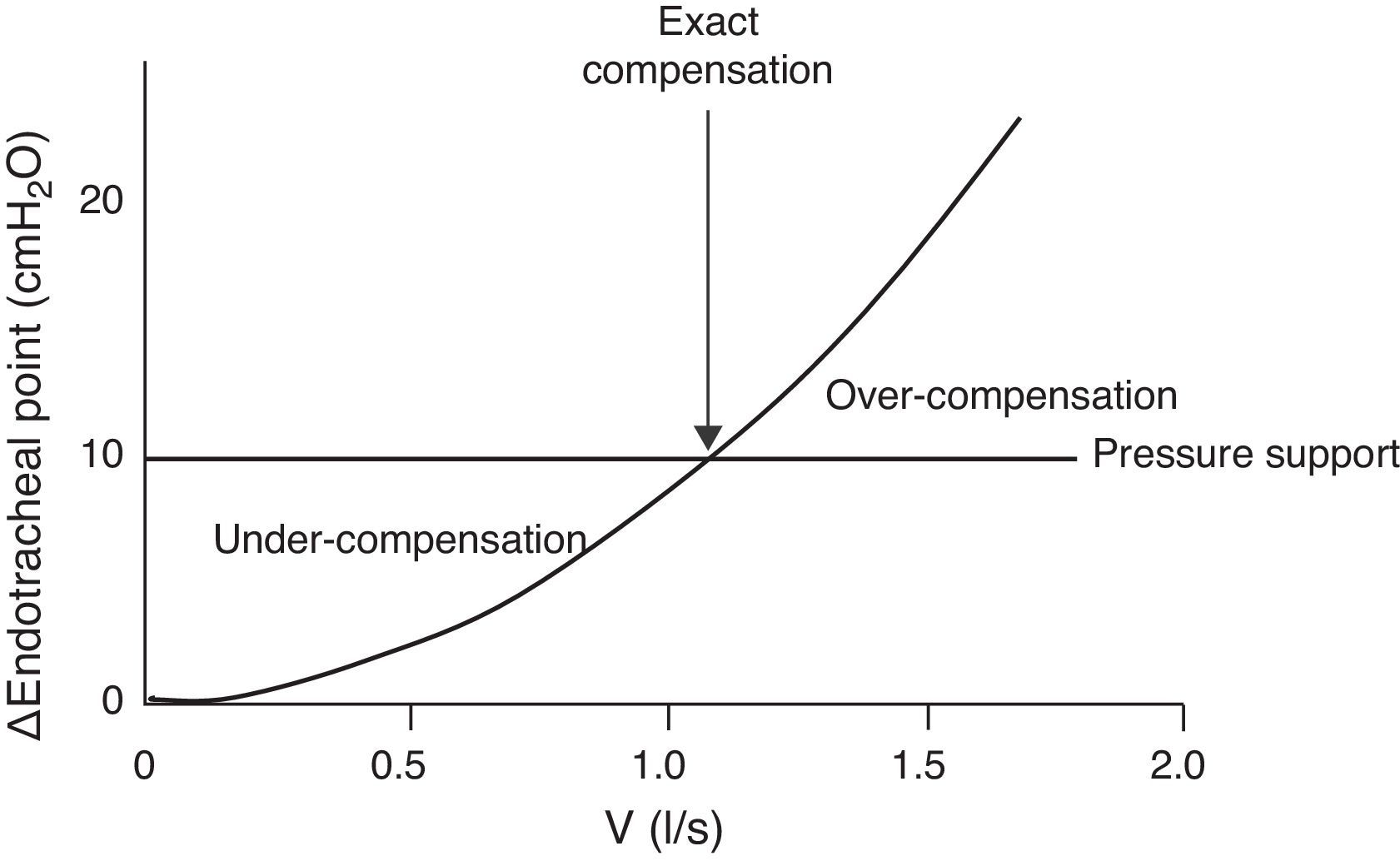
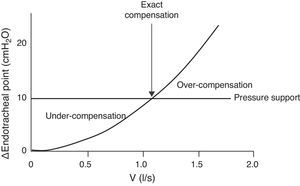
The endotracheal tube imposes inspiratory resistance during the spontaneous breathing test.36 The use of low pressure support levels (5–8cmH2O) has been proposed to compensate this effect.37 Unfortunately, it is not easy to determine or predict the precise level required to compensate the inspiratory load imposed upon an individual patient. With the same level of support, failure of the spontaneous breathing test may occur due to insufficient compensation of the imposed effort. In turn, overcompensation can allow the patient to successfully pass a spontaneous breathing test but may not allow precise evaluation of whether the patient is truly capable of breathing without support. All these depend on the size of the endotracheal tube and on the inspiratory flow (Fig. 2).
Inspiratory flow/pressure curve in a patient with an endotracheal tube measuring 7.5mm in internal diameter (modified from Refs. 38,39). Due to the variations in flow pattern in spontaneous breathing, the resistance imposed by the tube is variable. If we apply a constant pressure support of 10cmH2O (horizontal line), we can have three situations: (1) with low inspiratory flows, the pressure support under-compensates the resistance of the tube; (2) with medium inspiratory flows, the pressure support compensates the resistance (intersection between the resistance–pressure curve of the tube (ΔPendotracheal tube) and the pressure support line); and (3) with high flows, the pressure support over-compensates the resistance.
Automatic tube compensation (ATC), available with the Evita 4, Evita XL (Draeger Medical) and 840 Puritan Bennett (Covidien) ventilators, is a mode in which the ventilator continuously measures the pressure drop that occurs through the endotracheal tube, followed by programming of the level of pressure support that compensates the pressure drop.39,40 The studies that have compared ATC with pressure support suggest that ATC is more effective in compensating respiratory effort, more comfortable, and with fewer patient-ventilator asynchronies.39,41 Programming requires entering the size of the endotracheal tube and the desired percentage compensation (generally 100%).
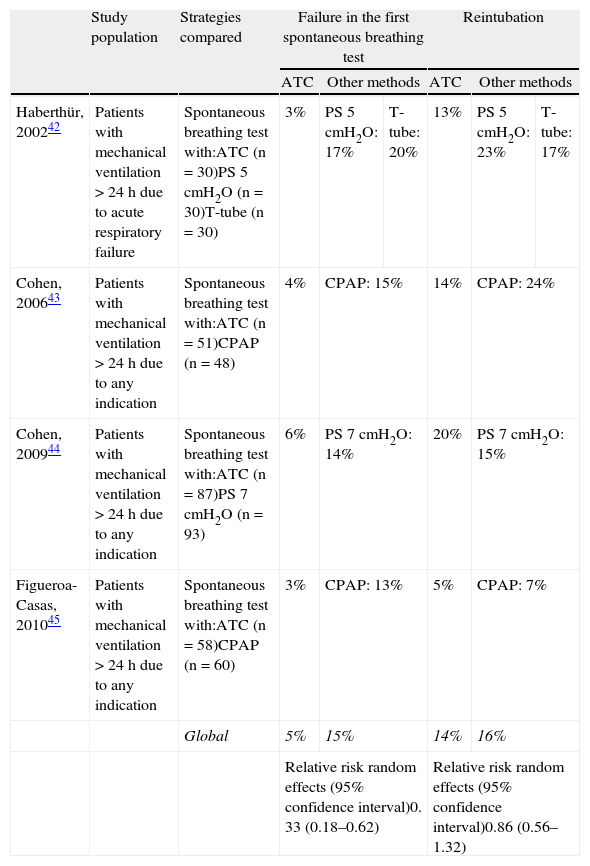
Automatic tube compensation has been evaluated as a method for the spontaneous breathing test in a number of clinical trials,42–45 with very favorable results regarding the success rate of the first spontaneous breathing test, but with reintubation rates similar to those of the control group (Table 4). On the other hand, another study46 has evaluated weaning time comparing gradual reduction of pressure support with ATC versus gradual reduction of pressure support alone. The study included 41 patients requiring mechanical ventilation due to acute respiratory failure secondary to poisoning from snakebites. In the group receiving pressure support with ATC, the median weaning time was 8h (interquartile range 7–12) versus 12h (interquartile range 7–17) in the group receiving pressure support isolatedly (p=0.12).
Clinical trials comparing automatic tube compensation (ATC) with other methods for the spontaneous breathing test.
| Study population | Strategies compared | Failure in the first spontaneous breathing test | Reintubation | |||||
| ATC | Other methods | ATC | Other methods | |||||
| Haberthür, 200242 | Patients with mechanical ventilation>24h due to acute respiratory failure | Spontaneous breathing test with:ATC (n=30)PS 5cmH2O (n=30)T-tube (n=30) | 3% | PS 5cmH2O: 17% | T-tube: 20% | 13% | PS 5cmH2O: 23% | T-tube: 17% |
| Cohen, 200643 | Patients with mechanical ventilation>24h due to any indication | Spontaneous breathing test with:ATC (n=51)CPAP (n=48) | 4% | CPAP: 15% | 14% | CPAP: 24% | ||
| Cohen, 200944 | Patients with mechanical ventilation>24h due to any indication | Spontaneous breathing test with:ATC (n=87)PS 7cmH2O (n=93) | 6% | PS 7cmH2O: 14% | 20% | PS 7cmH2O: 15% | ||
| Figueroa-Casas, 201045 | Patients with mechanical ventilation>24h due to any indication | Spontaneous breathing test with:ATC (n=58)CPAP (n=60) | 3% | CPAP: 13% | 5% | CPAP: 7% | ||
| Global | 5% | 15% | 14% | 16% | ||||
| Relative risk random effects (95% confidence interval)0. 33 (0.18–0.62) | Relative risk random effects (95% confidence interval)0.86 (0.56–1.32) | |||||||
Abbreviations: ATC, automatic tube compensation; CPAP, continuous positive airway pressure; PS, pressure support.
Despite the good results obtained in the studies published to date, further research is needed, involving larger patient samples and, especially, patients with difficulties in passing the first spontaneous breathing test, in order to be able to recommend ATC as the method of choice in performing the spontaneous breathing test.47,48
Automatic weaning with closed circuit systemsMandatory minute ventilationMandatory minute ventilation (MMV), described by Hewlett et al.,49 was the first ventilation mode in which the ventilator modified the support based on the response of the patient, or the first computerized ventilation control mode. At present, this ventilation mode is available with the Evita 4, Evita XL (Draeger Medical), CPU-1 Intensive Care ventilator (Ohmeda Medical), Sechrist IV-100B (Sechrist Industries, Inc.) and Hamilton Veolar ventilator (Hamilton Medical) systems. In this modality, we program the tidal volume and a mandatory frequency, thereby defining a minute ventilation target. The ventilator adapts the mandatory frequency to the minute ventilation target, taking into account the spontaneous respiratory frequency of the patient. The difference with synchronized intermittent mandatory ventilation (SIMV) is that while the mandatory frequency is fixed in the latter technique, it is variable in MMV. If spontaneous breathing of the patient, with previously established pressure support, reaches or exceeds the defined target, the ventilator produces no mandatory respiration. In contrast, if the minute ventilation of the patient drops below the predetermined level, the ventilator offers the respirations necessary to reach the target. One of the main limitations is that the ventilator performs the adjustments only according to the determined minute ventilation. In other words, it does not distinguish between a tidal volume of 500ml with a respiratory frequency of 12rpm (normal ventilatory pattern) and a tidal volume of 200ml with a respiratory frequency of 30rpm (rapid and superficial ventilatory pattern).
Very few studies have evaluated MMV as an alternative method of weaning from mechanical ventilation, and most of the existing publications involve newborn infants. There is only one clinical trial in adults,50 which randomized 22 patients to weaning with MMV and 18 patients to weaning with intermittent mandatory ventilation. MMV significantly shortened the weaning time (5 versus 33h; p<0.001), with no increase in the extubation failure rate 4h after extubation (89% versus 86%).
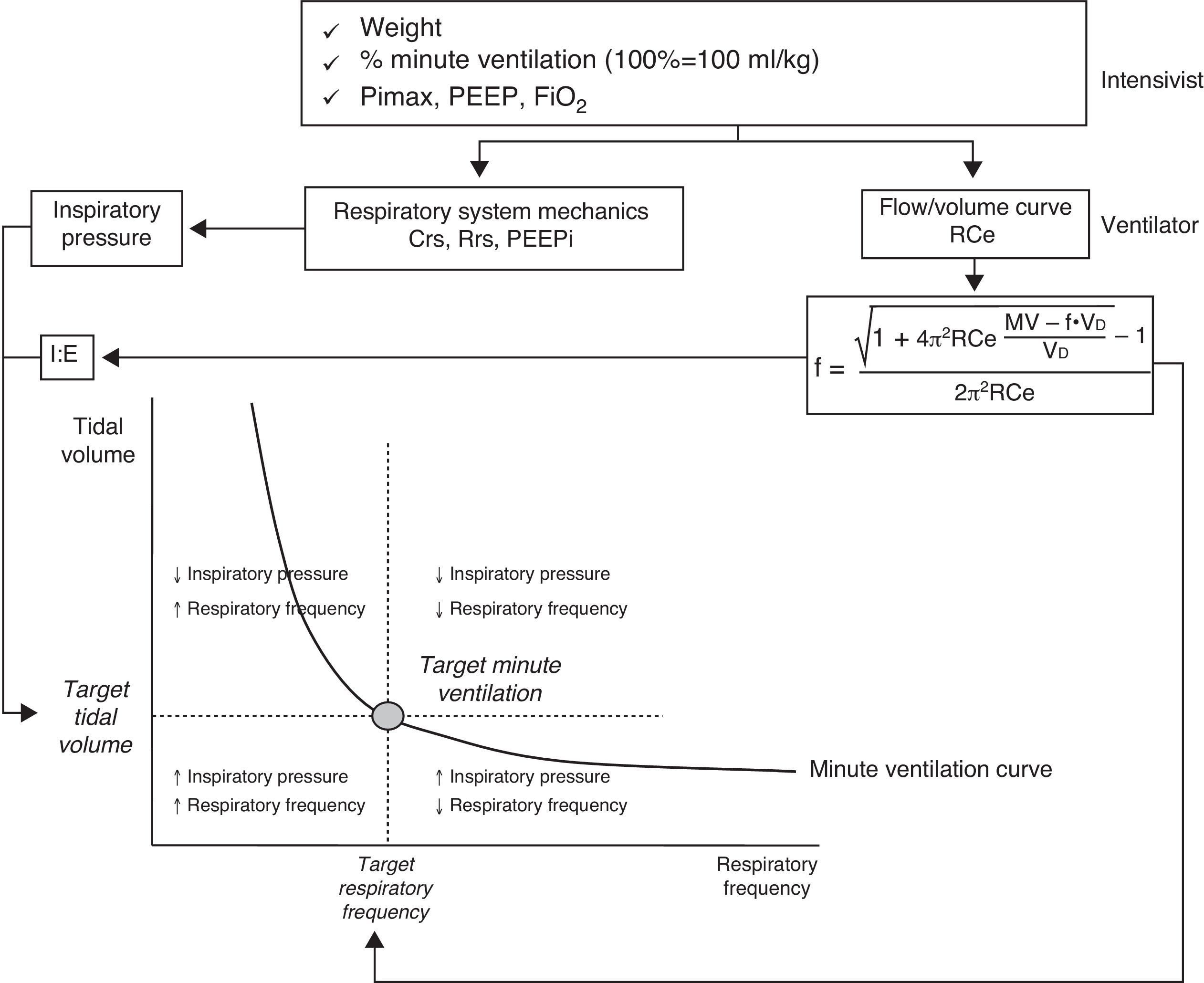
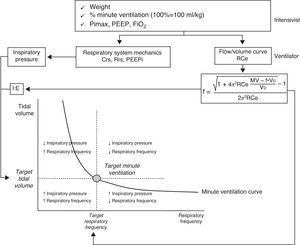
Adaptive support ventilationThis ventilation mode was initially described as adaptive lung ventilation by Laubscher in 1994.51,52 It is a pressure-controlled assisted mode with automatic regulation of the ventilatory parameters in response to changes in the respiratory mechanics and spontaneous ventilation pattern. This ventilation mode is available with the Galileo, Raphael, Hamilton S1, Hamilton C2 and Hamilton G5 respirators (Hamilton Medical). Its essential operating principal is based on the formula of Otis.53 This equation calculates the ideal respiratory frequency, which is that associated to lesser energy expenditure. To this effect, the equation takes into account the dead space, minute ventilation and the expiration time constant. The process starts by entering a series of parameters into the ventilator: patient weight, the desired minute ventilation percentage (100% being equivalent to 100ml/kg/min), the inhaled oxygen fraction, PEEP, and the maximum inspiratory pressure (Pimax). The system then determines the expiration time constant through analysis of the expiratory flow-volume curve. Posteriorly, the automatic algorithm adjusts the inspiratory pressure, the inspiratory/expiratory time ratio and the respiratory frequency, in order to maintain the minute ventilation target (Fig. 3) within an established range to avoid rapid and superficial breathing or an excessive insufflation volume. In determining the optimum respiratory frequency, the ventilator assumes a dead space according to the Radford nomogram55 of 2.2ml/kg. In patients undergoing weaning from mechanical ventilation, the algorithm of the adaptive support ventilator (ASV) gradually and automatically reduces the inspiratory pressure. Weaning is complete when all respirations are spontaneous and the patient maintains adequate gas exchange for some hours with an inspiratory pressure of under 8cmH2O.
Schematic representation of the basic operating principles of adaptive support ventilation (ASV)(modified from Ref. 54). Abbreviations: Crs, compliance of the respiratory system; f, respiratory frequency; FiO2, inhaled oxygen fraction; MV, minute ventilation; PEEP, positive end-expiratory pressure; PEEPi, intrinsic positive end-expiratory pressure; PiMax, maximum inspiratory pressure; Rce, expiratory time constant; Rrs, resistance of the respiratory system; VD, anatomical dead space.
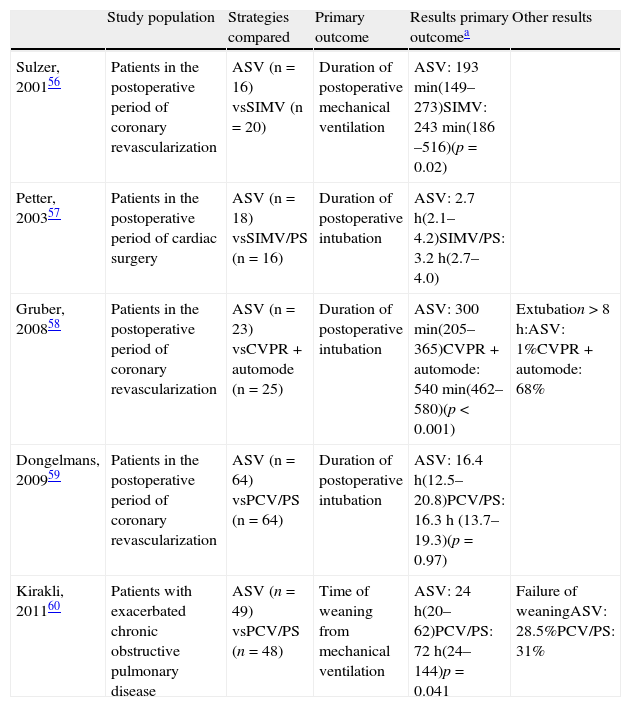
The studies that have evaluated the ASV as a weaning method56–60 suggest that it could simplify ventilatory management and shorten the time to extubation (Table 5). The limitations of these studies are that they have been carried out in small groups of mainly postoperative patients, with a short duration of mechanical ventilation.
Clinical trials that have evaluated adaptive support ventilation (ASV) as mechanical ventilation weaning method.
| Study population | Strategies compared | Primary outcome | Results primary outcomea | Other results | |
| Sulzer, 200156 | Patients in the postoperative period of coronary revascularization | ASV (n=16) vsSIMV (n=20) | Duration of postoperative mechanical ventilation | ASV: 193min(149–273)SIMV: 243min(186 –516)(p=0.02) | |
| Petter, 200357 | Patients in the postoperative period of cardiac surgery | ASV (n=18) vsSIMV/PS (n=16) | Duration of postoperative intubation | ASV: 2.7h(2.1–4.2)SIMV/PS: 3.2h(2.7–4.0) | |
| Gruber, 200858 | Patients in the postoperative period of coronary revascularization | ASV (n=23) vsCVPR+automode (n=25) | Duration of postoperative intubation | ASV: 300min(205–365)CVPR+automode: 540min(462–580)(p<0.001) | Extubation>8h:ASV: 1%CVPR+automode: 68% |
| Dongelmans, 200959 | Patients in the postoperative period of coronary revascularization | ASV (n=64) vsPCV/PS (n=64) | Duration of postoperative intubation | ASV: 16.4h(12.5–20.8)PCV/PS: 16.3h (13.7–19.3)(p=0.97) | |
| Kirakli, 201160 | Patients with exacerbated chronic obstructive pulmonary disease | ASV (n=49) vsPCV/PS (n=48) | Time of weaning from mechanical ventilation | ASV: 24h(20–62)PCV/PS: 72h(24–144)p=0.041 | Failure of weaningASV: 28.5%PCV/PS: 31% |
Abbreviations: ASV, adaptive support ventilation; CVPR, controlled ventilation with pressure regulation; PS, pressure support; PCV, pressure controlled ventilation; SIMV, synchronized intermittent mandatory ventilation.
In contrast to the two above described methods, automated weaning systems do not constitute new ventilation modes but automatic mechanical ventilation weaning strategies based on an already existing ventilation mode (pressure support). Two automated weaning systems are currently found on the market: SmartCare®,61 available with the Evita XL ventilator (Draeger Medical), and the mandatory rate ventilation (MRV) system, available with the Taema Horus ventilator (Air Liquid).
The SmartCare® system continuously applies a weaning protocol with changes in pressure support based on measurements of the respiratory frequency, tidal volume and end-expiration partial CO2 pressure (etCO2). To start the system, the physician enters data referred to the patient (weight, antecedents of chronic lung disease and/or neurological disorders), the type of artificial airway (endotracheal tube or tracheostomy) and the type of humidification (heat-humidity exchanger or active heat humidification). The weaning process then starts when the system adapts to maintain patient respiratory comfort (normal ventilation) by adjusting the pressure support with increments or decrements of 2–4cmH2O, according to the respiratory frequency (normal limits 15–30rpm; in patients with neurological disease the upper limit is increased to 34rpm), tidal volume (normal limits>300ml) and etCO2 (normal limits<55mmHg; in patients with chronic obstructive pulmonary disease the limit is increased to 65mmHg)–with averaging of the values every 2–5min. Once the patient shows normal ventilation, the system reduces or increases the pressure support, depending on the patient needs, every 15, 30 or 60min, according to the previous pressure support level, until reaching a variable pressure support depending on the type of artificial airway and the type of humidification (5cmH2O for active heat humidification and tracheotomy, 7cmH2O for active heat humidification and endotracheal tube, 9cmH2O for heat-humidity exchanger and tracheotomy, and 12cmH2O for heat-humidity exchanger and endotracheal tube). Once this level of support has been reached, the patient is considered to start a spontaneous breathing test with a duration determined by the respiratory pattern and the level of pressure support at which the weaning process was started. If during this time the patient shows a respiratory pattern different from normal ventilation or hyperventilation, the system assumes that the spontaneous breathing test has failed, and increases pressure support until a normal ventilation pattern is obtained. In the case of a stable respiratory pattern, the ventilator displays a message indicating that the patient is ready for extubation.
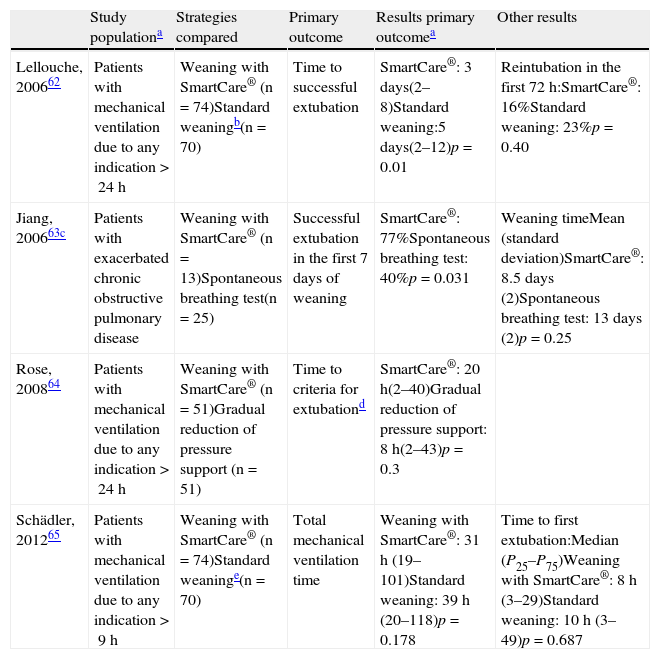
This system is not recommended in patients with neurological disease affecting respiratory control, in over-sedated individuals, in patients with severe bronchospasm, in patients with delirium/agitation, or in patients with severe polyneuropathy/myopathy. It has been evaluated in a series of clinical trials,62–65 with different results (Table 6), due to the assessment of different outcomes and differences in the weaning from mechanical ventilation protocol in the non-automatic weaning group.
Clinical trials that have evaluated the SmartCare® system as mechanical ventilation weaning method.
| Study populationa | Strategies compared | Primary outcome | Results primary outcomea | Other results | |
| Lellouche, 200662 | Patients with mechanical ventilation due to any indication>24h | Weaning with SmartCare® (n=74)Standard weaningb(n=70) | Time to successful extubation | SmartCare®: 3 days(2–8)Standard weaning:5 days(2–12)p=0.01 | Reintubation in the first 72h:SmartCare®: 16%Standard weaning: 23%p=0.40 |
| Jiang, 200663c | Patients with exacerbated chronic obstructive pulmonary disease | Weaning with SmartCare® (n=13)Spontaneous breathing test(n=25) | Successful extubation in the first 7 days of weaning | SmartCare®: 77%Spontaneous breathing test: 40%p=0.031 | Weaning timeMean (standard deviation)SmartCare®: 8.5 days (2)Spontaneous breathing test: 13 days (2)p=0.25 |
| Rose, 200864 | Patients with mechanical ventilation due to any indication>24h | Weaning with SmartCare® (n=51)Gradual reduction of pressure support (n=51) | Time to criteria for extubationd | SmartCare®: 20h(2–40)Gradual reduction of pressure support: 8h(2–43)p=0.3 | |
| Schädler, 201265 | Patients with mechanical ventilation due to any indication>9h | Weaning with SmartCare® (n=74)Standard weaninge(n=70) | Total mechanical ventilation time | Weaning with SmartCare®: 31h (19–101)Standard weaning: 39h (20–118)p=0.178 | Time to first extubation:Median (P25–P75)Weaning with SmartCare®: 8h (3–29)Standard weaning: 10h (3–49)p=0.687 |
Standard weaning consists of: daily evaluation of the weaning criteria, spontaneous breathing test (T-tube or with pressure support) upon meeting the criteria, and extubation after passing the spontaneous breathing test.
On the other hand, the mandatory rate ventilation (MRV) system progressively reduces the pressure support based on a concrete respiratory frequency target or goal. This respiratory frequency represents the respiratory frequency that is expected of the patient. In each cycle, the ventilator compares the desired respiratory frequency with the mean respiratory frequency corresponding to the last four cycles. If the average is greater than the respiratory frequency target, the pressure support automatically increases 1cmH2O. If the mean respiratory frequency is less than the desired respiratory frequency, the system reduces the pressure support 1cmH2O. This system has only been evaluated in a study66 involving 106 postoperative patients assigned to weaning with gradual reduction of the pressure support (53 patients) or weaning with the MRV system (53 patients). In the intent-to-treat (ITT) analysis, the mean time to extubation was 221±192min in the group with gradual reduction of pressure support versus 271±369min in the MRV group (p=0.375).
In summary, although the automatic mechanical ventilation weaning systems represent an important step forward, further studies are needed to define which ventilated patients can benefit from these systems versus the conventional weaning modes.
Other modesOther new ventilation modes have been developed, based on the same concept as some of the aforementioned modes (“the ventilator adapts to the patient instead of the patient to the ventilator”): the amplified spontaneous pattern (ASP),67 proportional assisted ventilation (PAV),68 neurally adjusted ventilation assist (NAVA)69 and automode70–though there are not enough studies to establish their possible role in weaning from mechanical ventilation.
In conclusion, and for the time being, it seems that all the modes that are being introduced for weaning from mechanical ventilation are alternative methods. We therefore consider that the most effective and simplest approach is to perform spontaneous breathing tests on a daily basis, each lasting about 30min, with the T-tube.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Frutos-Vivar F, Esteban A. Desconexión de la ventilación mecánica. ¿Por qué seguimos buscando métodos alternativos? Med Intensiva. 2013;37:605–617.
This review article belongs to the series «Update in Mechanical Ventilation» of the Acute Respiratory Failure Working Group (GT-IRA).