Currently, the aim of the resuscitation of burn patients is to maintain end-organ perfusion with fluid intake as minimal as possible. To avoid excess intake, we can improve the estimation using computer methods. Parkland and Brooke are the commonly used formulas, and recently, a new, an easy formula is been used, i.e. the ‘Rule of TEN’. Fluid resuscitation should be titrated to maintain the urine output of approximately 30–35mL/h for an average-sized adult. The most commonly used fluids are crystalloid, but the phenomenon of creep flow has renewed interest in albumin. In severely burn patients, monitoring with transpulmonary thermodilution together with lactate, ScvO2 and intraabdominal pressures is a good option. Nurse-driven protocols or computer-based resuscitation algorithms reduce the dependence on clinical decision making and decrease fluid resuscitation intake. High-dose vitamin C, propranolol, the avoidance of excessive use of morphine and mechanical ventilation are other useful resources.
El objetivo de la reanimación de los pacientes quemados es mantener la perfusión tisular con el menor aporte de fluidos posible. Para evitar un aporte excesivo podemos usar métodos de estimación computarizados. La fórmula de Parkland y la de Brooke son las más usadas y recientemente se ha propuesto una fórmula sencilla que es la «regla de los diez». Los fluidos de reanimación deben intentar mantener una diuresis de 30-35ml/h. Los fluidos más usados son los cristaloides, pero el fenómeno del «fluid creep» ha renovado el interés por el uso de la albúmina. En pacientes quemados críticos, la monitorización con termodilución transpulmonar junto con lactato, SvcO2 y presión intraabdominal es una buena opción. Protocolos de enfermería y algoritmos de reanimación informáticos reducen la dependencia de las decisiones de los clínicos y disminuyen el aporte requerido. Otras actuaciones útiles son: usar altas dosis de vitamina C, emplear propranolol y evitar el uso excesivo de morfina y de ventilación mecánica.
Over the recent years, the improved survival rates in the critically burn patients are because of the development of resuscitation protocols together with early burn wound closure, improved respiratory and renal support, control of the hypermetabolic response and early enteral nutrition.
Emergency management follows the principles of the Advanced Trauma Life Support Guidelines for the assessment and stabilisation of airway, breathing, circulation, disability, exposure and environment control. Instantaneously, we must assess for the severity and extent of the burn.
The goal of the initial resuscitation of critically burn patients is to replace extracellular fluid losses to maintain end-organ perfusion and prevent burn shock. These patients have a much higher capillary leak than that in septic or trauma patients; thus, they require more aggressive fluid resuscitation. Furthermore, in critically burn patients, only partial compensation can be achieved by fluid resuscitation because of a generalised reduction in sodium ATPase activity and disruption of the cellular transmembrane ionic gradient.1
Clinically, this is manifested by hypovolaemia, haemoconcentration, oedema, reduced urine output and cardiovascular dysfunction.
Under-resuscitation can limit perfusion to potentially recoverable burns, grafted tissue, kidney and other organs that are not directly injured. Over-resuscitation is as deleterious as under-resuscitation. Excessive fluid administration can produce complications such as the conversion of superficial burns into deep burns; abdominal, extremity and orbital compartment syndromes; myocardial oedema; infectious complications; impaired gas exchange, prolonged mechanical ventilation, prolonged hospital stay and multiple organ dysfunction.2
Fluid resuscitationAll critically burn patients should receive formal fluid resuscitation. Delayed or insufficient fluid resuscitation increases mortality. Patients with inhalation injury, electrical burns and those in whom resuscitation was delayed have greater fluid requirement than others. Other factors that increase the fluid requirement are age, narcotic use and ventilator dependence.
Several studies have shown that many patients with major burns receive more fluid than that recommended by the Parkland formula.3,4 Friedrich et al. found that fluid requirements of their current patients were double that of those before.5 This phenomenon had been described by Pruitt, and it was named as ‘fluid creep’.6 To avoid fluid creep, patients should be given the least amount of fluid necessary to maintain adequate organ perfusion.2 Fluid creep usually results from inaccuracies in calculating fluid requirement, from clinician inattention to reducing unnecessary fluid infusions, from the increased use of sedation and narcotic pain medication and from the excess administration of crystalloid. Finally, despite the growing awareness of fluid creep, Cartotto et al. confirmed that their patients were continuing to receive more fluid as expected.7
Fluid selectionThe ideal burn resuscitation fluid is the one that effectively restores plasma volume, with no adverse effects. There are no level I or II publications to guide the choice of resuscitation fluid in the burn patient. The most commonly used fluids are crystalloid solutions. High-volume administration of normal saline can produce dilutional hyperchloraemic acidosis; to avoid it, we use Ringer Lactate (RL) solution. However, RL is not free of some adverse effects such as an increase in neutrophil activation. d-Lactate in RL solution containing a racemic mixture of the d-lactate and l-lactate isomers has been found to be responsible for the increased production of reactive oxygen species and acute respiratory distress syndrome. Another adverse effect that has been demonstrated is that dilution with crystalloids resulted in a hypercoagulable state.
Other balanced solutions have demonstrated effectiveness in burn patients. Thus, Gille et al. showed that Ringer's acetate solution in severe burn is associated with lower SOFA-scores than RL solution.8
Hypertonic sodium solutions have proven to increase the plasma osmolality and limit cellular oedema. Patients resuscitated with hypertonic sodium solutions required lower total volume than that of isotonic solutions in the initial 24h, but after 48h, cumulative fluid loads were similar. The use of hypertonic saline as a resuscitation fluid decreases the risk of abdominal compartment syndrome but does not appear to provide better outcomes than isotonic solutions and has been associated with increased rates of renal failure and death in one retrospective observational study.9
Colloids are more expensive, and in critically ill patients, do not improve survival when compared with crystalloids.10 Some studies were unable to observe increase in multiple organ failure rates or direct association between died and fluid resuscitation in critically burn patients, whereas other studies found that colloid-resuscitated patients required less fluid than those who received crystalloid alone.11 Thus, there is a great controversy about the use of colloids in burn patients, but the American Burn Association (ABA) accepted the addition of colloid-containing fluid following burn injury, particularly after the first 12–24h post-burn, because it may decrease the overall fluid requirements. The fluid creep phenomenon has renewed interest in the use of colloids and even for its early use. A recent meta-analysis suggests that albumin can improve outcomes of burn shock resuscitation.12,13
It has also shown efficacy in reducing the need for fluid intake with other colloids as 6% hydroxyethyl starch (HES) or fresh frozen plasma. Vlachou et al. found that patients in the HES arm required less overall fluid volumes in the first 24h.14 Another study found that resuscitation using both colloid and crystalloid has a better outcome, although they recommended caution when using higher concentrations of artificial colloid and lactated Ringer's solution, of which some adverse effects have been observed. Recently, it has been recommended that HES should be avoided; however, there are no appropriately designed studies in burn patients that show increased acute kidney injury (AKI) or mortality. The incidence of AKI in burns patients resuscitated with and without HES has only been compared in studies by Bechir; although in the first study with ‘old’ HES, they found a greater tendency for renal replacement therapy (RRT) requirement, in the second study performed with ‘modern’ HES, they did not find early renal dysfunction.15,16 Similarly, our group studied 165 patients with HES supplementation and we did not show any increase in the incidence of AKI than expected. Moreover, patients who received HES in the first 12h by hypotension and hypovolemia did not show increased AKI rate.17 Present data is insufficient to conclude that the supplementation of HES during the resuscitation phase may cause renal dysfunction. More studies to clarify the safe doses of HES, its effectiveness in reducing fluid requirements, its effectiveness as rescue treatment in complicated resuscitation or its effectiveness in reducing complications such as compartmental syndromes are needed.
Plasma-resuscitated patients maintained an intra-abdominal Pressure (IAP) below the threshold of complications of intra-abdominal hypertension. This appears to be a direct result of the decrease in volume.18 Although, because of the risk of blood-borne infectious transmission fresh frozen plasma is not recommend without active bleeding or coagulopathy.
In conclusion, it seems that today albumin is more accepted colloid than HES or fresh frozen plasma. However, the best time to use it for treatment is yet to be elucidated. It was thought that colloid in the first 24h of resuscitation would pass through the ‘leaky’ capillaries. However, investigators have recently found that albumin extravasation stops 8–12h after injury, advocating the use of colloid in burn for the first 24h of the resuscitation.19 Some studies with resuscitation including albumin in the early post-burn period reported decreased resuscitation volume requirements. Further studies are required to determine the most appropriate colloid along with formulation and timing for its use.
Blood transfusion are generally unnecessary because of the hemoconcentration. It has been associated with increased mortality in patients with severe thermal burns; therefore, it has been suggested only if the haemoglobin falls below 8g/dL, except for patients at significant risk for an acute coronary syndrome. A prospective, randomised trial of restrictive vs. liberal blood transfusion policy in burns of >20% TBSA is currently being undertaken by the American Burn Association.
Initial fluid requirementsThe first step in resuscitation is careful calculation of burn sizeIt is because it is crucial for decisions about fluid resuscitation. It is known that burn size is often overestimated by as much as 2× by referring hospitals. Computer-aided methods have improved the accuracy of estimating burned areas by including data analysis and reducing subjective differences. Computer planimetry and three-dimensional (3D) scanning allows to determine body dimensions rapidly and reproducibly. Representative efforts are the BurnCasev 3D, EPRI 3D Burn Vision, BAI, Chang Gung Whole Body Scanner and BurnCalc.20
The second step is to choose the optimal route of resuscitationBurn injuries of <20% TBSA can generally be resuscitated with oral hydration, except in cases of facial, hand and genital burns as well as burns in children and the elderly. Adults and children with burns of >20% TBSA should undergo formal intravenous fluid resuscitation.
The third step is to initiate fluid resuscitation by means of a formulaSeveral formulas have been developed to optimise fluid delivery. We can use formulas:
- -
based only in the contribution of crystalloids (Parckland, modified Brooke). Usually we use lactated Ringer's solution.
- -
based in the contribution of colloids from the beginning (Evans, Brooke, Slater)
- -
and based in hypertonic solutions (Monafo) or dextran (Demling).
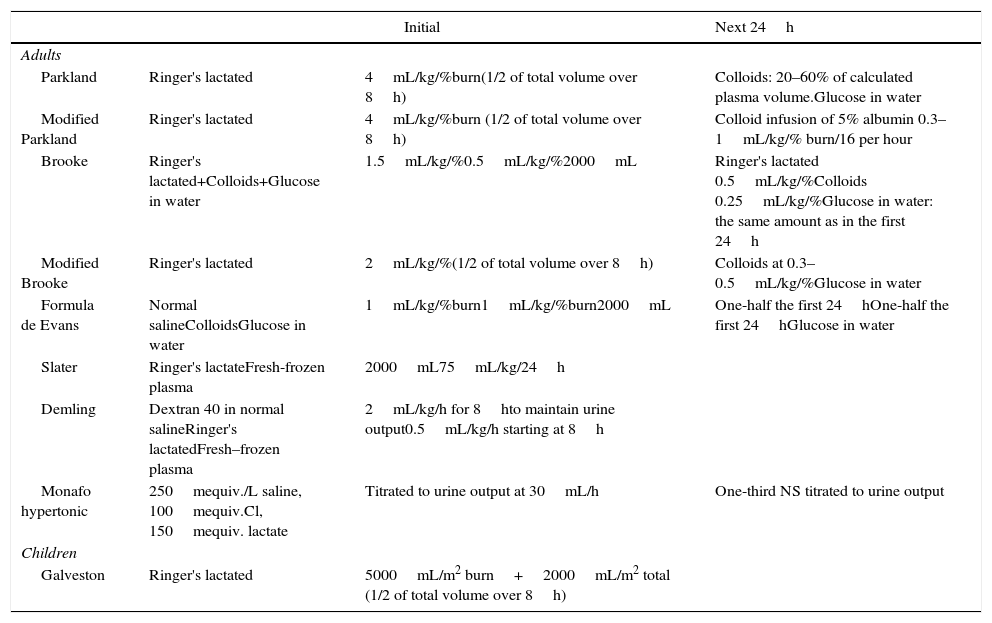
Common formulas used to initiate resuscitation estimate a crystalloid need for 2–4mL/kg body weight/% TBSA during the first 24h (Table 1). The Parkland formula, which calculates the amount of fluid required to resuscitate a patient based on body size and surface area burned, is the most commonly used formula. According to this formula, the fluid requirement during the initial 24h of treatment is 4mL/kg of body weight for each percent of TBSA burned, given intravenously. Superficial burns are excluded from this calculation. An alternative to Parkland is the modified Brooke formula. According to one review, use of the modified Brooke formula may reduce the total volume used in fluid resuscitation without causing harm.21
Burn resuscitation formulas.
| Initial | Next 24h | ||
|---|---|---|---|
| Adults | |||
| Parkland | Ringer's lactated | 4mL/kg/%burn(1/2 of total volume over 8h) | Colloids: 20–60% of calculated plasma volume.Glucose in water |
| Modified Parkland | Ringer's lactated | 4mL/kg/%burn (1/2 of total volume over 8h) | Colloid infusion of 5% albumin 0.3–1mL/kg/% burn/16 per hour |
| Brooke | Ringer's lactated+Colloids+Glucose in water | 1.5mL/kg/%0.5mL/kg/%2000mL | Ringer's lactated 0.5mL/kg/%Colloids 0.25mL/kg/%Glucose in water: the same amount as in the first 24h |
| Modified Brooke | Ringer's lactated | 2mL/kg/%(1/2 of total volume over 8h) | Colloids at 0.3–0.5mL/kg/%Glucose in water |
| Formula de Evans | Normal salineColloidsGlucose in water | 1mL/kg/%burn1mL/kg/%burn2000mL | One-half the first 24hOne-half the first 24hGlucose in water |
| Slater | Ringer's lactateFresh-frozen plasma | 2000mL75mL/kg/24h | |
| Demling | Dextran 40 in normal salineRinger's lactatedFresh–frozen plasma | 2mL/kg/h for 8hto maintain urine output0.5mL/kg/h starting at 8h | |
| Monafo hypertonic | 250mequiv./L saline, 100mequiv.Cl, 150mequiv. lactate | Titrated to urine output at 30mL/h | One-third NS titrated to urine output |
| Children | |||
| Galveston | Ringer's lactated | 5000mL/m2 burn+2000mL/m2 total (1/2 of total volume over 8h) | |
Children may, in addition, require a maintenance fluid and dextrose in the resuscitation fluid.
To simplify calculations, Chung et al. have recently developed the ‘Rule of TEN’ (only applicable for adults). The estimated burn size in % TBSA is multiplied by 10 to derive the initial fluid rate in mL/h. For every 10kg above 80kg, 100mL is added to this rate.22
The forth step is to monitor and titrate fluid resuscitationNeither Parkland formula nor others provides a precise method for determining the fluid requirements, these formulas provide only a starting point.4 Severity of burn, inhalation injury, associated injury, patient age and comorbidities can alter the fluid requirements of individual patients. Thus, the American Burn Association recommended monitoring urine output, pulse, blood pressure and oxygen saturations.23 Distal pulses, capillary refill and colour and turgor of uninjured skin along with serial laboratory determinations can assist the monitoring. Non-invasive blood pressure measurements by cuff are rendered inaccurate because of the interference of tissue oedema. Pulse rate is a much more sensitive monitoring parameter than arterial blood pressure. A pulse rate >120 beats/min usually indicates hypovolemia. However, urine output is the monitoring method most commonly used. Fluid resuscitation should be titrated to maintain an urine output of approximately 0.5–1.0mL/kg/h in adults and 1.0–1.5mL/kg/h in children, and in those patients at risk of rhabdomyolysis due to electrical burn or due to the association with crush injury, Although, now goals are reduced to 30–35mL/h for an average-sized adult.5 The infusion rate should be increased by approximately 20–30% when the urine output is insufficient or other clinical parameters suggest inadequate resuscitation. We can give fluids bolus, but any change to the infusion rate must be made as gradually as possible.
It is important to recognise when fluid resuscitation is not going well. If low urine output persists despite increasing fluid infusion rates, we must consider starting a continuous infusion of albumin 5%, use of vasopressors (vasopressin, norepinephrine) to support blood pressure and/or inotropes to support cardiac function. In these situations, the monitoring of cardiac output and filling pressures has shown a decrease in mortality with a trend towards less renal failure. Various studies demonstrated that urine output as a measure of resuscitation may not provide sensitive or specific monitoring and may not necessarily achieve the best prevention of organ dysfunction either.24,25 This is the reason because severity burned patients should be monitored by other methods. In addition, critically burn patients can have three simultaneous types of shock: cardiogenic, hypovolemic and distributive, and therefore, the use of hemodinamic monitoring is very important.
The finding that pulmonary artery occlusion pressure and central venous pressure are not good indicators of preload has led to increased resuscitation guided by data from the transpulmonary thermodilution (TPTD) method.26–28 This method measures cardiac output (CO), intrathoracic blood volume (ITBV), extravascular lung water (EVLW) and other dynamic parameters. Csontos et al. found that the mean ScvO2 was significantly lower and multiple organ dysfunction (MODS) was significantly higher in the hourly urine output group than in the ITBV group (with resuscitation goal of maintain ITBV Index between 800–850mL/m2). They suggest that ITBV Index may be a better target parameter than urine output.29 Our group found that monitoring TPTD and lactate was safe and avoided excessive fluid intake.30 Endorf et al. recommended monitoring dynamic data such as SVV and the response to the contributions of fluid (increased ITBVI percentage after a given contribution) without neglecting other supplementary data such as lactic acid clearance or intraabdominal pressure.31 In every critically burn patient, bladder pressures should be monitored to identify intraabdominal hypertension. Infusion of large fluid volumes (>250mL/kg) of crystalloid in the first 24h during burn resuscitation are considered a high risk for the development of Abdominal compartment syndrome. Early recognition can allow for timely descompressive (percutaneous or laparotomy).
There are patients with inadequate perfusion despite having achieved the objectives of blood pressure (compensated shock or microcirculatory shock). Thus, it is useful to add global perfusion parameters. A decrease in mixed venous oxygen saturation and an increase in serum lactate suggest inadequate end-organ perfusion. Base deficit and lactate have been shown to correlate with mortality and fluid resuscitation volumes. But so far physiologic manipulation does not change the outcome.32 Futhermore, in critically ill patients arteriovenous DeltaCO2 has been used as tissular perfusion parameter, but there are no studies on burn patients. Other technologies to guide fluid resuscitation are being used such as echocardiography, esophageal echo-Doppler and Doppler ultrasound measurement of renal perfusion. Another recently incorporated technology is the computer-guided infusion based upon urine output33.
Cancio et al. found that clinicians directing the resuscitation were significantly less likely to reduce the rate of fluid infusion when urine output was high than they were to increase fluid rates when urine output was low. In an effort to reduce the dependence on clinical decision making, some centres have been incorporating nurse-driven protocols or computer-based resuscitation algorithms. Some nurse-driven resuscitation showed a significant decrease in fluid volumes during resuscitation.
The fifth steep is to look forward the optimal endpointsWhen it began the monitoring with pulmonary artery catheter high contributions of fluid were needed because we were trying to reach a normal preload. Some studies have shown that TPTD monitors results in more aggressive therapeutic strategies and is associated with a significant increase in fluid administration, but it does not improve preload. However, there are some study which found that when a fluid resuscitation is based on the arterial waveform analysis, the initial fluid volume provided was significantly lower than that delivered on the basis of physician-directed fluid resuscitation (by urine output and mean arterial pressure).
TPTD is a very beneficial tool in the estimation of amounts of fluid resuscitation, although the values of ideal end points need to be adjusted. The traditional values of ITVB, EVLW and cardiac index (CI) are associated with significant tissue oedema.34 In a study, Arlati et al. used a permissive hypovolemia protocol and reduced the volume given as low as possible by titrating the infusion rate to a minimum ITBV value that allowed for at least 2.2L/min/m2 of the CI. They found that this protocol was effective in reducing MODS.35 Zhang et al. did not found superiority in TPTD monitoring compared with that for central venous pressure, but in their algorithm, before administering vasoactive drugs seeks to achieve a minimum preload of 850mL/m2 of ITBV Index. We assume that a severely burn patient may not be adequate to achieve the optimal ITBV Index level36; therefore, it would be better to accept a slightly below-normal level to avoid excessive fluid intake. We studied 165 severely burn patients and found that CI and clearance of lactic acid significantly improved when ITBV Index levels reached 750mL/m2; however, further increases did not considerably improve the haemodynamics.37
The sixth step is pharmaceutical resuscitation- -
Control of oxidative stress with high dose ascorbic acid (vitamin C, 66mg/kg/h) decreases systemic inflammation, decreases fluid requirements and decreases the days under mechanical ventilation.38 There is only one study in which high-dose vitamin C supplementation has been shown to cause secondary calcium oxalate nephropathy. In relation to its infusion is important to note that routine point-of-care glucose analysis can show fictitious hyperglycemia. Membrane-stabilising agents such as zinc, selenium and vitamin E could help in the recovery of burn patients.
- -
Remove proinflammatory cytokines: whether continuous RRT or plasmapheresis exerts a beneficial effect during burn shock resuscitation remains to be determined.
- -
Low doses of propranolol to severely burn patients reduce myocardial oxygen requirements without adversely affecting oxygen delivery, enhance wound healing and decrease the surface area requiring skin grafting.
- -
Intensive insulin treatment improved biochemical markers of inflammation, decreased the catabolic response and decreased the incidence of infection and sepsis. Other drugs, as oxandrolone, are not yet standard-of-care therapies despite clear potential clinical benefit.
- -
Other important aspects are pain management with morphine but avoiding excess that causes fluid creep. It should be also avoided over-treated with routine ventilation.39 Most severe patients need alternative ventilation modes and lung protective ventilation strategy combining lung recruitment maneuverer. Patients with inhalation injury may require mechanical ventilation and adjunctive therapies such as nebulised heparin and tissue plasminogen activator that have demonstrated efficacy through the breakdown of fibrin deposition, maintenance of alveolar structure and reduced obstruction.40 Antithrombin III has also been used in inhalation injury. Other therapies that should be considered are aggressive pulmonary toilet, nitric oxide, N-acetylcysteine, modified tetracycline, nebulised vitamin E and/or bronchodilators.
Finally, new therapeutic strategy to prevent microvascular permeability should be emphasised and developed in future, which may hopefully act as the most basic approach to prevent burn shock and the complications associated with it.
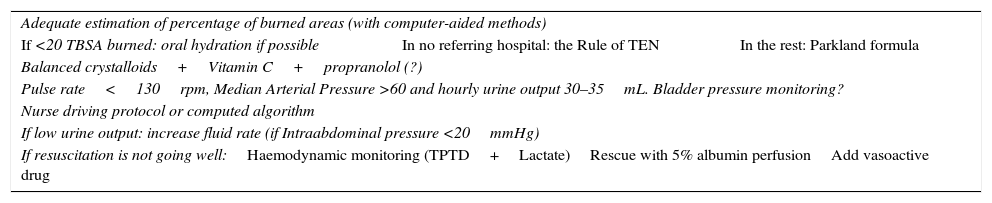
Advances in resuscitation41,42After this review we conclude that advances in resuscitation are aimed at avoiding excessive fluid intake (Table 2). Major advances are as follows:
- 1.
The use of computer-aided methods for improving the estimation of the burned areas.
- 2.
The ‘Rule of TEN’ is an easy formula for the estimation of fluid needs of the patient.
- 3.
The most commonly used fluids are crystalloid, but the phenomenon of creep flow has renewed interest in colloids, even earlier than the first 12h. But there is no consensus about it.
- 4.
The use of vasoactive drugs and colloids may help reduce excess crystalloid administration.
- 5.
The colloid of choice at the moment is albumin; however, further studies are required to determine the most appropriate colloid along with formulation and timing for its use.
- 6.
Now goals of urine output are reduced to roughly 30–35mL/h for an average-sized adult.
- 7.
In severely burn patients when fluid resuscitation is not going well haemodynamic monitoring is necessary. TPTD together with lactate or ScvO2 is the recommended option in some studies.
- 8.
Bladder pressures should be monitored to identify intraabdominal hypertension.
- 9.
More important than monitoring technique is the goal to achieve. With all monitoring techniques, particularly with the TPTD, the target should be to achieve slightly below-normal preload parameters.
- 10.
Control of oxidative stress with high-dose vitamin C should be started in high-risk patients as soon as possible after admission.
- 11.
Other remove proinflammatory cytokines such continuous RRT or plasmapheresis are proposals that have not yet sufficiently proven beneficial.
- 12.
Avoid the excessive use of morphine and mechanical ventilation.
- 13.
Other pharmaceutical resuscitation options include propranolol, nebulised heparin, tissue plasminogen activator, and antithrombin III.
Step for a burn resuscitation.
| Adequate estimation of percentage of burned areas (with computer-aided methods) | ||
| If <20 TBSA burned: oral hydration if possible | In no referring hospital: the Rule of TEN | In the rest: Parkland formula |
| Balanced crystalloids+Vitamin C+propranolol (?) | ||
| Pulse rate<130rpm, Median Arterial Pressure >60 and hourly urine output 30–35mL. Bladder pressure monitoring? | ||
| Nurse driving protocol or computed algorithm | ||
| If low urine output: increase fluid rate (if Intraabdominal pressure <20mmHg) | ||
| If resuscitation is not going well:Haemodynamic monitoring (TPTD+Lactate)Rescue with 5% albumin perfusionAdd vasoactive drug | ||
No financial support.
Conflict of interestThe authors declare no conflict of interest.