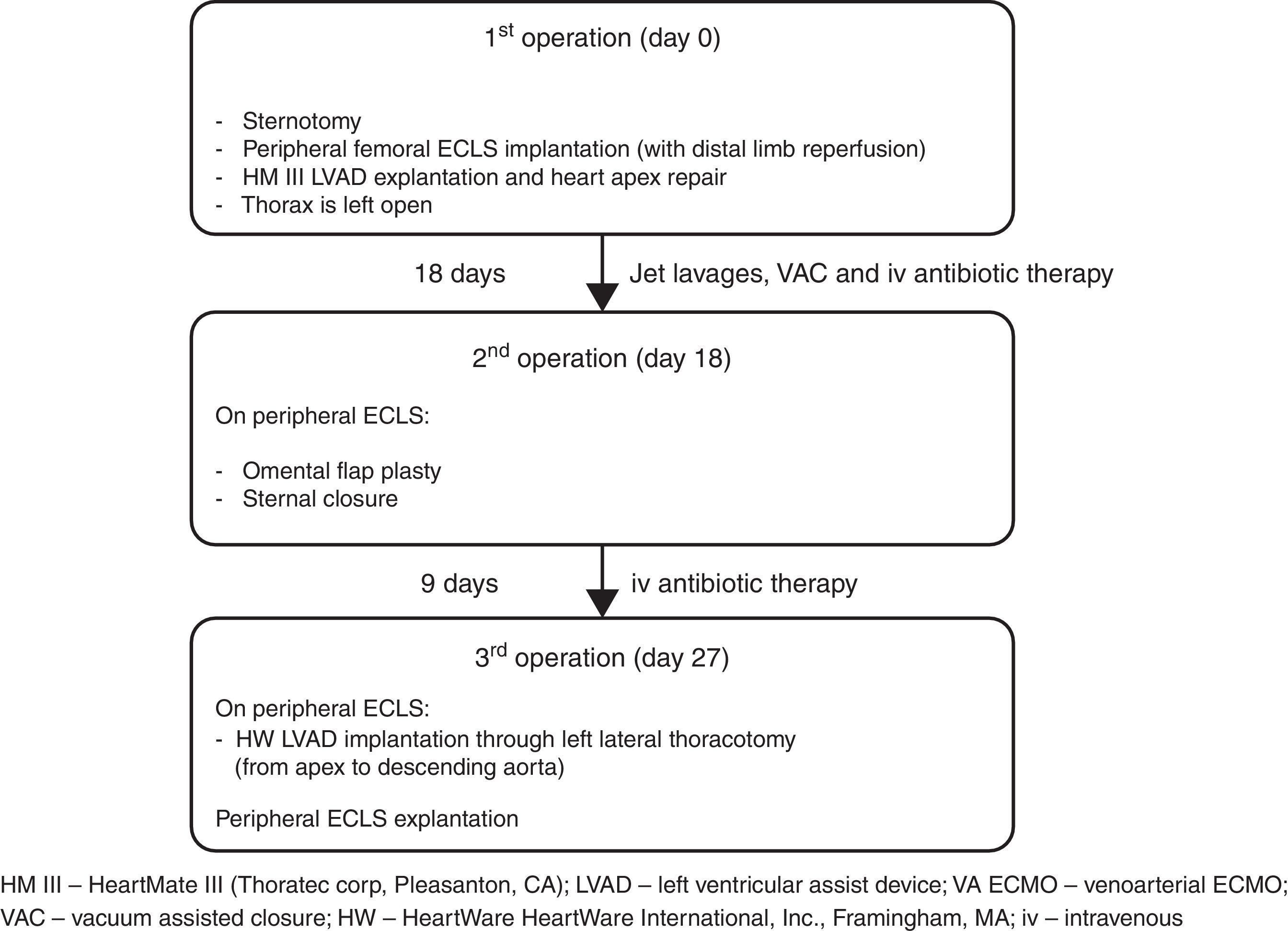
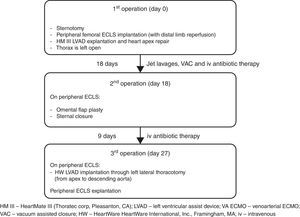
Left ventricular assist device (LVAD) infection is a major cause of re-admission during long-term follow-up and is associated with very high morbidity and mortality. The VAD-related mediastinitis mortality rate is up to 70%.1–3 We describe successful three-stage treatment: 1st stage, explantation of the infected device and implantation of peripheral extracorporeal life support (ECLS), followed by surgical toilet of the infected mediastinum employing jet lavage and vacuum drainage; 2nd stage, mobilization of the great omentum and wrapping around the heart including apex; and 3rd stage, implantation of a new LVAD outside of mediastinum and explantation of ECLS (Fig. 1).
A 42-year-old man (175cm and 110kg-BSA 2.24m2) with ischemic cardiomyopathy, type 2 diabetes mellitus, arterial hypertension, chronic renal failure (stage III), and paroxysmal atrial fibrillation was supported with Heart Mate III LVAD (Thoratec Corp, Pleasanton, CA) at Deutsches Herzzentrum Berlin.
Six months after hospital discharge, the patient presented with a subxyphoidal abscess and signs of systemic infection (white blood cells 12.6×109/L, C reactive protein 24mg/dl, procalcitonin 23.1 micrograms/L, and body temperature 38.9°C). The exit site of the driveline was completely healed and free of infection. The thoracic CT scan revealed presternal collection of fluid with no evidence of intrathoracic involvement. Over 300ml of purulence was evacuated during surgical drainage. The cable was involved in the abscess. Additionally, jet lavage was performed with 3 liters of warm 0.9% NaCl solution and a vacuum assisted closure (VAC) system was applied. Systemic antibiotics (fosfomycin and rifampicin) were administered to address methicillin-sensitive Staphylococcus aureus (MSSA) isolated from purulent drainage and blood samples obtained at hospital admission. The patient was extubated and orally fed. The dosage of the antimicrobic therapy was the maximal dose recommended according to the organ function throughout the whole clinical course.
The patient showed clinical improvement with reduction of infection markers and no fever, but, during 3rd VAC exchange, he presented continuous bleeding from the wound originating from the proximal part of the cable exiting. Loosening of the apical fixation ring connection was suspected. Ten days after hospital admission, re-sternotomy was performed. The apical cuff of the device was found to be partially detached from the apex causing bleeding. Macroscopic evidence of infection was found. Peripheral ECLS was implanted, including distal leg perfusion, and all the components of the LVAD were explanted. The apex of the heart was repaired with prolene sutures and a pericardial patch. In order to achieve full sterilization of the thorax, the sternum was left open and treated with repeated jet lavage and VAC. Systemic multidrug intravenous antibiotic therapy, including gentamicin, flucloxacillin, and linezolid, was administered for several weeks. Fourteen days after surgery limb ischemia occurred and required surgical treatment including thrombectomy and cannulation of the contralateral femoral artery. Already after few days after the first operation the wound samples were sterile in the absence of bacteremia, therefore, after 18 days of ECLS, omental flap plasty and sternal closure were performed.
One week later and 27 days after removal of infected LVAD, with the patient breathing spontaneously on ECLS and without signs of systemic infection (white blood cells 7×109/L, C reactive protein 16.6mg/dl, procalcitonin 0.31 micrograms/L, and body temperature 37.3°C) he underwent re-implantation of an LVAD (HVAD HeartWare International, Inc., Framingham, MA) from apex to descending aorta through left lateral thoracotomy, as described previously.4,5 The new driveline course does not intersect with the previous one and has a new exit site; in the same session the ECLS was explanted.
The postoperative course was complicated by pump thrombosis on day 7 with subsequent pump exchange as described,6 and by groin infection treated with surgical revision and VAC therapy. The patient was stable, without signs of systemic infection and partially rehabilitated. Antibiotic therapy continued although blood cultures were negative, while intraoperative samples from the left groin identified Candida glabrata and Klebsiella pneumonia. According to the antibiogram, rifampicin and flucloxacillin were discontinued (total duration of therapy was 43 and 36 days, respectively), and caspofungin and ceftazidime were started. Unfortunately, after further 35 days of hospitalization the patient died due to sudden intracerebral bleeding.
If the pump is infected, surgical cleaning of the mediastinum, debridement and vacuum treatment and, finally, transposition of the great omentum with wrapping around the pump have been successfully used in our institution and also described by other authors.7–10 Retention of potentially infected inflow or outflow cuffs could contribute to infection recurrence, and apex involvement with loosening of the fixation ring is still an unsolved problem. Severe pump infection may be controlled by device explantation if the cardiac function has fully recovered,11,12 wrapping with the great omentum7–10 or by pump exchange.13 However, in a devastating case of apex infection with bleeding, switching the inflow cannula from apex to left atrium is an option. In our case temporary implantation of the Berlin Heart Excor (Berlin Hear AG, Berlin, Germany) or Levitronix Centrimag (Levitronix, Framingham, MA) from left atrium to ascending aorta14 would bring foreign material into the infected mediastinum. Connection to the descending aorta may lead to infection of the left pleural space precluding the implantation of the long-term pump through a left lateral thoracotomy. Therefore we decided to use peripheral ECLS to avoid opening the pleural space or introducing any additional cannula in or close to the infected situs. The thorax was free from any MCS components and the choice to leave it open allowed application of all topical treatments in addition to iv antimicrobic drugs.
For ECLS, cannulation of the subclavian artery and jugular vein to allow better mobilization of the patient would also have been an option.
It is important that the new pump does not come into contact with the previously infected tissue – in the present case the new pump was implanted, with new access to the thorax, 9 days after the omentum was wrapped around the sterile apex acting as a biological barrier to infection.
In conclusion, pump infection with apex involvement is rare but can be disastrous. A staged surgical approach including complete removal of infected foreign material, sterilization of the wound by surgical debridement and VAC, closure of the wound with the highly immune competent great omentum and, finally, re-implantation of an LVAD outside the infected field may be effective.
Conflicts of interests and source of fundingNo conflicts of interest. The work was supported by departmental funds