Cancer patients are a vulnerable group exposed to numerous and serious risks beyond cancer itself. In recent years, the prognosis of these individuals has improved substantially thanks to several advances such as immunotherapy, targeted molecular therapies, surgical techniques, or developments in support treatment. This coincides with the prolonged survival of oncological patients admitted to the ICU due to critical complications, and under the supervision of intensivists. The time has therefore come to revisit the intensive care support of these patients, which poses new professional as well as organizational challenges. An agreement was signed in 2017 between the SEOM and SEMICYUC with the aim of improving the quality of care of cancer patients with critical complications. The initiative seeks to aid in decision-making, standardize criteria, decrease subjectivity, generate channels of communication, and delve deeper into the ethical and scientific aspects of these situations. This document sets forth the most important reasons that have led us to undertake this initiative.
Los pacientes con cáncer constituyen un colectivo vulnerable expuesto a numerosos riesgos graves, más allá del cáncer en sí. En los últimos años, el pronóstico de estos individuos ha mejorado sustancialmente gracias a varios avances, como la inmunoterapia, las terapias moleculares específicas, las técnicas quirúrgicas o el desarrollo de los tratamientos de soporte. Esto se traduce en un aumento de la supervivencia de los pacientes oncológicos hospitalizados en la UCI y que son llevados por intensivistas. Por lo tanto, ha llegado el momento de revisar el apoyo de cuidados intensivos para estos pacientes, lo que plantea nuevos desafíos profesionales y de organización. En este marco, en 2017 se firmó un acuerdo entre la SEOM y la SEMICYUC con el objetivo de mejorar la calidad de la atención de pacientes oncológicos con complicaciones críticas. Esta iniciativa busca ayudar en la toma de decisiones, estandarizar criterios, disminuir la subjetividad, generar canales de comunicación y profundizar en los aspectos éticos y científicos de estas situaciones. Este documento establece las razones más importantes que nos han llevado a emprender esta iniciativa.
Cancer patients, even those who respond to treatment and become long-term survivors, are exposed to numerous potential risks, including the most obvious one: progression of their cancer. To name just a few of these dangers, respiratory failure is estimated to affect between 10 and 50%1; febrile neutropenia in 5–50%, with severe complications or acute organ failure in some 25%2; thromboembolic disease develops in 20% and is associated with hemodynamic instability in a high percentage of cases.3 Putting end-of-life palliative care aside, many of these patients with severe complications, such as sepsis, respiratory failure, severe toxicities, etc. are cared for on Oncology wards.
Recently, several observational registries have estimated a cumulative incidence of Intensive Care Unit (ICU) admissions of cancer patients of approximately 5–7%, although this figure varies considerably depending on tumor type and other variables.4–6 To translate this into absolute terms, we must bear in mind that cancer is a leading cause of morbi-mortality worldwide, with approximately 14 million new cases per year.7,8 The trend is growing and population estimates point toward a rising number of new cases in the coming two decades to reach 22 million diagnoses every year. Therefore, more and more cancer patients are expected to be eligible for ICU admission.9 At present, roughly 15% of all ICU admissions are oncological patients and, according to the SEER (Surveillance, Epidemiology, and End Results) data, a yearly increase of 6.6% was recorded between 1993 and 2002.10 Although part of that can be attributed to the epidemiological patterns of cancer,11 as oncological treatments become more and more efficacious and patient prognosis improves, the need for critical care is likely to intensify.
Improved prognosis for oncological patientsCancer patients have typically been regarded as highly vulnerable. Nevertheless, for the last 15 years, population registries have consistently reported improved survival.12,13 Beyond the contribution of early diagnosis and palliative and supportive care, this prolonged survival is due to a new generation of targeted cancer treatments.
Since the Food and Drug Administration (FDA) approved the use of the first targeted drug, trastuzumab in September 1998,14 tens of molecules have been integrated into our therapeutic arsenal.15 For instance, imatinib now enables more than 50% of patients with gastrointestinal stromal tumors (GIST), previously deemed rapidly fatal, to survive for more than 5 years.16 The first CTLA-4-blocking antibody for the treatment of melanoma, ipilimumab, was approved in 2011. Since then, new immuno-oncology strategies have revolutionized the treatment of various neoplasms, including pre-treated individuals, previously considered refractory to any therapy. Thus, the KEYNOTE-010 trial has recently proven that pembrolizumab (anti-PD1 antibody) could achieve long-term survival rates of 21–25% in previously treated lung cancer patients, in comparison with the anecdotal survival of those who receive conventional chemotherapy.17 In melanoma, ipilimumab, anti-PD1 antibodies, and anti-MEK or anti-BRAF drugs yield 12-month survival rates of some 75%, which is very uncommon with chemotherapy alone.18 In another common tumor, colorectal cancer, median survival has gone from 6 to more than 30 months in recent years,19 with the possibility of cure following metastasectomy and combined treatments.20 Essentially, all prevalent tumors have attained similar improvements in survival. Furthermore, the research agenda into new targets is vast and other novelties include poly(ADP-ribose) polymerase (PARP) inhibitors, cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors, antiangiogenics, or multi-targeted tyrosine kinase inhibitors, among others.
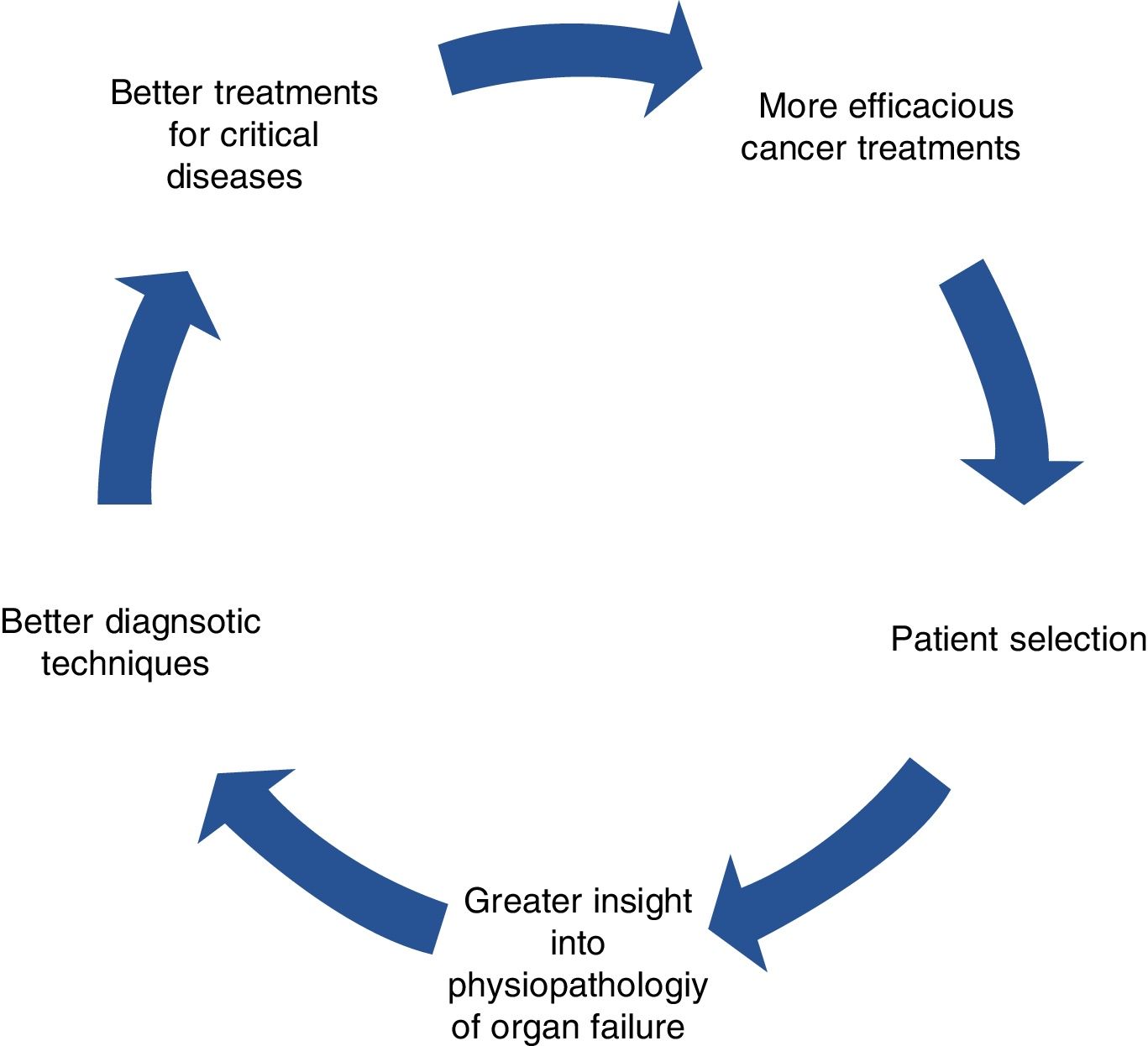
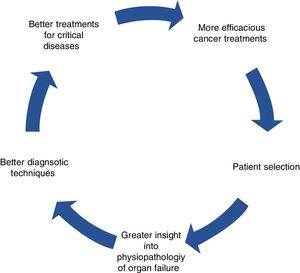
The benefit of these strategies surpasses that of the intrinsic effect of the drugs. The availability of effective therapies comprises a stimulus that opens the way to progress on other levels, including more ambitious indications and surgical techniques for primary tumors and metastases, individualized treatments, and better diagnostic processes and outcome evaluation.
This is a virtuous circle, seen as a feedback process in which a beneficial event sets off a chain of favorable outcomes that spreads to other levels, stimulating efforts of other specialists, which, in turn, results in more cancer patients benefitting from therapies and coming back to amplify the original positive effects (Fig. 1).
Novel drugs not only lead to greater expectations, but also new challenges and toxicities.15,21 In the previous decade, a generation of oncologists had learned that some of the most intense and aggressive antineoplastic strategies (e.g., taxanes for adjuvant use in breast cancer) were theoretically efficacious, but would only truly be applicable when they could properly prevent and treat the most severe complications and toxicities.22,23 Today, it is crucial that these lessons from the past not be squandered; instead, we must endeavor to apply them in the new emerging scenario.
Cause for ICU admission of oncological patientsMore and more of these patients are being admitted to the ICU to manage infectious or non-infectious complications associated with the disease itself, treatment side effects, or for an ailment unrelated to their cancer requiring ICU care. Shock, respiratory failure, neurological impairment, and acute kidney failure are the leading causes of ICU admissions of cancer patients.24,25 Moreover, approximately 30% of all oncological patients (including those with hematologic cancers) who are admitted to the ICU present neutropenia,26 traditionally believed to associate extremely high mortality that advised against their admission to the ICU, given its futility. Moreover, another growing plethora of reasons for admission is currently accepted, such as the management of specific syndromes (e.g., tumor lysis, airway obstruction, severe hydroelectrolyte imbalance, immune-mediated adverse effects, or desensitization to cytostatics).
Admission is currently deemed reasonable for patients with non-small-cell lung cancer with epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) mutations who debut with respiratory failure and require mechanical ventilation (MV). In these conditions, tyrosine kinase inhibitors (TKI), such as erlotinib or crizotinib, are correlated with rapid tumor response and dramatic recovery.27,28 Urgent chemotherapy has even been administered together with intensive support treatments that include MV. The need for chemotherapy for patients with recently diagnosed an advanced tumor should not contraindicate ICU admission; in fact, it may well be feasible in selected cases.29
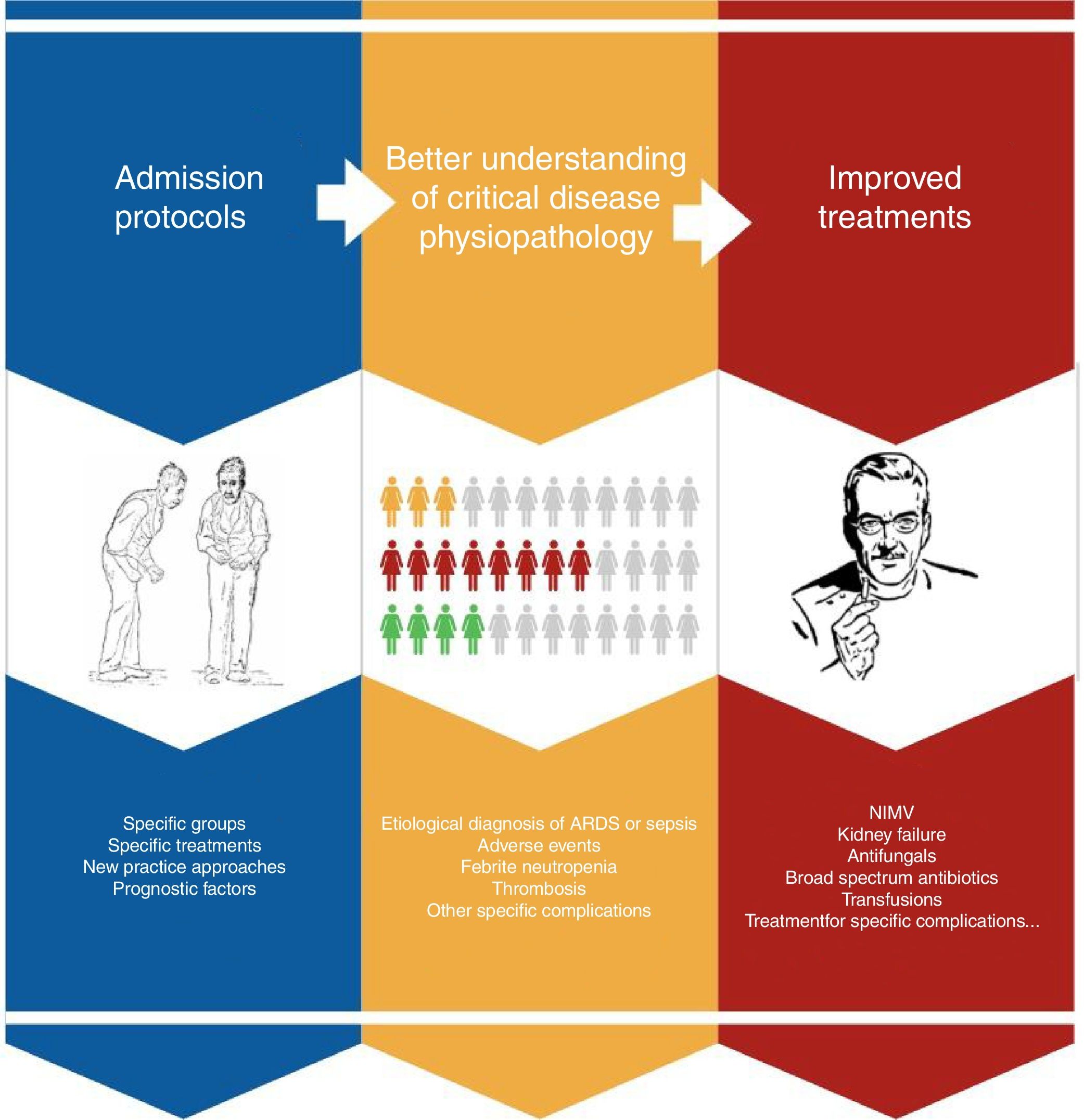
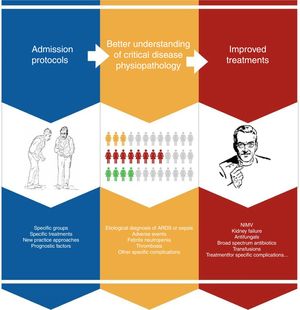
Improved prognosis for oncological patients admitted to ICUAdvances in early diagnosis and the development of new treatments have significantly prolonged survival in cancer patients in the ICU runs by intensivists. This is due to a series of incremental insights into the physiopathology of the diseases, particularly, multiorgan failure, and breakthroughs in diagnostics and treatment (Fig. 2). Progress includes better diagnostic techniques (e.g., diagnosis of respiratory distress), stratification scales, sepsis and febrile neutropenia algorithms, the development of antifungals, or non-invasive MV.30–33 The outcome of all this is that, one by one, results have also begun to improve for subgroups with specific complications, confirming general trends.
In contrast, it has not been easy to demonstrate a general increase in survival in time series of patients with cancer admitted in ICUs.34 There is rarely a direct answer to this type of question, since both complications and patients are diverse and we still lack data from the post-immunotherapy era and long-term outcomes, enriched with accurate descriptions of the oncological context.34 Most of the survival data published in these conditions still lack all the contextualized information from an oncological perspective that we would like, so as to have a clear idea of what is indeed going on.
Nevertheless, series in the 1990s reported extremely high intrahospital mortality rates in this population, especially in individuals with neutropenic sepsis, bone marrow transplant, or ventilatory failure. In recent decades, we have witnessed an important fall in mortality among cancer patients admitted into these units, from 80% (all but universal when MV was needed) to 40% (∼60% when MV is required) at present.35–40 One meta-analysis that pooled 38 studies of cancer patients necessitating admission to ICU examined a total of 6054 patients (2097 neutropenic). During the study period (2005–2015), mortality declined by 11%.26 Interestingly, the crude mortality rate for neutropenic vs. non-neutropenic patients rose by 10%. However, after adjusting for confounding variables, neutropenia did not significantly impact mortality. In fact, it is currently thought that cancer patients benefit from ICU admission just as much as individuals with other underlying medical conditions, such as cirrhosis of the liver or chronic heart failure.40
On the other hand, MV was previously considered to be futile, but given the improved survival rate achieved in the last decade thanks to positive pressure ventilation (PPV),41,42 current thought is that invasive or non-invasive PPV should be used in cancer patients with acute respiratory failure receiving curative or palliative treatment, with good functional status, and consistent with patients’ wishes, since hospital survival is 60% when it is implemented. A recent study conducted in England, Wales, and Northern Ireland of more than 90,000 patients with solid tumors admitted to intensive care between 1997 and 2013 revealed greater survival between 2009 and 2013 as opposed to the previous period and a mortality rate of only 26%.43
Early treatment of the critical cancer patientEarly admission of critical oncological patients improves their prognosis and has been identified as an independent factor associated with higher survival rates.44,45 We know that many of the techniques applied in the ICU achieve better outcomes when initiated early and that delaying treatments entails a worse prognosis for all patients.46,47 This is evident in the case of sepsis that cancer patients often develop, as well as in cases of acute respiratory failure that benefit from early ventilatory support.39,48 Early administration of non-invasive respiratory support (non-invasive MV or high-flow nasal oxygen) has proven to be efficacious in these patients in preventing the need for invasive MV and lower mortality in cancer patients with respiratory failure.49,50
Shared decision-making for cancer patient admission to intensive careWe are currently seeing a paradigm shift as regards treatment approach and admission of patients with active oncological disease to ICU42,51 owing to different circumstances, such as a change in prognosis for cancer in recent years even after being admitted to an ICU,52,53 improved quality of life after receiving treatment, and the evolution of monitoring systems and treatment in Intensive Care, enabling less invasive and aggressive support, adapting treatment to the patient, as well as possibly to a change in thinking about treatment objectives in caring for critical patients.54
In 2011, an international consensus was published on ICU admission criteria in patients with cancer.42 The consensus established different groups of possible alternatives and treatment steps, ranging from admission for the use of all treatment measures available to different treatment options to suit each patient's true aims, including alternatives for treatments that can potentially cause immediate complications and alternatives for palliative treatment.
In general, we can state that at present, admission of this group of patients to the ICU would be justified in four circumstances:
- (1)
The reason for ICU admission must be reversible, regardless of the cancer.
- (2)
The prognosis for the cancer itself justifies applying potentially aggressive treatments, given the expectation of being able to maintain adequate subsequent quality of life.
- (3)
The patient, or their legal representatives when necessary, does not refuse treatment in the ICU.
- (4)
The patient has good quality of life prior to the complication, with feasible cancer treatment options and reasonable expectation for survival.
Be that as it may, we believe that at present, these patients can clearly benefit from the existence of a closer relationship between Oncology and Intensive Care Medicine. This would entail setting up top quality, structured consultations between both services. The prognosis for each patient must be adequately and accurately established, planning ahead (together with the patient and their family) for possible complications, creating plans for future treatment, and contemplating early admission or adequate treatment for organ dysfunction, as well as palliative treatment plans. The ICU trial admission policy, with rapid transition to Palliative Care if evolution is unfavorable, is a particularly appropriate admission model for some patients with advanced cancer.55
Progress undoubtedly implies new responsibilities for all professionals involved in treating cancer; however, they also call for important organizational repercussions for each center's daily activity. Additionally, in terms of pharmacoeconomics, the only way to amortize the huge investments of resources in anti-cancer treatments is through proper, efficient, and rational decision-making that also factors in support for toxicities and complications.
One possibility that has grown in recent years is multi-professional, inter-specialty collaboration in managing these individuals in the initial stages on regular wards, with follow-up by means of systems that alert of both clinical and analytical decline, allowing us to get a jump on complications.50,56
We must all be aware of the fact that we, as professionals as well as all parties concerned, have cognitive biases that will definitely affect decision-making. Being cognizant of the impact of irrational decision-making on such a vulnerable group of patients is the only way to find a truly better alternative in each case. Therefore and insofar as possible, decisions should be protocolized ahead of time, by means of practicable algorithms accepted by all the hospital teams; goals should be discussed ahead of time with the patients whenever possible, taking into account the families’ wishes and values in other situations of uncertainty.
Given that intensive care physicians do not participate in the patient's overall treatment strategy, they will only be aware of the tip of the iceberg; i.e., a specific critical condition. In contrast, the oncologist, lacking sufficient objective elements, confronts a highly subjective decision. Thus, a 15% discrepancy has been reported between oncologists/hematologists as regards the suitability of transferring a patient to the ICU.25
Furthermore, the general trend is to look for strategies to lessen the negative impact of ICU admission in this vulnerable population, thereby enabling more patients to benefit from this kind of care. These efforts include open ICU policies, preventive ICU admissions, and the use of critical treatments in controlled conditions outside of the ICU.42,55 Management is probably clear for the long-term survivor, complications from adjuvant treatment, or sustained response to immunotherapy over time. However, it is less obvious how these strategies should be individually transmitted to other cases (e.g., unevaluated response) and will probably continue to be open to debate.
A new clinical research agenda has therefore been opened that aspires to gain greater insight into the conditions that lead to full recovery and continuation of anti-neoplastic therapy, subjective endpoints, such as health-related quality of life or symptomatic control, the application of shared decision-making models, and admission policies, in light of new options, as well as investigating the effect of new care modalities and critical management outside of the ICU in a contextualized manner.57
The role of the oncologist when the patient is admitted to the ICUIn the age of immunotherapy, determining who is eligible for full code admission (decision to administer all advanced life support techniques if necessary, including cardiopulmonary resuscitation maneuvers and MV), ICU trial (full code for 3–4 days followed by reevaluation of the level of support going forward), or non-admission to ICU can be extremely complex, given the uncertainty surrounding the patient's possibility of becoming a long-term survivor. The oncologist's fundamental role is to contribute their prior knowledge about the patient, prognostic factors, and possibilities for recovery.45,58 Cancer is not a single clinical and molecular reality. At present, the mere label of cancer does not suffice and calls for greater knowledge about its molecular alterations, stage, available treatment options, etc. However, it is worth mentioning that recent developments in treating cancer not only fail to ameliorate uncertainty, but instead, actually increase it, given the impossibility of knowing ahead of time which patient is most likely to become a long-term survivor.59 Once the patient has been admitted to the ICU, the oncologist's role must be to aid in the prevention, diagnosis, and management of specific complications (e.g., drug-induced toxicity, neutropenia, etc.). During follow-up, the oncologist must collaborate with the intensive care physician in decision-making (e.g., initiation of aggressive support measures, transition to Palliative Care, etc.).
SEOM-SEMICYUC collaborationThe time has come to revisit the ICU admission policy of some of these patients to optimize their support treatment. As a result of these reflections between professionals of both societies, in June 2017 the first SEOM-SEMICYUC Framework Collaboration Agreement was signed, with the aim of improving the quality of care of cancer patients with critical complications. This agreement is in line with recent work and consensus with institutions around the world.57 Among the most salient aspects of this agreement are to:
- 1.
Contribute to improving care for cancer patients with critical complications for whom intensification of support treatment is indicated.
- 2.
Aid in shared decision-making between Oncology and Intensive Care Medicine, by jointly drafting Evidence-Based Clinical Practice Guidelines, setting forth the main indications for ICU admission and treatment of cancer patients, addressing their differences and special characteristics with respect to other patients.
- 3.
Contribute to standardizing care for these patients, reducing unjustified variability, subjectivity, and bias in decision-making.
- 4.
Generate channels of communication to boost information exchange and dialog between both specialties, with the aim of delving deeper into the scientific and ethical aspects of decision-making.
- 5.
Contribute to creating working groups dedicated to specific treatment aspects (infections in immunodepressed individuals, non-invasive MV in cancer patients, etc.).
- 6.
Develop joint clinical registries and clinical or basic research projects that enable us to better understand prognostic factors of critical oncological patients and generating evidence of the best diagnostic and therapeutic approaches.
Oncological patients’ survival has improved markedly in recent years, coinciding with a decrease in intrahospital mortality among these individuals. With this comes the need for both oncologists and intensive care physicians to change their thinking once and for all about ICU admission for selected patients. Quality consultation among professionals must be fostered to decide on the best attitude to adopt in each case without bias. All patients with possibilities of being cured should be routinely admitted to the ICU (e.g., adjuvant or neoadjuvant therapies). Likewise, patients with chemosensitive tumors, mutations predictive of response with targeted therapies, possibilities of long-term tumor control with immunotherapy, and other specific situations should be deemed eligible for ICU admission, in a concerted manner, on the basis of their distinct characteristics.
Conflict of interestThe authors declare no conflict of interests.