Oversedation has adverse effects on critically ill patients. The Analgosedation and Delirium Committee of the FEPIMCTI (Pan-American and Iberian Federation of Critical Care Medicine and Intensive Care) conducted a cross-sectional study through a survey addressed to ICU physicians: PANDEMIC (Pan-American and Iberian Study on the Management of Analgosedation and Delirium in Critical Care [fepImCti]).
HypothesisWorsening of these practices in the course of the pandemic and that continued afterwards, with further oversedation.
ObjectivesPerception of analgosedation and delirium practices in Pan-American and Iberian ICUs before, during and after the COVID-19 pandemic, and factors associated with persistent oversedation after the pandemic.
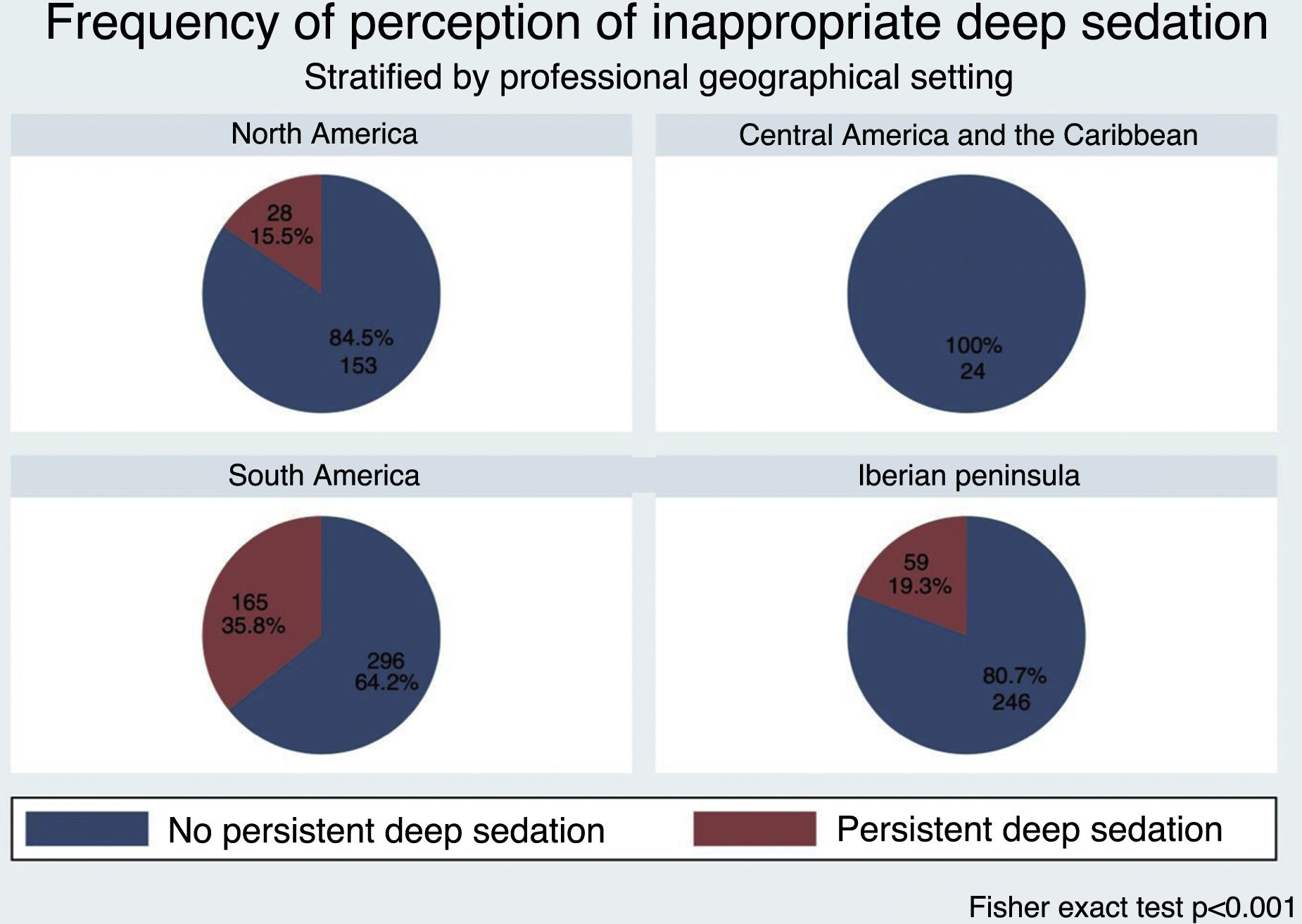
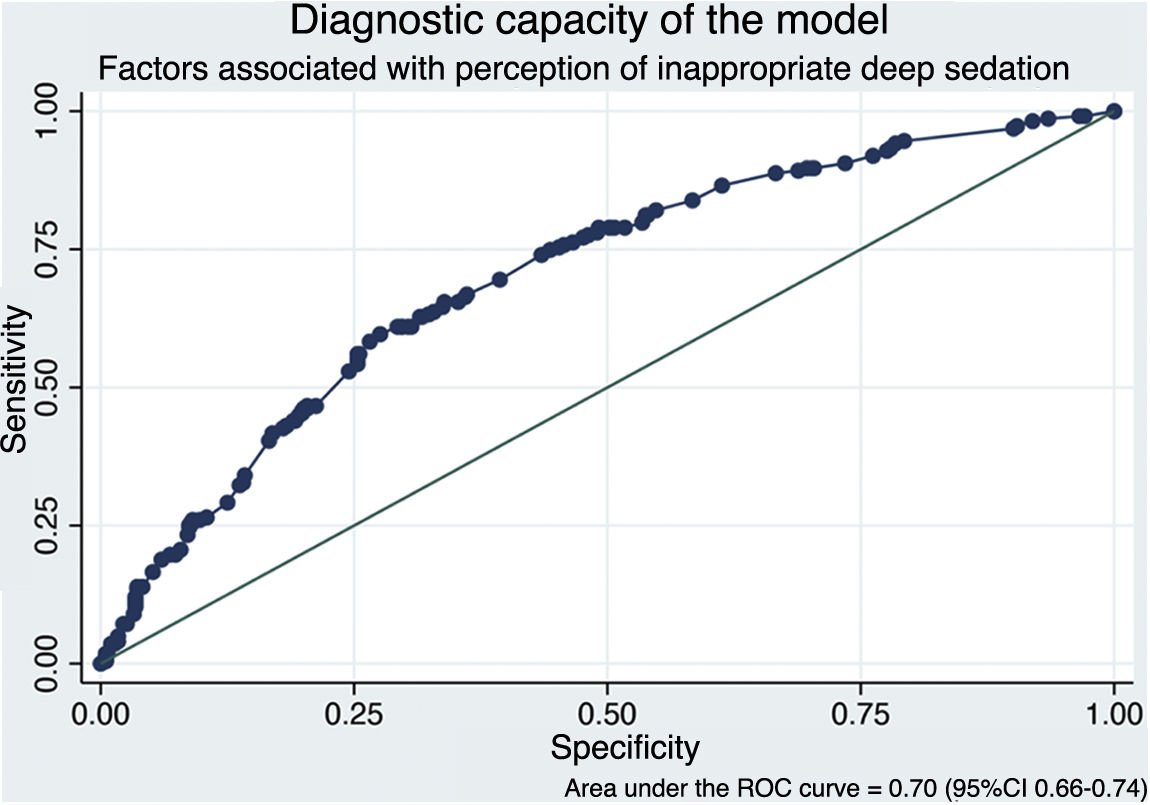
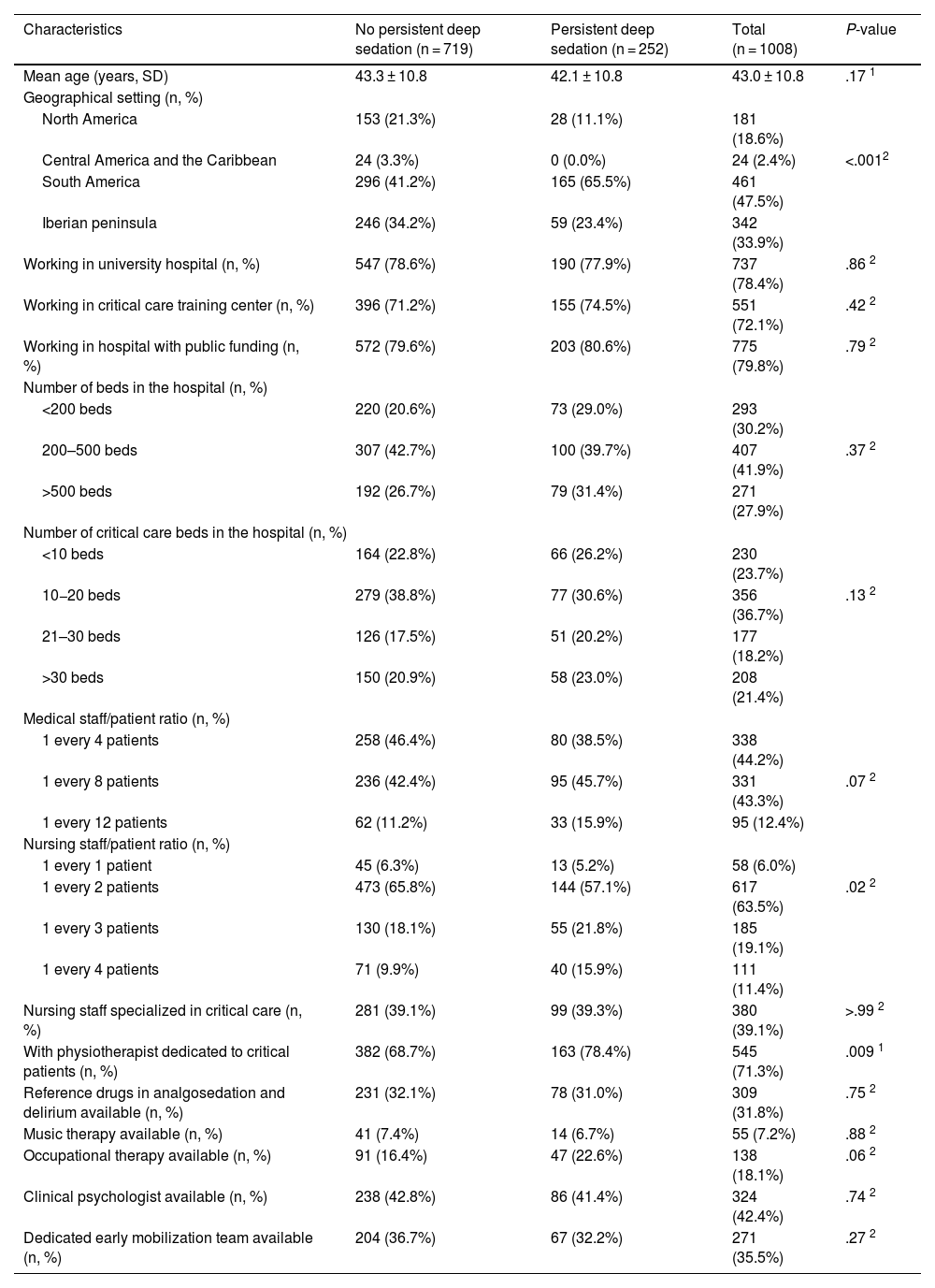
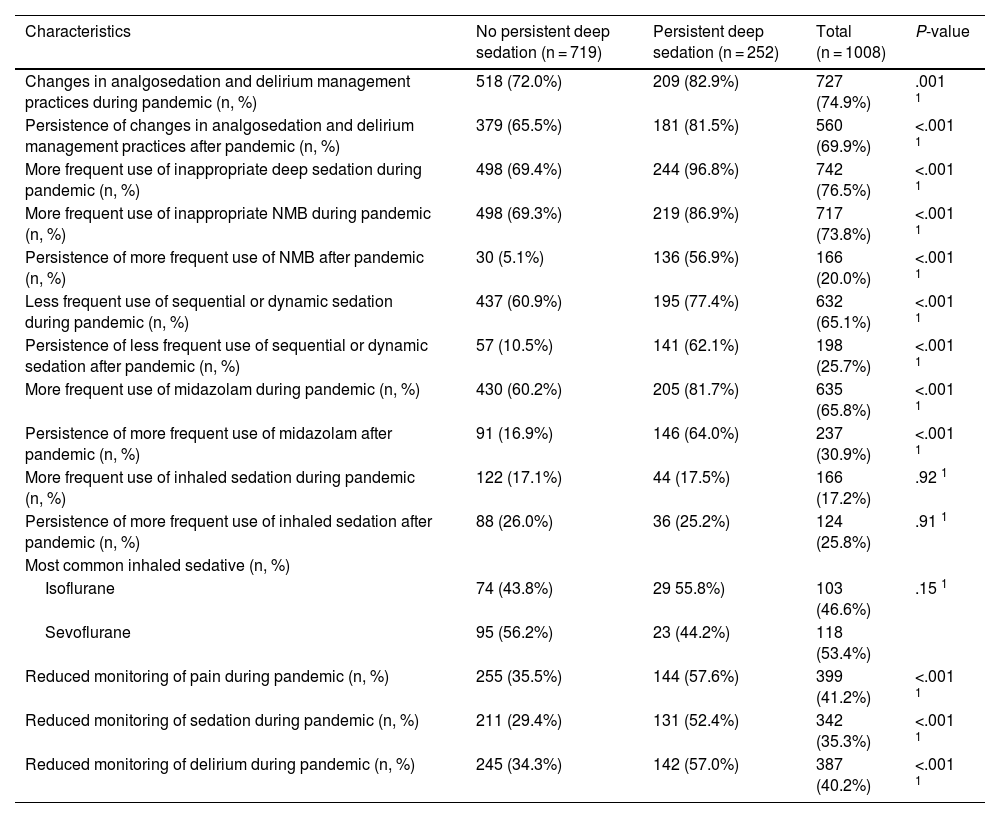
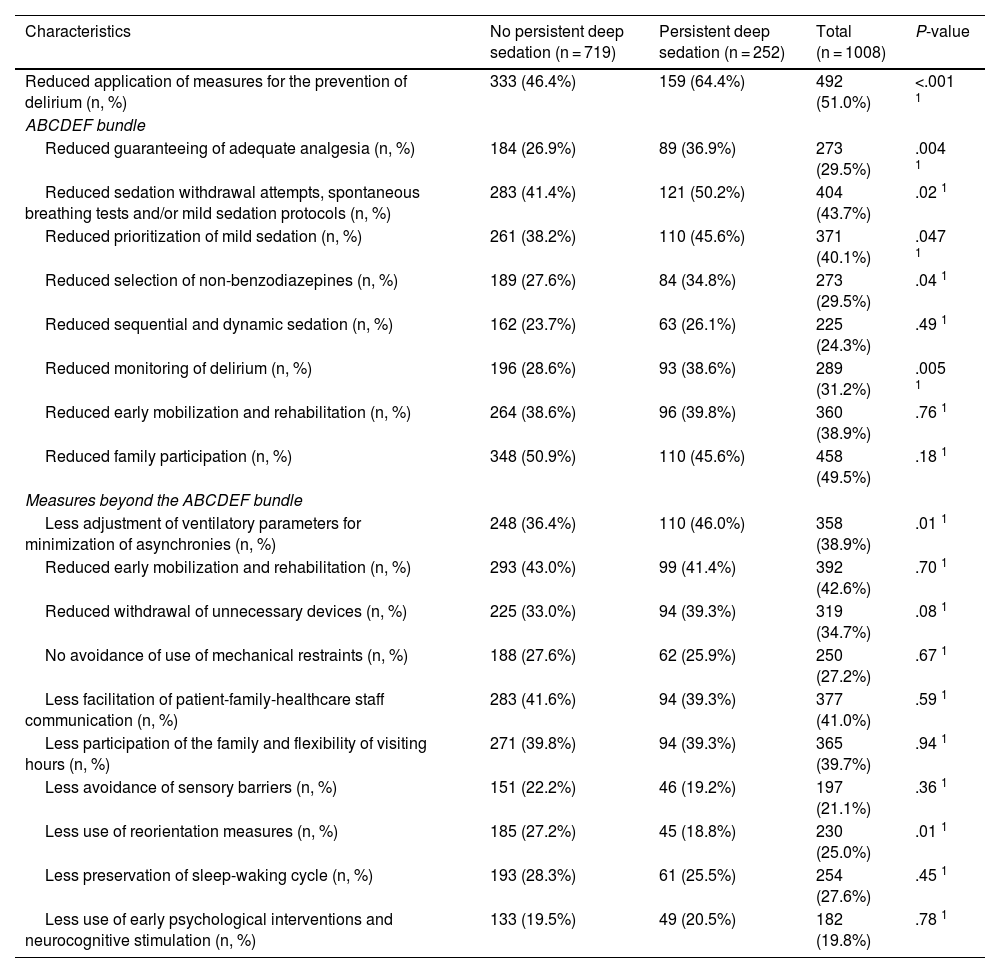
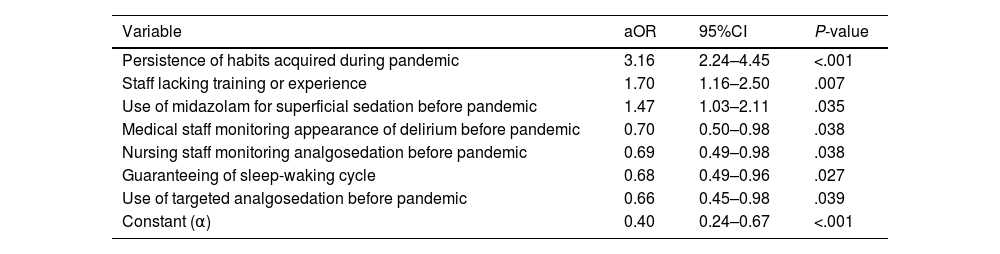
Of the 1008 respondents, 25% perceived oversedation after the pandemic (95%CI 22.4–27.8). This perception was higher in South America (35.8%, P < .001). Main risk factor: habit acquired during the pandemic (adjusted OR [aOR] 3.16, 95%CI 2.24–4.45, P < .001). Main protective factor: delirium monitoring before the pandemic (aOR 0.70, 95%CI 0.50−0.98, P = .038).
The factors identified in this study provide a basis for targeting future interventions.
La sobresedación tiene consecuencias negativas en pacientes críticos. Desde el Comité de Analgosedación y Delirium de la FEPIMCTI (Federación Panamericana e Ibérica de Medicina Crítica y Terapia Intensiva) diseñamos estudio transversal mediante encuesta dirigida a médicos/as de UCI: PANDEMIC [estudio Panamericano e Ibérico sobre manejo de ANalgosedación y DEliriuM en Cuidados Críticos (fepImCti)].
HipótesisEmpeoramiento de dichas prácticas durante la pandemia que persistieron tras ella, con mayor sobresedación.
ObjetivosPercepción de prácticas de analgosedación y delirium en las UCI de la región Panamericana e Ibérica, antes, durante y después de la pandemia COVID-19 y factores asociados a persistencia sobresedación post-pandemia.
De los 1008 encuestados, 25% informó percepción de sobresedación tras la pandemia (IC95% 22.4%–27.8%), mayor en Sudamérica (35.8%, P < .001). Principal factor riesgo: hábito adquirido durante la pandemia (OR ajustado [aOR] 3.16, IC95% 2.24–4.45, P < .001). Principal factor protector: monitorización delirium (aOR 0.70, IC95% 0.50−0.98, P = .038) previo a la pandemia.
Estos factores identificados en el estudio ofrecen una base para dirigir intervenciones futuras.
Article
Go to the members area of the website of the SEMICYUC (www.semicyuc.org )and click the link to the magazine.