To assess the changes in lung aeration and respiratory effort generated by two different spontaneous breathing trial (SBT): T-piece (T-T) vs pressure support ventilation (PSV).
DesignProspective, interventionist and randomized study.
SettingIntensive Care Unit (ICU) of Hospital del Mar.
ParticipantsForty-three ventilated patients for at least 24 h and considered eligible for an SBT were included in the study between October 2017 and March 2020.
Interventions30-min SBT with T-piece (T-T group, 20 patients) or 8-cmH2O PSV and 5-cmH2O positive end expiratory pressure (PSV group, 23 patients).
Main variables of interestDemographics, clinical data, physiological variables, lung aeration evaluated with electrical impedance tomography (EIT) and lung ultrasound (LUS), and respiratory effort using diaphragmatic ultrasonography (DU) were collected at different timepoints: basal (BSL), end of SBT (EoSBT) and one hour after extubation (OTE).
ResultsThere were a loss of aeration measured with EIT and LUS in the different study timepoints, without statistical differences from BSL to OTE, between T-T and PSV [LUS: 3 (1, 5.5) AU vs 2 (1, 3) AU; p = 0.088; EELI: −2516.41 (−5871.88, 1090.46) AU vs −1992.4 (−3458.76, −5.07) AU; p = 0.918]. Percentage of variation between BSL and OTE, was greater when LUS was used compared to EIT (68.1% vs 4.9%, p ≤ 0.001). Diaphragmatic excursion trend to decrease coinciding with a loss of aeration during extubation.
ConclusionT-T and PSV as different SBT strategies in ventilated patients do not show differences in aeration loss, nor estimated respiratory effort or tidal volume measured by EIT, LUS and DU.
Evaluar los cambios de aireación pulmonar y esfuerzo respiratorio generados por dos pruebas de respiración espontánea (PRE) diferentes: tubo en T (T-T) frente a ventilación con presión soporte (PS).
DiseñoEstudio prospectivo, intervencionista y aleatorizado.
ÁmbitoUnidad de Cuidados Intensivos (UCI) del Hospital del Mar.
ParticipantesCuarenta y tres pacientes ventilados durante al menos 24 horas y considerados elegibles para una PRE fueron incluidos en el estudio entre octubre de 2017 y marzo de 2020.
IntervencionesPRE de 30 minutos con T-T (20 pacientes) o PS de 8 cmH2O y presión positiva al final de la espiración de 5 cmH2O (23 pacientes).
Variables de interés principalesDatos demográficos, clínicos, variables fisiológicas, aireación pulmonar evaluada con tomografía de impedancia eléctrica (EIT) y ecografía pulmonar (LUS) y esfuerzo respiratorio mediante ultrasonografía diafragmática (DU) en diferentes momentos: basal (BSL), final de SBT (EoSBT) y una hora post-extubación (OTE).
ResultadosHubo pérdida de aireación medida con EIT y LUS en los diferentes tiempos de estudio, sin diferencias estadísticas de BSL a OTE, entre T-T y PS [LUS: 3 (1, 5.5) AU vs 2 (1, 3) AU; p = 0.088; EELI: −2516.41 (−5871.88, 1090.46) AU vs −1992.4 (−3458.76, −5.07) AU; p = 0.918]. El porcentaje de variación entre BSL y OTE fue mayor cuando se utilizó LUS en comparación con EIT (68.1% vs 4.9%, p ≤ 0,001). La excursión diafragmática tiende a disminuir coincidiendo con una pérdida de aireación durante la extubación.
ConclusionesT-T y PS como estrategias de PRE en pacientes ventilados no muestran diferencias en la pérdida de aireación, ni en el esfuerzo respiratorio estimado ni en el volumen corriente medido por EIT, LUS y DU.
The weaning process consists in liberate patients from mechanical ventilation (MV) removing the endotracheal tube after well tolerate spontaneous breathing.1,2 To determine the optimal moment for extubation remains a challenge, so current guidelines recommend the implementation of a spontaneous breathing trial (SBT) as a tool to predict weaning outcome.2–4 However, 10%–25% of patients who are extubated following a successful SBT need to be reintubated, which is associated with higher mortality.5,6 The T-piece trial (T-T) and low level of pressure support ventilation (PSV) are the most common modes of SBT used in clinical practice but there is still no consensus on which of them are associated with better outcomes.1,4,7,8 While some studies suggested that PSV is related to a higher proportion of successful extubation, some meta-analysis advocate for the T-T method.3,7,9,10 Recently, Li et al., have also published a meta-analysis suggesting comparable predictive power of successful extubation between them in critically ill patients.11
Since loss of aeration and diaphragm dysfunction have been related to weaning failure, they should be taken into account when evaluating an extubation attempt.12–21 Some authors evaluated the utility of electrical impedance tomography (EIT) to predict SBT failure during the weaning process.12–14 Specifically, EIT showed a significant decrease in tidal variation of impedance and end-expiratory lung volume after ventilator disconnection during a SBT.12,13 In this regard, SBT failure compared to success was characterized by a greater lung de-recruitment and more inhomogeneity of ventilation distribution measured by EIT.14 Transthoracic lung ultrasound (LUS) is also a non-invasive, radiation-free, bedside tool, that allows to detect the amount of lung aeration loss or derecruitment of the lung during the SBT and could be an accurate predictor of weaning failure.15,16,22 Different studies agree that there is a greater lung derecruitment evaluated by LUS in patients who develop weaning failure after successfully pass the SBT (both T-T and PSV), in comparison with other who are successfully weaned.16–18,23 Moreover, current literature have suggested that diaphragmatic ultrasonography (DU) could be an accurate tool to identify diaphragmatic dysfunction, to monitor respiratory work-load and, also, to predict extubation failure during weaning from MV and SBT.20,21,24
However, heterogeneous results have been published and there are no recommendations on which SBT to use based on patient characteristics to achieve better outcomes. Nevertheless, differences in aeration between different SBT have not been compared, so further investigation is needed to determine the best approach. In that sense, we hypothesized that both SBT, T-T and PSV, could generate different changes in aeration and respiratory drive which could be related to differences in successful extubation rates. Therefore, this study aimed to evaluate the changes in lung aeration evaluated with EIT and LUS, and respiratory effort estimation using DU, generated by two different SBT (T-T vs PSV).
Materials and methodsStudy designThis is a prospective, interventionist and randomized single center study, conducted at the Critical Care Department of the Hospital del Mar in Barcelona, between October 2017 and March 2020. It was designed and conducted in accordance with the amended Declaration of Helsinki and approved by the Institutional Review Board of the PSMAR (Hospital del Mar, Barcelona, Spain, number 2015/6444/I). Informed consent was obtained from each patient or next of kin.
Patients and selection criteriaAll patients admitted to the Intensive Care Unit (ICU) undergoing MV for at least 24 h who were considered eligible for an SBT were included in the study. SBT eligibility criteria are detailed in the Online Supplement and were based on previous literature.2 The exclusion criteria were age <18 years, pregnancy, neuromuscular disease, presence of tracheostomy and patients with surgical incisions likely to interfere with ultrasound or impedance examination.
Study groups and definitionsEnrolled patients were randomized in a 1:1 ratio by using closed-envelope technique to the following groups: (1) T-T: 30-min SBT with T-piece, or (2) PSV: 30-min SBT with 8-cmH2O PSV and 5-cmH2O positive end expiratory pressure (PEEP). Oxygenation parameters remained unchanged from the mechanical ventilation period before the SBT and during the 30 min of trial in both groups. The intervention was not blinded for the investigators or attending physicians. Since one of the objectives was to assess the impact of both SBT strategies in aeration changes and outcomes after extubation, patients who failed in the SBT were excluded. In this regard, SBT failure was considered when during the trial appeared at least one of the follows as described before2: agitation, anxiety or depressed mental status, diaphoresis, cyanosis, respiratory rate (RR) greater than 35 breaths/min, use of accessory muscles, dyspnea, persistent decrease in oxygen saturation (SpO2) by pulse oximetry <90% with inspiratory oxygen fraction (FiO2) ≥ 0.5, heart rate (HR) above 140 beats/min or greater than a 20% increase from baseline, systolic blood pressure (SBP) lower than 90 mmHg or higher than 180 mmHg, or development of arrhythmia. Failure in extubation was considered when reintubation or the use of any kind of non-invasive respiratory support (NIRS) after the extubation process due to acute respiratory failure (ARF) was needed within the first 48 h after extubation.2,25,26 Those decisions were left to the attending physicians who were blinded to the ultrasonographic neither EIT results. Patients who required reintubation in the first 24 h for other reason than respiratory failure (e.g. surgical intervention) were also excluded.
Study protocolOnline Supplement (OS) Fig. 1 shows the study protocol followed in all the patients included in both groups. Experimental procedures as electrical impedance (EIT) and ultrasonography (LUS and DU measures) were performed at inclusion before randomization (Basal or BSL) and one hour after orotracheal extubation (OTE). In the T-T group in which intrathoracic pressures changed by protocol during the SBT, they were also performed at the end of the 30 min SBT (EoSBT), just before the extubation process. In the PSV group, these measurements were not repeated during the SBT since it was assumed no aeration changes when PEEP was maintained.
Electrical impedance tomography measurements (EIT)EIT was performed using the Swisstom BB2® device (Swisstom, Landquart, Switzerland). The inner surface of the belt containing 32 electrodes (SensorBelt®, Swisstom, Landquart, Switzerland) was placed along the sixth intercostal space around the chest. EIT data were continuously recorded and analysed offline using Swisstom’s EIT Data Analyser (ibeX 1.1, Swisstom, Landquart, Switzerland). EIT-derived parameters as end-expiratory lung impedance (EELI) and EELI variation (ΔEELI) were measured following previous literature.27,28 EELI variation between the different timepoints (ΔEELI or delta) was also recorded and calculated as the EELI difference between the two analysed timepoints. The percentage of variation (Δ%EELI) between two different timepoints was calculated as the ΔEELI divided by the EELI value at the first timepoint and multiplied by 100. Further details are shown in the Online Supplement.
Lung ultrasound measurements (LUS)LUS was performed by trained operators using the Vivid I® ultrasound device (General Electric, Fairfield, CT, USA). Measurements were performed with a 3.5–5 MHz convex probe. Four quadrants of each hemithorax were examined (anterior superior, anterior inferior, lateral superior and lateral inferior) and each one was rated according to the aeration pattern evidenced as previously described22: 0 = normal aeration (A lines or <3 B lines); 1 = moderate loss of aeration (multiple B lines); 2 = severe loss of aeration (confluent B lines); 3 = alveolar consolidation (tissue pattern) which correspond to complete loss of aeration. The LUS score was obtained from the sum of all the quadrant rates, ranging from 0 to 24. LUS variation or delta (ΔLUS) between different analysed timepoints was also recorded, as well as percentage of variation (Δ%LUS).
Diaphragm ultrasound measurements (DU)DU was performed by trained operators using the Vivid I® ultrasound device (General Electric, Fairfield, CT, USA) while patients were lying in a semi-recumbent position with the head of the bed elevated at an angle between 30º and 45º. The images during forced breaths, sighs and cough were excluded. Images were recorded and analysed later by a trained blind investigator. Diaphragm excursion (DE), thickening fraction (TF) and inspiratory time (TI) were measured as previously described.29,30 Further details are shown in the Online Supplement. Variation of all these parameters between the different timepoints were also recorded again as delta (ΔDE, ΔTF, ΔTI).
Data collectionAt inclusion, the following data were collected for each patient: age, gender, body mass index (BMI), toxic habits, comorbidities, chronic treatment, reason for admission to the ICU and severity scores such as Acute Physiology and Chronic Health Evaluation II score (APACHE II) and Sequential Organ Failure Assessment score (SOFA) at ICU admission. ICU treatment, ICU length of stay (LOS) until SBT, duration of MV and ventilator parameters at inclusion were also recorded. Physiological variables as RR, SBP, HR and SpO2 were registered at the three timeline points: BSL, EoSBT and OTE (see supplementary material OS Fig. 1). Need of NIRS as prophylaxis or treatment for ARF at the moment of OTE measurements [non-invasive mechanical ventilation (NIV) or high-flow nasal cannula (HFNC)] were also recorded, as well as the need for reintubation. Finally, ICU LOS and ICU mortality were collected.
Statistical analyisisFor the baseline descriptive analysis of the two study groups, categorical variables were expressed as frequencies and percentages. Following the results of the Kolmogorov–Smirnoff test, continuous variables were expressed as means and standard deviation (SD) when the data followed a normal distribution, or as medians and interquartile range (IR 25, 75%) otherwise. The differences between groups were analysed with the chi-square test for categorical variables and Student’s t-test or Mann–Whitney U test for continuous variables. Paired group analysis was used to compare EIT, LUS and DU data between different timepoints. Statistical significance was established at p ≤ 0.05. Data were analysed using the statistical package for social sciences 19.0 (IBM® SPSS Stadystics®, Chicago, IL, USA) for Windows.
Accepting an alpha risk of 0.05 and a beta risk of 0.2 in a two-sided test, 28 subjects are necessary in first group and 28 in the second to recognize as statistically significant a difference greater than or equal to 4 units. The common standard deviation is assumed to be 5. It has been anticipated a drop-out rate of 10%.
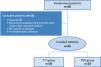
ResultsA total of 58 patients were enrolled in the study. Among them, 43 were finally included, 20 (46%) being randomized to the T-T group and 23 (53%) to the PSV group (Fig. 1). Table 1 shows the principal characteristics of included patients. Briefly, there were no differences in demographic variables, comorbidities and chronic treatment as well as, in the reason for ICU admission, severity scores, and treatment received during ICU stay. Furthermore, no differences were found in extubation failure, in the NIRS used after extubation (used in almost 50% of patients in each group), ICU LOS, or in-ICU mortality between groups.
Baseline characteristics of included patients regarding the study group.
| T-T | PSV | p-Value | |
|---|---|---|---|
| n = 20 | n = 23 | ||
| Demographics | |||
| Age, years | 60 (12) | 58 (19) | 0.654 |
| Gender (male), n (%) | 9 (45.0) | 15 (65.2) | 0.183 |
| BMI, kg/m2 | 23.6 (4.7) | 26.3 (7.0) | 0.152 |
| Toxic habits, n (%) | |||
| Current or former smoker | 15 (75.0) | 12 (52.2) | 0.122 |
| Current alcoholism | 5 (25.0) | 4 (17.4) | 0.541 |
| Comorbidities, n (%) | |||
| Hypertension | 7 (35.0) | 8 (34.8) | 0.988 |
| Liver disease | 5 (25.0) | 5 (21.7) | 0.801 |
| Diabetes mellitus | 4 (20.0) | 4 (17.4) | 0.826 |
| COPD | 5 (25.0) | 3 (13.0) | 0.315 |
| Obesity | 3 (15.0) | 4 (17.4) | 0.832 |
| Neurological disease | 4 (20.0) | 3 (13.0) | 0.538 |
| Heart failure | 1 (5.0) | 4 (17.4) | 0.206 |
| Endocrinological disease | 4 (20.0) | 1 (4.3) | 0.110 |
| Mental illness | 3 (15.0) | 2 (8.7) | 0.520 |
| Coronary artery disease | 2 (10.0) | 2 (8.7) | 0.883 |
| Chronic kidney disease | 0 (0.0) | 2 (8.7) | 0.177 |
| VIH | 2 (10.0) | 0 (0.0) | 0.120 |
| Asthma | 0 (0.0) | 1 (4.3) | 0.345 |
| Chronic treatment, n (%) | |||
| Corticosteroids | 4 (20.0) | 4 (17.4) | 0.826 |
| Statins | 3 (15.0) | 5 (21.7) | 0.571 |
| Insulin | 1 (5.0) | 0 (0.0) | 0.278 |
| Reason for ICU admission, n (%) | 0.687 | ||
| Neurologic | 7 (35.0) | 10 (43.5) | |
| Acute respiratory failure | 6 (30.0) | 9 (39.1) | |
| Shock | 2 (10.0) | 2 (8.7) | |
| Intoxication | 3 (15.0) | 1 (4.4) | |
| Cardiologic | 2 (10.0) | 1 (4.4) | |
| Severity scores at admission | |||
| APACHE II score | 24 (7) | 21 (8) | 0.180 |
| SOFA score | 6 (4) | 6 (3) | 0.521 |
| ICU treatment, n (%) | |||
| Corticosteroids | 10 (50.0) | 13 (56.5) | 0.669 |
| Neuromuscular blockers | 2 (10.0) | 4 (17.4) | 0.485 |
| Insulin | 2 (10.0) | 2 (8.7) | 0.883 |
| Patients’ evolution | |||
| ICU LOS until SBT, days | 7 (4) | 9 (6) | 0.472 |
| Time from MV starting until SBT, days | 6 (3) | 8 (5) | 0.101 |
| Extubation failure, n (%) | 3 (15.0) | 2 (8.7) | 0.650 |
| Reintubation | 2 (10.0) | 0 (0.0) | |
| NIRS due to ARF | 1 (5.0) | 2 (8.7) | |
| NIRS at OTE measurement, n (%) | 10 (50.0) | 11 (47.8) | 0.948 |
| NIV | 2 (10.0) | 2 (8.7) | |
| HFNC | 8 (40.0) | 9 (39.1) | |
| Outcomes | |||
| ICU LOS, days | 14 (10) | 12 (7) | 0.584 |
| ICU mortality, n (%) | 2 (10.0) | 2 (8.7) | 1.000 |
Data expressed as frequencies and percentages (n (%)) or mean (SD).
Abbreviations: SBT: spontaneous breathing trial; T-T: T-piece; PSV: pressure support ventilation; BMI: body mass index; COPD: chronic obstructive pulmonary disease; APACHE II: Acute Physiology And Chronic Health Evaluation II; SOFA: Sequential Organ Failure Assessment; ICU: Intensive Care Unit; LOS: length of stay; MV: mechanical ventilation; NIRS: non-invasive respiratory support; ARF: acute respiratory failure; OTE: one hour after orotracheal extubation; NIV: non-invasive mechanical ventilation; HFNC: high-flownasal cannula; LOS: length of stay.
No differences were found in physiological parameters (RR, SBP, HR and SpO2) evaluated at any study timepoints between groups (see supplementary material OS Table 1).
Aeration changesAeration changes evidenced by EIT and LUS in the two SBT groups at the different protocol monitoring times are shown in Fig. 2. At baseline, no significant differences were found in EELI nor LUS between PSV and T-T (Fig. 2A). During the study period in both SBT groups, EELI and LUS score showed a trend to loss of aeration (trend to decrease EELI score and to increase LUS score) from BSL to EoSBT and OTE in PSV and T-T although without differences between them (Fig. 2A). Fig. 2B shows the ΔEELI and ΔLUS between the different study timepoints in both study groups, showing in all the cases a loss of aeration. In the T-T group, the greatest aeration loss occurred during the SBT period (EELI and LUS). Only differences between study groups in ΔEELI between EoSBT and OTE were statistically significant, showing higher loss of aeration in PSV compared to T-T [−1992.40 (−3458.76, −5.07) AU vs 48.09 (−1319.71, 1084.07) AU, p = 0.027] (Fig. 2B). Percentage of variation calculated between BSL and OTE, was greater when LUS was used (Δ%LUS 60.6%) in comparison with EIT (Δ%EELI 4.7%), p ≤ 0.001 (Fig. 2C).
Aeration according to EIT and LUS by SBT (A), aeration changes by SBT (B) and percentage of variation between BSL and OTE of EELI and LUS (C).
PSV: pressure support ventilation; T-T: T-piece trial; EELI: end-expiratory lung impedance; LUS: lung ultrasound; AU: arbitrary units; BSL: basal; EoSBT: end of spontaneous breathing trial; OTE: one hour after orotracheal extubation.
The subanalysis of aeration by groups of SBT regarding the use of NIRS (NIV or HFNC) at one-hour post-extubation is shown in supplementary material OS Fig. 2. There were no significant differences between groups according to receive NIRS at OTE.
Diaphragmatic ultrasound measuresFig. 3 shows the data of DU in both study groups at different timepoints (DE, TF and TI). There were no differences between any of them at baseline, neither in the other timepoints (Fig. 3A). However, DE tended to decrease during extubation in both SBT groups, coinciding with a loss of aeration. During the weaning process, the decrease trend in DE was greater in the T-T group. Furthermore, there were no differences regarding ΔTF between SBT groups (Fig. 3B).
Diaphragm ultrasound measurements by SBT groups (A) and delta diaphragm thickening fraction by SBT (B).
PSV: pressure support ventilation; T-T: T-piece trial; BSL: basal; EoSBT: end of spontaneous breathing trial; OTE: one hour after orotracheal extubation; TF: diaphragm thickening fraction.
The main findings of this study are that: 1) there are no differences between PSV and T-T in generating aeration loss measured by EIT and LUS, and 2) there are no differences between PSV and T-T regarding respiratory effort or tidal volume estimated using TF and DE respectively.
For this study and in order to assess changes in aeration and respiratory drive between two SBT strategies, we used the two previously described with the greatest difference in respiratory effort and degree of demand between them: T-piece and PSV with 5-cmH2O PEEP.7,8,11,31 In addition, we performed the SBT during 30 min since no differences have been found between 30 min and longer duration (120 min) SBT on checking breathing capacity related to successful extubation rates.32,33 Supporting that, in our study we observed that using the T-T test for 30 min there was no greater loss of aeration than PSV (measured by EIT and LUS), despite in this group the greatest aeration changes occurred during the SBT itself. Moreover, there were no differences in the estimated tidal volume with DE or estimated respiratory effort with TF, compared to the PSV group.
Different studies have found significant lung derecruitment after an SBT based on both, EIT or LUS as a tool to measure pulmonary aeration. Those studies showed that lung derecruitment is greater among patients who develop weaning failure after successfully pass the SBT, in comparison with patients who are successfully weaned.13,14,16,17,23 In the present study, we found a loss of aeration during the weaning process regardless the tool used to evaluate lung derecruitment, or the SBT strategy used. Until now, there is no agreement in the literature regarding superiority of a specific SBT to others in determining extubation failure. Although it has been suggested that SBT with a ventilator support could overestimate patients capability to maintain the cardiorespiratory load after extubation,34 we found no aeration differences between the two SBT during the weaning process. However, while we included a general population, other studies as Cabello et al.8 included specific subpopulation of difficult-to-wean subjects with a high prevalence of left ventricular heart failure where ventilator support could have modified cardiovascular and respiratory response in comparation with T-piece. Moreover, our absence of differences in aeration between both SBT groups could also be explained by using a T-T of only 30 min duration, as well as the small sample size.
Analyses at different study timepoints, showed that the greatest aeration loss occurred in the T-T group was during the SBT period. These results are in accordance with previous studies,7,25,32 where only 30 min SBT allows discrimination of patients who are probably going to fail without producing a greater loss of aeration and could justify the benefit of a rest period with reconnection to the MV after a successful T-T trial before extubation.35 Moreover, we found a higher loss of aeration measured by EELI in PSV compared to T-piece between EoSBT and OTE, probably related with the higher aeration loss during the SBT in the T-T group, as commented. That could also support findings of Subirà et al. who recommended a less demanding ventilation strategy during weaning trial to avoid delay on extubation in patients able to spontaneously breathe without the ventilator, although without assessing pulmonary aeration.7
In addition, literature supports both, EIT and LUS, to measure pulmonary aeration. However, they have not been compared between them. Our results showed that between BSL and OTE timepoints, the Δ%LUS was greater than Δ%EELI which could reflect a higher sensitivity of LUS over EELI in order to detect loss of aeration. This phenomenon could be explained by a greater number of thoracic regions evaluated using LUS.
Current literature suggests that DU could be an accurate tool to identify diaphragmatic contractility in patients under MV to predict extubation failure, to measure loss of aeration through DE changes as an estimator of tidal volume, as well as, to monitor diaphragm effort.24,30,36,37 On the one hand, DE performed during an SBT in intubated patients has proven to be useful to predict weaning failure. In this regard, the cut-off ranged from 10 to 14 mm during normal spontaneous breathing have been selected to diagnose diaphragmatic disfunction.24,38 On the other hand, TF has shown significant correlation with invasive techniques such as oesophageal and transdiaphragmatic pressure monitoring (the gold-standard to assess inspiratory diaphragmatic effort in mechanical ventilated patients)36,39 as well as to predict extubation failure during an SBT (cut-off ranged between 30 and 36%).24,37 In this line, Xia et al.40 showed that patients with a greater loss of pulmonary aeration, had increased diaphragmatic contractility, indicating an additional respiratory effort to compensate lung volume loss. In our study, there were no differences in respiratory effort between groups, in accordance with no differences in aeration loss. Furthermore, in the present study we observed that when MV were disconnected during the SBT in the T-T group, DE decreased. In fact, excursion is directly proportional to inspired volume in both the supine and sitting position, regardless of whether it depends on the force of the diaphragm contraction by itself or passive displacement by ventilator support pressure.24 Consequently, in our study we observed that DE as an estimator of lung tidal volume, tended to decrease with extubation, suggesting a loss of aeration in both groups.
Our study has several limitations to consider. First, prophylactic use of NIV or HFNC after extubation was not protocolized, but at the discretion of the treating physician. Then, it could have been used associated to patients with more comorbidities and higher risk factors for extubation failure. Therefore, since these are not homogeneous criteria, the results of lung aeration could be influenced by the use of NIRS. However, in our cohort of patients, there were no differences between groups regarding the use of NIRS after extubation and this would not be a confusion factor when comparing both strategies. Second, investigators and attending physicians were not blinded to weaning trial randomization group, but clinicians were blinded to their results and clinical management was not affected by the result of the techniques. Third, although it was not the aim of this study, the sample size and the study design did not allow conclusions to be drawn on clinically relevant outcomes such as weaning failure, hospital mortality, nor stratify aeration loss according to weaning failure. Besides, due to this study included general ICU patients, the results could not be extrapolated to specific group of patients as difficult weaning population, in whom SBT modality may have more clinical importance. Finally, this is an unicentric study with a limited number of included patients and further studies should be designed to generalize the results.
ConclusionsT-piece and PSV as different strategies to perform SBT do not show differences in aeration loss, nor estimated respiratory effort or tidal volume measured by EIT, LUS and DU. Further studies will be needed to evaluate the impact of those SBT strategies in a higher sample size to generalize these results.
Author’s contributionsRBC, JMC and PPT conceived and designed the study.
RBC, RM, ID, CC, JMC and PPT, contributed to patient and data recruitment.
RBC, FJP, JRM, JMC and PPT made important intellectual contributions and actively participated in the interpretation of the data.
RBC wrote the paper.
JMC and PPT done contributions to the writing and conceptual exposition of the results.
All authors contributed to critical examination of the paper for important intellectual content and approval of the final manuscript.
FundingThe authors declare that no funds, grants, or other support were received for this study.
Conflict of interest statementsThe authors have no relevant financial or non-financial interests relevant to this article to disclose.
Ethics committee approvalThe study was conducted in accordance with the ethical principles of the Declaration of Helsinki, and approved by the Institutional Review Board of the PSMAR (Hospital del Mar, Barcelona, Spain, number 2015/6444/I).
Acknowledgments to Marta Gas and all the healthcare personnel (physicians, nurses, auxiliaries and the entire logistical team involved) for their dedication and commitment in helping to collect the data that have made this study possible. This research will be part of the doctoral thesis of one of the coauthors. We thank the Department of Medicine of the Universitat Autònoma de Barcelona for the logistical support for its development.