Edited by: Rosario Amaya Villar - Unidad de Cuidados Intensivos, Hospital Universitario Virgen del Rocio, Sevilla, España
Last update: December 2023
More infoTo evaluate the benefits and harmful effects of conservative versus liberal oxygen therapy in patients admitted to the Intensive Care Unit (ICU).
DesignA systematic review and meta-analysis was carried out.
SettingICU.
ParticipantsAdult patients (aged 18 years or older) were randomized to either a lower oxygenation target strategy (conservative oxygen therapy) or a higher oxygenation target strategy (liberal oxygen therapy) in the ICU.
InterventionsPatients received different oxygenation target strategies.
ResultsTen studies involving 5429 adult patients admitted to the ICU were included in the meta-analysis. The pooled results showed no decreased all-cause mortality at 28 days (RR 0.90; 95%CI 0.75–1.09; p = 0.28), 90 days (RR 1.02; 95%CI 0.92–1.13; p = 0.71) or longest follow-up (RR 0.97; 95%CI 0.88–1.08; p = 0.63) among patients administered conservative oxygen therapy. Secondary outcomes were comparable between the two groups. The results of sensitivity analyses and subgroup analyses were consistent with the main analyses.
ConclusionNo beneficial or harmful effects of conservative oxygen therapy were found compared to liberal oxygen therapy in relation to all-cause mortality among adult patients in the ICU. Conservative oxygen therapy did not reduce all-cause mortality at 28 days, 90 days or longest follow-up. Other important clinical outcomes were also comparable between the two groups.
Evaluar los beneficios y los daños de la oxigenoterapia conservadora frente a la liberal para los pacientes de la unidad de cuidados intensivos (UCI).
DiseñoRevisión sistemática y metanálisis.
LugarUnidad de cuidados intensivos (UCI).
ParticipantesLos pacientes adultos (de 18 años o más) fueron asignados al azar para recibir una estrategia de objetivo de oxigenación más baja (terapia de oxígeno conservadora) o una estrategia de objetivo de oxigenación más alta (terapia de oxígeno liberal) en la UCI.
IntervencionesLos pacientes recibieron diferentes estrategias de objetivos de oxigenación.
ResultadosEn este metanálisis se incluyeron diez estudios con 5429 pacientes adultos ingresados en la UCI. Los resultados agrupados no mostraron una disminución de la mortalidad total a los 28 días (RR 0,90; IC del 95%: 0,75 a 1,09; P = 0,28), 90 días (RR 1,02; IC del 95%: 0,92 a 1,13; P = 0,71) y el seguimiento más prolongado (RR 0,97; IC del 95%: 0,88 a 1,08; P = 0,63) para los pacientes tratados con oxigenoterapia conservadora. Los resultados secundarios fueron comparables entre los dos grupos. Los resultados de los análisis de sensibilidad y los análisis de subgrupos fueron consistentes con los análisis principales.
ConclusiónNo se encontraron efectos beneficiosos o perjudiciales de la oxigenoterapia conservadora en comparación con la oxigenoterapia liberal sobre la mortalidad total entre los pacientes adultos en la UCI. La oxigenoterapia conservadora no redujo la mortalidad por todas las causas a los 28 días, a los 90 días ni en el seguimiento más prolongado. Otros resultados clínicos importantes también fueron comparables entre los dos grupos.
Hypoxemia refers to low oxygen tension arterial blood gases or partial pressure of oxygen (PaO2) in the blood, occurring when oxygen supplies fail to meet oxygen demands.1 Hypoxemia is common and generally viewed as deleterious, especially in critically ill patients. Supplementary oxygen is the main strategy for the prevention and treatment of hypoxemia, either by invasive ventilation or non-invasive ventilation, and is wildly used in a hospital setting.
In clinical practice, providing supplemental oxygen for almost all acutely or critically ill patients, regardless of blood oxygen levels, is a longstanding cultural norm. However, this practice is not based on clinical evidence.2,3 A significant proportion of patients are exposed to an excessive oxygen administration. Undoubtedly, hypoxia can lead to cell injury and even death, and adequate oxygen supplementation is necessary,4 while hyperoxia may also cause cell, tissue or organ injury due to enhanced oxidative stress and inflammation.5,6 In recent years, more studies have investigated the relevant between hyperoxia and clinical outcomes in acutely or critically ill patients, while the results are contradictory. Some studies indicated that hyperoxia can be associated with poor clinical outcomes in different patients, such as patients with mechanically ventilation,7 traumatic brain injury (TBI),8 after resuscitation from cardiac arrest,9 and myocardial infarction,10 whilst other studies have not.11–14
In the intensive care unit (ICU), oxygen therapy is administrated for most patients. A number of studies have focused on the fraction of inspired oxygen or targets of arterial oxygenation in these patients, however, the management of oxygenation targets remains challenging in critically ill patients. Four previous systematic reviews all reached the conclusions that a liberal oxygen therapy strategy in adult patients admitted to the ICU could increase mortality and the number of adverse events compared with a conservative oxygen therapy strategy.15–18 However, two multicenter randomized control trials (RCTs) recently published in the New England Journal of Medicine involving ICU patients with hypoxemic respiratory failure, or mechanical ventilation found no significant differences in clinical outcomes between the conservative oxygen therapy groups and liberal oxygen therapy groups.19,20 Additionally, another similar RCT involving ICU patients with acute respiratory distress syndrome (ARDS) found conservative oxygen therapy may increase 90-day mortality and mesenteric ischemic events.21
As new high-quality RCTs have been published and the results are inconsistent with previous studies, we performed this systematic review of RCTs with meta-analysis and trial sequential analysis (TSA) to evaluate the benefits and harms of conservative versus liberal oxygen therapy in critically ill patients in the ICU.
MethodsThis study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA statement) guidelines.22 This meta-analysis was registered on PROSPERO (Registration number: CRD42021234555).
Eligibility criteriaStudies were included if they met the following criteria: (1) population: adult patients (aged 18 years or older) admitted to ICU; (2) patients were randomized to receive either a lower oxygenation target strategy (conservative oxygen therapy) or a higher oxygenation target strategy (liberal oxygen therapy), the aim of which was measured by any one of the following: fraction of inspired oxygen (FiO2), PaO2, peripheral oxygen saturation (SpO2), or arterial oxygen saturation of hemoglobin (SaO2); (3) studies reported at least one of the following outcomes of interest: including 28-day all-cause mortality, 90-day all-cause mortality, or the longest follow-up all-cause mortality; (4) study type: RCT. Studies that only including patients with chronic respiratory diseases were excluded.
Search strategy and selection processStudies were identified by searching the Cochrane Central Register of Controlled Trials Library database, PubMed and EMBASE from inception through to February 1, 2021. We did not put any restrictions on publication language. The detail of search strategy for PubMed was provided in the Additional File 1. To find additional citations, the reference lists of the included studies and recent reviews were also screened. Two authors (X.L. and D.L.) independently screened titles and abstracts of all citations. Studies deemed potentially relevant were further assessed by reading full-text. Disagreements between two authors were resolved through discussion or by consulting a third author (F.Z.) when necessary.
Data extraction and risk of bias assessmentTwo authors (X.L. and D.L.) independently extracted the following information in a standard form: the first author, country, study center, publication year, participants (mean age of the patient, number of patients randomized, number of missing patients, number of patients finally analyzed, male percentage, type of population, inclusion criteria, and exclusion criteria), details of intervention (types of oxygen intervention, FiO2, oxygenation target, oxygen delivery system, and duration of intervention), all clinical outcomes. Two authors (X.L. and D.L.) independently evaluated the risk of bias for each of these studies according to the Cochrane risk of bias assessment tool.23 The study would be classified as high risk of bias if any of bias domains were assessed as high risk. Disagreements between two authors were resolved through discussion or by consulting a third author (F.Z.) when necessary.
OutcomesThe primary outcomes were all-cause mortality (at 28 days, 90 days, and the longest follow-up). The secondary outcomes included ICU all-cause mortality, length of hospital stay, length of ICU stay, mechanical ventilation free days through day 28, new-onset pneumonia, new-onset infection, new-onset ARDS, new-onset atelectasis, and new-onset pneumothorax, new-onset mesenteric ischemia.
Statistical analysisFor dichotomous outcomes, we calculated the risk ratios (RRs) and 95% confidence intervals (CIs) by the Mantel–Haenszel method. For continuous outcomes, we used the Inverse Variance method to pool the mean differences (MDs) and 95% CIs. Concerning potential heterogeneity, we used a random effect model in all analyses. Heterogeneity among the included studies was assessed using the I2 statistic, which estimates the proportion of total variation across studies due to heterogeneity rather than chance.24 We performed funnel plots to assess publication bias by inspecting its asymmetry. And Egger’s test was also performed to detect publication bias.25
Subgroup analyses for the primary outcome were performed according to oxygen delivery system (invasive mechanical ventilation, others) and duration of intervention (more than 48 h, less than 48 h). Sensitivity analyses for the primary outcomes included the following: using a fixed-effects model, excluding the study of specific diseases, excluding the study dividing groups by FiO2, and excluding the study conducted by Schjørring et al.
To assess the potential impact of the missing participants for the primary outcomes, we performed a best-worst scenario analysis, in which we assumed all missing participants in the conservative oxygen therapy group would survive, and all missing participants in the liberal oxygen therapy group would die. A worst-best scenario analysis was also performed, in which all missing participants in the conservative oxygen therapy group were assumed to die, and all missing participants in the liberal oxygen therapy group were assumed to survive. All above statistical analyses were performed by Review Manager (version 5.3) and STATA (version 14.0). If a two-sided P value was less than 0.05, the results were considered statistically significant.
TSA was performed to control both type I and type II errors due to multiple testing and sparse data.26 TSA was done using TSA software (version 0.9 Beta, Copenhagen Trial Unit). We used a random effect model to construct the cumulative z curve. TSA was performed to maintain an overall 5% risk of a type I error. An anticipated relative risk reduction (RRR) of 20.0% with a power of 90% was used to calculate the required information size to detect or reject an intervention effect. And the control event rate was adjusted according to the relevant rate of the liberal oxygen therapy group in our meta-analysis. When the cumulative Z-curve crossed the trial sequential monitoring boundary, reached the required information size, or entered the futility area, a firm evidence for accepting or rejecting the anticipated intervention effect may have been established, indicating that further trials may be superfluous. In contrast, if the boundary was not surpassed, and the required information size had not been reached, it indicated that more trials would be required.27,28
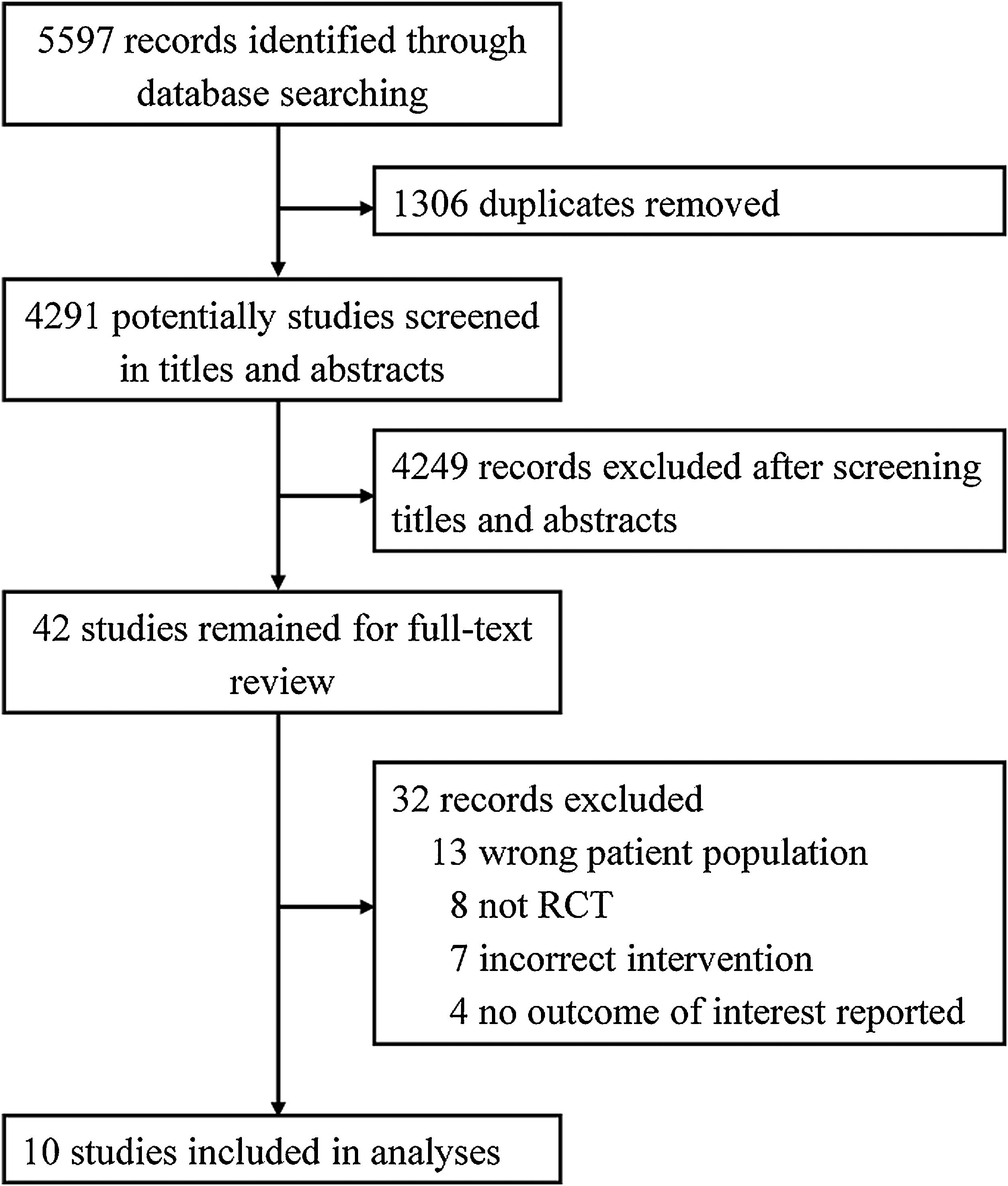
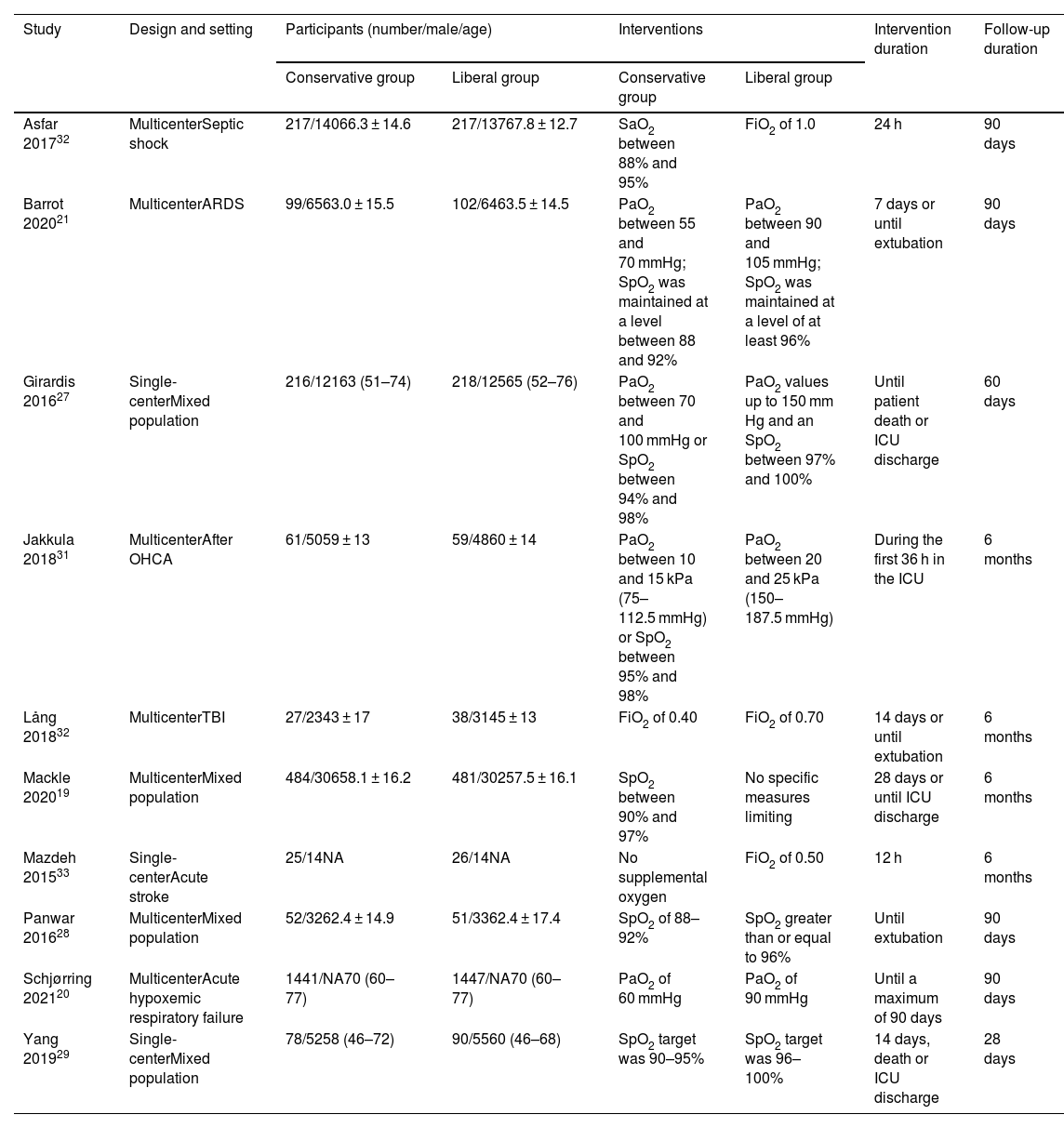
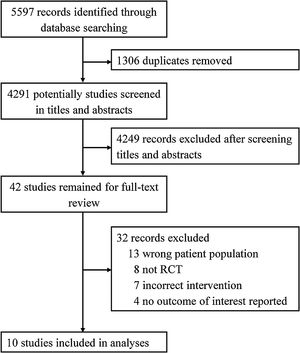
ResultsAccording to our search strategy, 5597 potentially studies were identified. After removing duplicates, 4291 studies were screened by titles and abstracts and 42 studies were further screened by reading full-text. Finally, ten studies involving 5429 adult patients admitted to the ICU with critical illness,19,29–31 septic shock,32 ARDS,21 out-of-hospital cardiac arrest (OHCA),33 traumatic brain injury (TBI),34 severe acute stroke,35 or acute hypoxemic respiratory failure20 were included in this meta-analysis (Fig. 1). The studies were published from 2015 to 2021. The number of participants ranged from 65 to 2888. Most studies were assessed as low risk of bias (see Additional file 2). Six studies only included patients who received invasive mechanical ventilation at randomization.19,21,30,32,34 All other studies randomized patients to liberal versus conservative oxygen therapy using oxygenation target, except for two studies using FiO2.34,35 Details of the included individual studies characteristics were shown in Table 1 and Appendix B Additional file 3.
Characteristics of included studies.
| Study | Design and setting | Participants (number/male/age) | Interventions | Intervention duration | Follow-up duration | ||
|---|---|---|---|---|---|---|---|
| Conservative group | Liberal group | Conservative group | Liberal group | ||||
| Asfar 201732 | MulticenterSeptic shock | 217/14066.3 ± 14.6 | 217/13767.8 ± 12.7 | SaO2 between 88% and 95% | FiO2 of 1.0 | 24 h | 90 days |
| Barrot 202021 | MulticenterARDS | 99/6563.0 ± 15.5 | 102/6463.5 ± 14.5 | PaO2 between 55 and 70 mmHg; SpO2 was maintained at a level between 88 and 92% | PaO2 between 90 and 105 mmHg; SpO2 was maintained at a level of at least 96% | 7 days or until extubation | 90 days |
| Girardis 201627 | Single-centerMixed population | 216/12163 (51–74) | 218/12565 (52–76) | PaO2 between 70 and 100 mmHg or SpO2 between 94% and 98% | PaO2 values up to 150 mm Hg and an SpO2 between 97% and 100% | Until patient death or ICU discharge | 60 days |
| Jakkula 201831 | MulticenterAfter OHCA | 61/5059 ± 13 | 59/4860 ± 14 | PaO2 between 10 and 15 kPa (75–112.5 mmHg) or SpO2 between 95% and 98% | PaO2 between 20 and 25 kPa (150–187.5 mmHg) | During the first 36 h in the ICU | 6 months |
| Lång 201832 | MulticenterTBI | 27/2343 ± 17 | 38/3145 ± 13 | FiO2 of 0.40 | FiO2 of 0.70 | 14 days or until extubation | 6 months |
| Mackle 202019 | MulticenterMixed population | 484/30658.1 ± 16.2 | 481/30257.5 ± 16.1 | SpO2 between 90% and 97% | No specific measures limiting | 28 days or until ICU discharge | 6 months |
| Mazdeh 201533 | Single-centerAcute stroke | 25/14NA | 26/14NA | No supplemental oxygen | FiO2 of 0.50 | 12 h | 6 months |
| Panwar 201628 | MulticenterMixed population | 52/3262.4 ± 14.9 | 51/3362.4 ± 17.4 | SpO2 of 88–92% | SpO2 greater than or equal to 96% | Until extubation | 90 days |
| Schjørring 202120 | MulticenterAcute hypoxemic respiratory failure | 1441/NA70 (60–77) | 1447/NA70 (60–77) | PaO2 of 60 mmHg | PaO2 of 90 mmHg | Until a maximum of 90 days | 90 days |
| Yang 201929 | Single-centerMixed population | 78/5258 (46–72) | 90/5560 (46–68) | SpO2 target was 90–95% | SpO2 target was 96–100% | 14 days, death or ICU discharge | 28 days |
ARDS, acute respiratory distress syndrome; SaO2, arterial oxygen saturation of hemoglobin; FiO2, fraction of inspired oxygen; PaO2, partial pressure of arterial oxygen; SpO2, peripheral oxygen saturation; ICU, intensive care unit; OHCA, out-of-hospital cardiac arrest; TBI, traumatic brain injury; NA, not available.
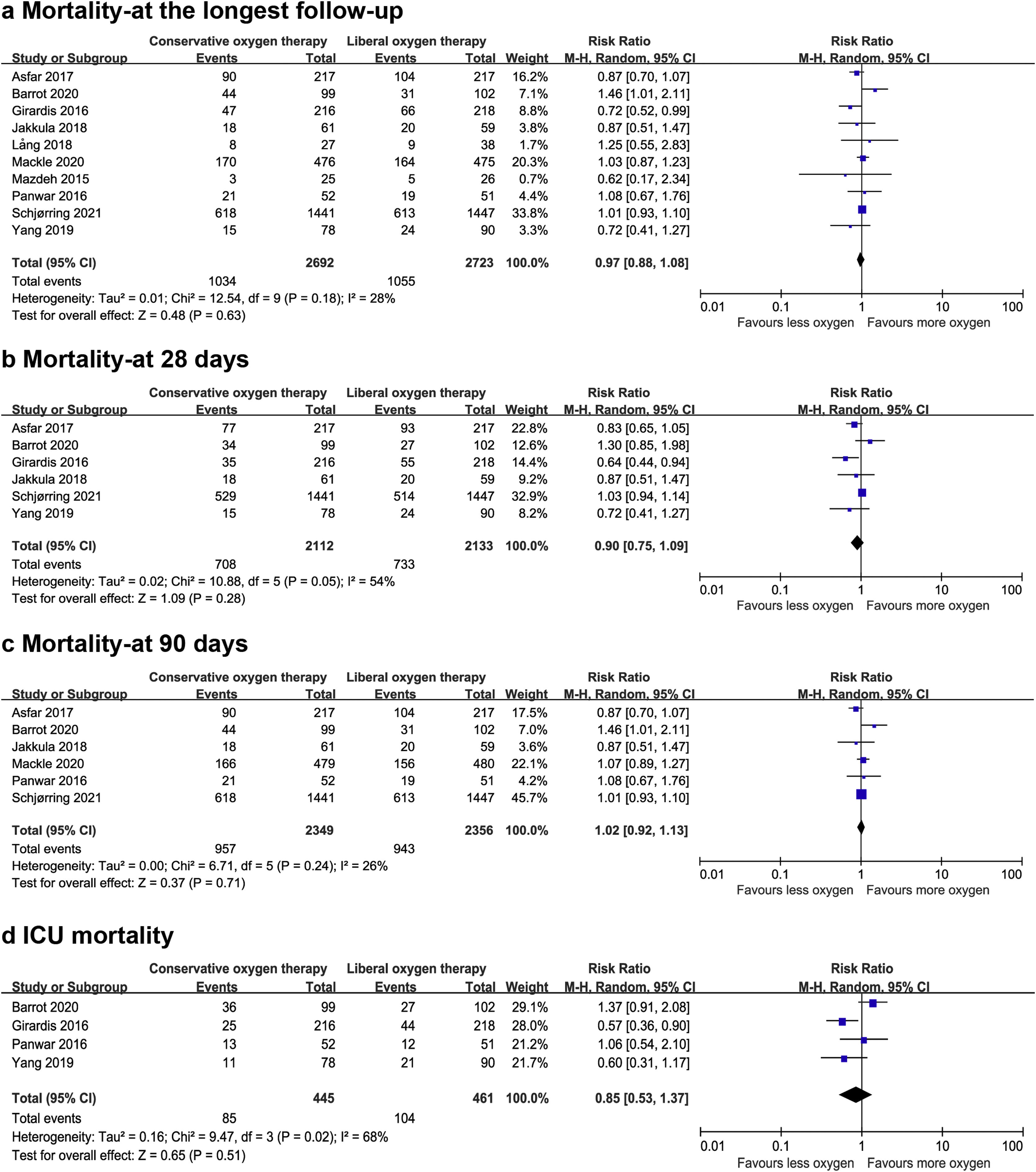
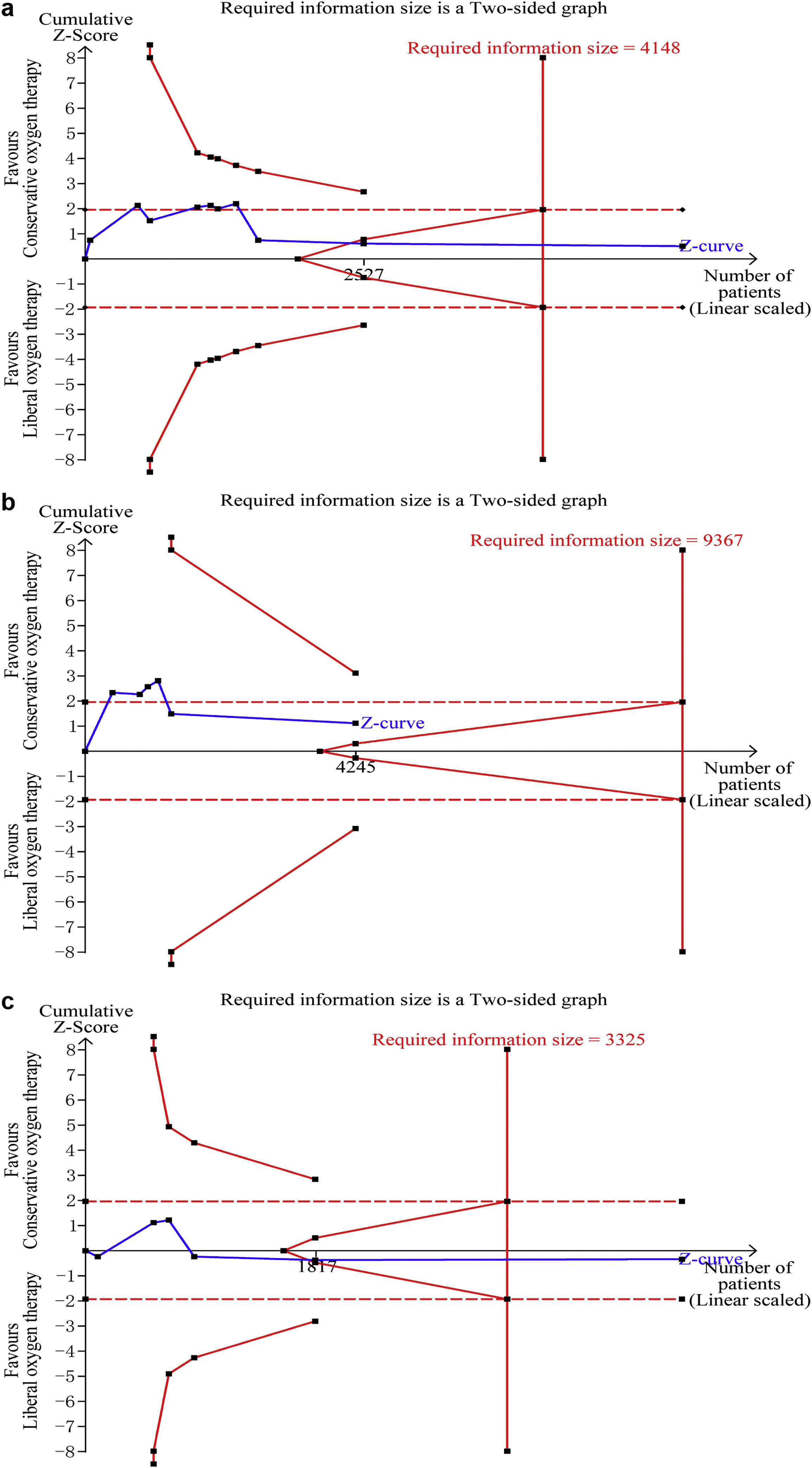
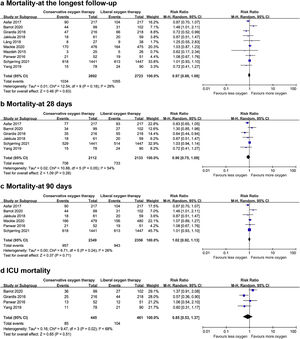
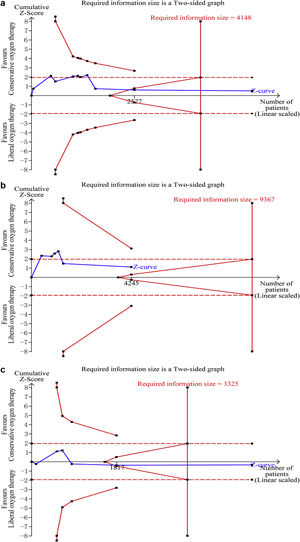
Data on mortality were available for all ten studies. The mortality in the conservative oxygen therapy group and the liberal oxygen therapy group were 38.4% (1034 of 2692 patients) and 38.7% (1055 of 2723 patients) at the longest follow-up, respectively. No significant difference was detected between two groups (RR 0.97; 95% CI 0.88–1.08; p = 0.63; I2 = 28%; Fig. 2a). Moderate heterogeneity was detected. There was no obvious asymmetry in funnel plots by visually inspecting, while Egger’s test indicated that publication bias may exist (p = 0.014, see Additional file 4a). TSA result showed that the required information size was 4148. The cumulative Z curve reached the required information size and crossed the futility boundary, suggesting that a RRR of 20% or greater could be rejected (Fig. 3a).
Trial sequential analysis. (a–c) The cumulative Z curve (complete blue line) was constructed using a random effect model. Etched red line shows conventional test boundary. Complete red line represents the trial sequential monitoring boundary. (a) TSA for mortality at the longest follow-up. A diversity-adjusted information size of 4148 patients were calculated on the basis of using alfa = 0.05 (two sided), beta = 0.10 (power 90%), an anticipated relative risk reduction (RRR) of 20.0%, and a control event rate of 38.7%. The cumulative Z curve crossed the futility boundary and reached the required information size. (b) TSA mortality at 28 days. A diversity-adjusted information size of 9367 patients was calculated on the basis of using alfa = 0.05 (two sided), beta = 0.10 (power 90%), an anticipated relative risk reduction (RRR) of 20.0%, and a control event rate of 34.4%. The cumulative Z curve did not cross any boundaries, and did not reach the required information size. c. TSA for mortality at 90 days. A diversity-adjusted information size of 3325 patients was calculated on the basis of using alfa = 0.05 (two sided), beta = 0.10 (power 90%), an anticipated relative risk reduction (RRR) of 20.0%, and a control event rate of 40.0%. The cumulative Z curve crossed the futility boundary and reached the required information size.
The pooled results showed that a conservative oxygen therapy strategy could not decrease mortality compared with a liberal oxygen therapy strategy at 28 days (RR 0.90; 95% CI 0.75–1.09; p = 0.28; I2 = 54%; 4245 participants, 6 studies, Fig. 2b), 90 days (RR 1.02; 95% CI 0.92–1.13; p = 0.71; I2 = 26%; 4705 participants, 6 studies, Fig. 2c), and in ICU (RR 0.85; 95% CI 0.53–1.37; p = 0.51; I2 = 68%; 906 participants, 4 studies, Fig. 2d). For 28-day all-cause mortality, TSA showed that the cumulative Z-curve did not cross any boundaries for benefit and harm, nor the futility boundary (Fig. 3b). For 90-day all-cause mortality, the cumulative Z curve reached the required information size and crossed the futility boundary (Fig. 3c).
Length of ICU stay and hospital stay, and mechanical ventilation-free days up to day 28Four studies reported the length of hospital stay19,29,30,34 and six studies reported the length of ICU stay.19,29,30,32–34 The pooled results showed that oxygen therapy strategy could not affect the length of hospital stay (MD 0.74; 95% CI –1.48 to 2.95; p = 0.51; I2 = 38%; 1567 participants; see Additional file 5a) or ICU stay (MD 0.14; 95% CI –0.65 to 0.94; p = 0.72; I2 = 59%; 2121 participants; see Additional file 5b). Only three studies involving 1502 patients reported mechanical ventilation-free days up to day 28.19,30,32 No significant difference was detected between two groups (MD 0.25; 95% CI –1.78 to 2.27; p = 0.81; I2 = 59%; see Additional file 5c).
Adverse eventsFour studies reported the number of new-onset pneumonia.21,29,32,34 Three studies reported the number of new-onset infection.29,32,34 Four studies reported the number of new-onset ARDS.29,30,33,34 The data of new-onset atelectasis was available in two studies.32,34 The data of new-onset pneumothorax was reported in two studies21,32 and the occurrence of mesenteric ischemia was reported in three studies.20,21,32 There were no significant differences in terms of new-onset pneumonia (RR 0.92; 95% CI 0.71–1.21; p = 0.57; I2 = 0; 1134 participants, see Additional file 5d), new-onset infection(RR 0.91; 95% CI 0.71–1.18; p = 0.49; I2 = 0; 933 participants, see Additional file 5e), new-onset ARDS(RR 1.06; 95% CI 0.65–1.75; p = 0.81; I2 = 0; 722 participants, see Additional file 5f), new-onset atelectasis(RR 0.76; 95% CI 0.34–1.70; p = 0.50; I2 = 75%; 499 participants, see Additional file 5 g), new-onset pneumothorax(RR 0.74; 95% CI 0.35–1.60; p = 0.45; I2 = 0; 635 participants, see Additional file 5 h), and the occurrence of mesenteric ischemia (RR 1.11; 95% CI 0.43–2.82; p = 0.83; I2 = 46%; 3545 participants, see Additional file 5i) between two groups.
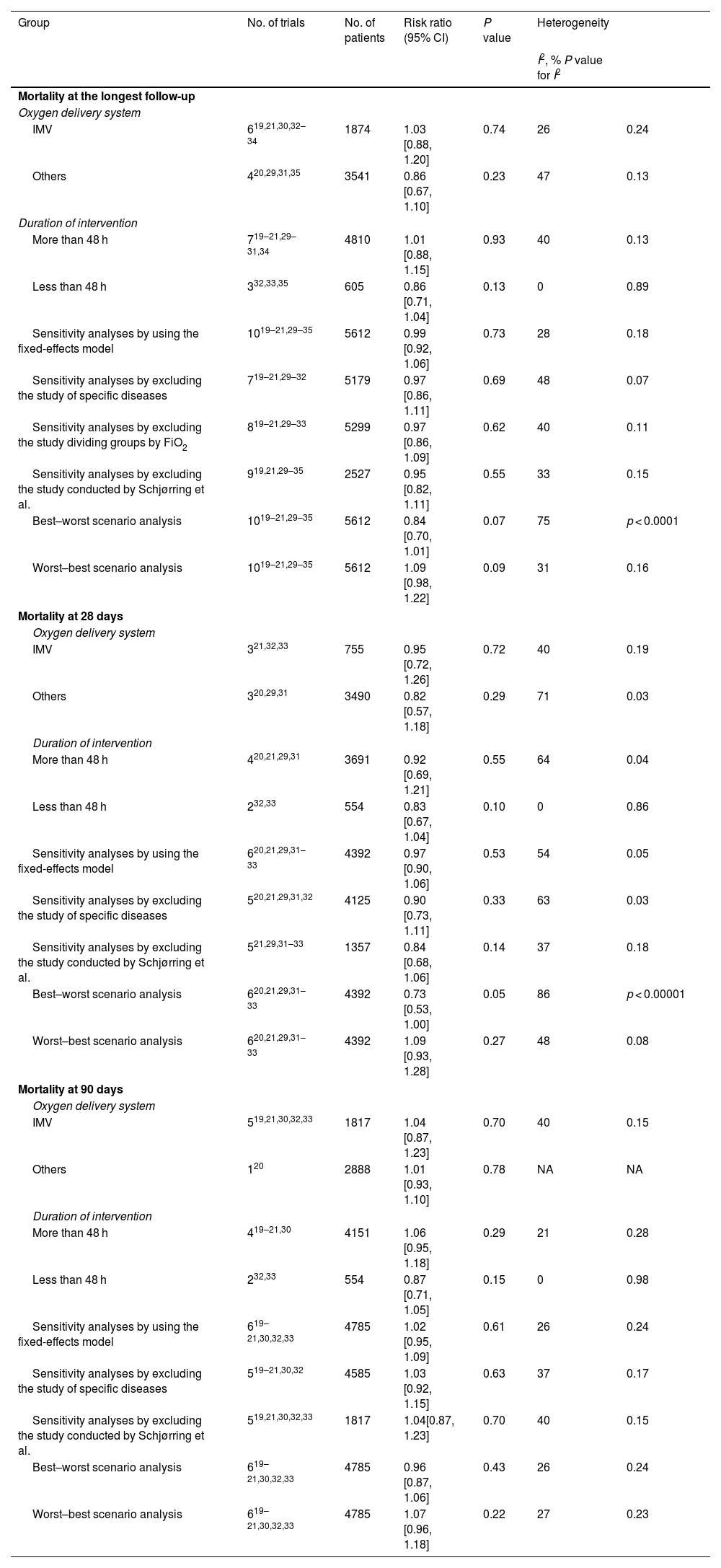
Subgroup analyses and sensitivity analysesFrom the subgroup analyses of the primary outcomes, we found that oxygen delivery system (invasive mechanical ventilation, others) and duration of oxygen intervention (more than 48 h, less than 48 h) had no significant effect on all-cause mortality at 28 days, 90 days, and the longest follow-up. Sensitivity analyses did not alter the conclusion of the main analyses. The results of sensitivity analyses on missing data through the best-worst scenario analysis and the worst-best scenario analysis were consistent with the main analyses. Detailed results about subgroup analyses and sensitivity analyses are presented in Table 2 and Appendix B Additional file 6.
Results of sensitivity analyses and subgroup analyses.
| Group | No. of trials | No. of patients | Risk ratio (95% CI) | P value | Heterogeneity | |
|---|---|---|---|---|---|---|
| I2, % P value for I2 | ||||||
| Mortality at the longest follow-up | ||||||
| Oxygen delivery system | ||||||
| IMV | 619,21,30,32–34 | 1874 | 1.03 [0.88, 1.20] | 0.74 | 26 | 0.24 |
| Others | 420,29,31,35 | 3541 | 0.86 [0.67, 1.10] | 0.23 | 47 | 0.13 |
| Duration of intervention | ||||||
| More than 48 h | 719–21,29–31,34 | 4810 | 1.01 [0.88, 1.15] | 0.93 | 40 | 0.13 |
| Less than 48 h | 332,33,35 | 605 | 0.86 [0.71, 1.04] | 0.13 | 0 | 0.89 |
| Sensitivity analyses by using the fixed-effects model | 1019–21,29–35 | 5612 | 0.99 [0.92, 1.06] | 0.73 | 28 | 0.18 |
| Sensitivity analyses by excluding the study of specific diseases | 719–21,29–32 | 5179 | 0.97 [0.86, 1.11] | 0.69 | 48 | 0.07 |
| Sensitivity analyses by excluding the study dividing groups by FiO2 | 819–21,29–33 | 5299 | 0.97 [0.86, 1.09] | 0.62 | 40 | 0.11 |
| Sensitivity analyses by excluding the study conducted by Schjørring et al. | 919,21,29–35 | 2527 | 0.95 [0.82, 1.11] | 0.55 | 33 | 0.15 |
| Best–worst scenario analysis | 1019–21,29–35 | 5612 | 0.84 [0.70, 1.01] | 0.07 | 75 | p < 0.0001 |
| Worst–best scenario analysis | 1019–21,29–35 | 5612 | 1.09 [0.98, 1.22] | 0.09 | 31 | 0.16 |
| Mortality at 28 days | ||||||
| Oxygen delivery system | ||||||
| IMV | 321,32,33 | 755 | 0.95 [0.72, 1.26] | 0.72 | 40 | 0.19 |
| Others | 320,29,31 | 3490 | 0.82 [0.57, 1.18] | 0.29 | 71 | 0.03 |
| Duration of intervention | ||||||
| More than 48 h | 420,21,29,31 | 3691 | 0.92 [0.69, 1.21] | 0.55 | 64 | 0.04 |
| Less than 48 h | 232,33 | 554 | 0.83 [0.67, 1.04] | 0.10 | 0 | 0.86 |
| Sensitivity analyses by using the fixed-effects model | 620,21,29,31–33 | 4392 | 0.97 [0.90, 1.06] | 0.53 | 54 | 0.05 |
| Sensitivity analyses by excluding the study of specific diseases | 520,21,29,31,32 | 4125 | 0.90 [0.73, 1.11] | 0.33 | 63 | 0.03 |
| Sensitivity analyses by excluding the study conducted by Schjørring et al. | 521,29,31–33 | 1357 | 0.84 [0.68, 1.06] | 0.14 | 37 | 0.18 |
| Best–worst scenario analysis | 620,21,29,31–33 | 4392 | 0.73 [0.53, 1.00] | 0.05 | 86 | p < 0.00001 |
| Worst–best scenario analysis | 620,21,29,31–33 | 4392 | 1.09 [0.93, 1.28] | 0.27 | 48 | 0.08 |
| Mortality at 90 days | ||||||
| Oxygen delivery system | ||||||
| IMV | 519,21,30,32,33 | 1817 | 1.04 [0.87, 1.23] | 0.70 | 40 | 0.15 |
| Others | 120 | 2888 | 1.01 [0.93, 1.10] | 0.78 | NA | NA |
| Duration of intervention | ||||||
| More than 48 h | 419–21,30 | 4151 | 1.06 [0.95, 1.18] | 0.29 | 21 | 0.28 |
| Less than 48 h | 232,33 | 554 | 0.87 [0.71, 1.05] | 0.15 | 0 | 0.98 |
| Sensitivity analyses by using the fixed-effects model | 619–21,30,32,33 | 4785 | 1.02 [0.95, 1.09] | 0.61 | 26 | 0.24 |
| Sensitivity analyses by excluding the study of specific diseases | 519–21,30,32 | 4585 | 1.03 [0.92, 1.15] | 0.63 | 37 | 0.17 |
| Sensitivity analyses by excluding the study conducted by Schjørring et al. | 519,21,30,32,33 | 1817 | 1.04[0.87, 1.23] | 0.70 | 40 | 0.15 |
| Best–worst scenario analysis | 619–21,30,32,33 | 4785 | 0.96 [0.87, 1.06] | 0.43 | 26 | 0.24 |
| Worst–best scenario analysis | 619–21,30,32,33 | 4785 | 1.07 [0.96, 1.18] | 0.22 | 27 | 0.23 |
IMV, invasive mechanical ventilation; CI, confidence interval; NA, not available.
In this meta-analysis for adult ICU patients, we found no beneficial or harmful effects of conservative oxygen therapy compared with liberal oxygen therapy. Both primary outcomes and secondary outcomes were comparable between two groups. TSA results indicated a RRR of 20% or greater could be rejected with respect to mortality at 90 days and at the longest follow-up, but in terms of mortality at 28 days, the required information size to detect or reject a RRR of 20% was not achieved.
Our results were at variance with the results of previous meta-analyses on this topic. Damiani et al. and Helmerhorst et al. have conducted meta-analyses including observational studies and drawn a similar conclusion that hyperoxia may increase mortality in critically ill patients.15,16 The pooled results of study conducted by Barbateskovic et al. including 10 RCTs indicated that higher oxygen supplementation was associated with increased mortality and the incidence of serious adverse events. However, the authors were very uncertain about the results due to very low-certainty evidence.17 Similarly, Hirase et al. have found conservative oxygen therapy administrated in the ICU could reduce mortality and new-onset non-respiratory organ failure compared to liberal oxygen therapy.18 We updated the meta-analysis on this topic, including results from three recently published high-quality RCTs, none of which found that ICU patients would benefit from conservative oxygen therapy as compared to liberal oxygen therapy, which may support the fact that our conclusions differ from those previous meta-analyses.19–21
More studies have gained increasing interest in investigating the effects of exposure to hyperoxia, and have found that excessive oxygenation had deleterious properties in various pathophysiological processes.36 In a recent meta-analysis including 25 RCTs involving 16,037 acutely ill adults, Chu and colleagues found that liberal oxygen therapy was associated with higher mortality than conservative oxygen therapy with no improvement on other important clinical outcomes.37 Our results showed no significant difference between the two groups. The different results between the two studies may be due to the following reasons-first, we only included studies involving critically ill patients admitted to the ICU. To our knowledge, both hypoxia and hyperoxia were independent risk factors of mortality in ICU patients.38,39 Patients assigned to the liberal oxygen therapy may be at higher risk of exposure to hyperoxia, while patients assigned to the conservative oxygen therapy may be at higher risk of exposure to hypoxia. Secondly, in our study, the mortality at the longest follow-up were 38.4% (1034 of 2692 patients) in the conservative oxygen therapy group and 38.7% (1055 of 2723 patients) in the liberal oxygen therapy group, while in the study conducted by Chu and colleagues, the mortality at the longest follow-up in the two groups were 9.5% (749 of 7857 patients) and 10.5% (828 of 7897 patients), respectively. It is reasonable to assume that the severity of disease in our study is higher. In addition, a significant proportion of patients in our study received invasive mechanical ventilation or had acute hypoxemic respiratory failure.19–21,30,32–34 For these patients, a liberal oxygen therapy strategy to ensure adequate oxygen supplementation may be necessary.
The strengths of our study are as follows: First, we used a comprehensive, up-to-date search strategy, which have identified three recently published well designed RCTs. Moreover, all studies were published in recent years and most of them were assessed as low risk of bias. Second, the methodology used in this study was rigorous. TSA was performed to control the risk of random errors. A best-worst scenario analysis and a worst–best scenario analysis were performed to assess the potential impact of the missing participants for the primary outcomes. Limitations existed in this study must also be considered. As with previous meta-analyses on this topic, the primary limitation was that the definitions of liberal and conservative oxygen therapy varied widely from study to study. For example, some studies used a fixed FiO2, while others used a particular oxygenation target by measuring PaO2, SaO2, or SpO2. Considering the differences in patients’ conditions and lung function, higher FiO2 oxygen supplementation does not necessarily lead to higher tissue oxygen saturation, so it may be more reasonable to define liberal and conservative oxygen therapy by a particular oxygenation target. We conducted sensitive analyses by excluding two studies using a fixed FiO2 and we found the results were consistent with main analyses. Second, we only included studies involving ICU patients, while some studies included mixed populations with specific conditions, such as TBI and severe acute stroke. And duration of intervention also existed differences among included studies. Nonetheless, the results of subgroup analyses and sensitive analyses were consistent.
Oxygen administration is part of a routine treatment in the ICU. Although no beneficial or harmful effects of conservative versus liberal oxygen therapy were detected in this study, it is possible that different oxygen therapy strategies have effect on clinical outcomes. More studies are required to find an appropriate oxygen therapy strategy for ICU patients. Considering the complexity of the ICU patient's condition, oxygen therapy strategy in future studies should be designed according to patients’ conditions.
ConclusionIn conclusion, no beneficial or harmful effects of conservative oxygen therapy were found compared with liberal oxygen therapy in adult ICU patients. Conservative oxygen therapy did not reduce all-cause mortality at 28 days, 90 days, and the longest follow-up. Other important clinical outcomes were also comparable between two groups.
Authors' contributionsXML and DSL conceived of the study, participated in the design, collected the data, performed statistical analyses and drafted the manuscript. CL participated in the design and performed statistical analyses. ZM helped to revise the manuscript critically for important intellectual content. YL collected the data and performed statistical analyses. HYY performed statistical analyses. FHZ conceived of the study, participated in the design and revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
FundingInnovation Research of Chinese PLA general hospital (CX19010).
Special Research of Military Medical Innovation (18CXZ026).
Conflict of interestThe authors declare that they have no conflict of interest.