Edited by: Alberto García-Salido - Pediatric Intensive Care Unit, Hospital Infantil Universitario Niño Jesús, Madrid, Spain
Last update: May 2024
More infoNoninvasive ventilation (NIV) failure it has been associated to worst clinical outcomes due to a delay in intubation and initiation of invasive mechanical ventilation (IMV). There is a lack of evidence in pediatric patients regarding this topic. The objective was to deter-mine the association between duration of IMV and length of stay, with duration of NIV prior tointubation/IMV in pediatric patients.
DesignA prospective cohort study since January 2015 to October 2019.
SettingA pediatric intensive care unit.
PatientsChildren under 15 years with acute respiratory failure who failed to noninvasive ventilation.
InterventionsNone.
Main variables of interestDemographic variables, pediatric index of mortality (PIM2), pediatric acute respiratory distress syndrome (PARDS) diagnosis, IMV and NIV duration, PICU LOS were registered and intrahospital mortality.
ResultsA total of 109 patients with a median (IQR) age of 7 (3–14) months were included. The main diagnosis was pneumonia (89.9%). PARDS was diagnosed in 37.6% of the sample. No association was found between NIV duration and duration of IMV after Kaplan–Meier analysis (Log rank P = .479). There was no significant difference between PICU LOS (P = .253) or hospital LOS (P = 0.669), when categorized by NIV duration before intubation. PARDS diagnosis was associated to an increased length of invasive ventilation (HR: 0.64 [95% IC: 0.42–0.99]).
ConclusionsNo association was found between NIV duration prior to intubation and duration of invasive ventilation in critical pediatric patients with acute respiratory failure.
En adultos el fracaso de la ventilación no invasiva (VNI) se ha asociado a peores resultados clínicos debido al retraso en la intubación y en el inicio de la ventilación invasive (VMI). Existe falta de evidencia de esta asociación en pediatría. Nuestro objetivo fue determinar la asociación entre la duración de la VMI y la estancia, con la duración de la VNI previo a laintubación/VMI en pacientes pediátricos.
DiseñoEstudio de cohorte prospectivo desde enero de 2015 a octubre de 2019.
ÁmbitoUnidad de cuidados intensivos pediátricos (UCIP).
PacientesNiños/as menores de 15 años con insuficiencia respiratoria aguda (IRA) que fracasaron a la VNI.
IntervencionesNinguna.
Variables de interés principalesSe registraron variables demográficas y clínicas, índice demortalidad pediátrica (PIM2), diagnóstico de síndrome de distrés respiratorio agudo pediátrico(SDRAP), duración de la VMI y la VNI, estancia en UCIP y mortalidad intrahospitalaria.
ResultadosSe incluyeron un total de 109 pacientes con una mediana de edad de 7 (3–14) meses. El diagnóstico principal fue neumonía (89,9%). El 37,6% de la muestra presentó SDRAP. No se encontró asociación entre duración de la VNI y duración de la VMI mediante el análisis de Kaplan–Meier (logrank test p = 0,479). No se encontraron diferencias significativas entre la estancia en UCIP (p = 0,253) y hospitalaria (p = 0,669) al categorizar por duración de la VNI. El SDRAP se asoció a mayor duración de la VMI (HR: 0,64 [IC 95%: 0,42–0,99]).
Conclusión: No se encontró asociación entre la duración de la VNI previo a la intubación y laduración de la VMI, ni en la estancia en pacientes pediátricos con IRA.
Noninvasive mechanical ventilation (NIV) is widely used for respiratory support in patients of all ages with acute respiratory failure (ARF).1 However, the failure of NIV has been identified as an independent risk factor for increased mortality in adults with de novo ARF (excluding exacerbation of existing chronic respiratory or heart disease) – though it is not clear whether this association reflects a causal relationship or constitutes a marker of increased patient severity.2
In the context of community-acquired pneumonia, a longer duration of NIV prior to intubation has been associated with decreased in-hospital survival3 – this finding having been related to delays in intubation and the start of invasive mechanical ventilation (IMV). The mortality rate associated with NIV failure is reported to be between 9%4 and 12%.5 In relation to the duration of IMV, and although the data found in the literature are not conclusive, the failure of NIV has been identified as a risk factor for IMV duration ≥96 hours.6,7
The failure of NIV in patients with pediatric acute respiratory distress syndrome (PARDS) has been associated with significantly increased mortality, particularly with the increasing severity of hypoxemia.8 For this reason, there is great interest in identifying factors that may allow the early prediction of NIV failure in pediatrics, with a view to avoiding its clinical consequences.5,9,10
An expert consensus suggests that the evaluation of patient response to NIV should be made within the first few hours in order to avoid the risk of delays in intubation – though no specific time period has been established.11 On the other hand, the median duration of NIV in patients that are subsequently intubated has been reported to be 13 hours (range 0.5−77 hours).5,10 Considering the above, it has not been possible to date to establish a time range or threshold beyond which deleterious clinical effects are to be expected.
The primary objective of the present study was to determine the association between the duration of NIV before intubation and the duration of IMV, as well as Pediatric Intensive Care Unit (PICU) and in-hospital length of stay among pediatric patients with ARF presenting NIV failure. As a secondary objective, an analysis was made to identify risk factors for a longer duration of IMV.
MethodStudy designA prospective cohort study was carried out between January 2015 and August 2019 at the PICU of Hospital Víctor Ríos Ruiz (Los Ángeles, Chile). The study was approved by the Scientific – Ethics Committee of the Biobio Health Department, and informed consent was requested from the legal guardians of the patients.
Study populationThe study was carried out in critical pediatric patients between 0–15 years of age presenting acute lower respiratory tract infection and requiring intubation due to failure of NIV. Patients with the following diagnoses were included: acute bronchiolitis defined as an acute respiratory infection in infants under two years of age, with rhinitis, tachypnea, wheezing, cough, crepitants, accessory muscle use and/or nasal flaring12; asthma crisis in children defined as progressive respiratory worsening due to asthma, with possible cough, wheezing, dyspnea, labored breathing and anxiety13; and pneumonia defined by the presence of fever, tachypnea, labored breathing, rhonchus, crepitants and wheezing.14 We excluded patients with cyanotic congenital heart disease, nocturnal NIV users, patients with neurological damage, and tracheostomized individuals. The sample was established based on a non-probabilistic sampling of consecutive cases.
NIV techniqueThe Unit has a protocol that was applied throughout the study (Appendix B Fig. 1 of the electronic supplementary material). The decision to start NIV was based on the presence of labored breathing (increase in respiratory frequency >2 standard deviations of the normal range for the age of the patient, and signs of moderate to severe dyspnea according to the modified Woods scale >4 points15) and/or FiO2 >50% to maintain SatO2 >94%16, despite standard medical care.
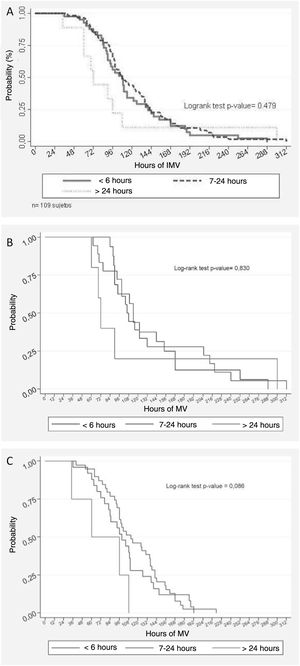
Kaplan–Meier analysis of the duration of invasive mechanical ventilation (IMV) following the failure of noninvasive mechanical ventilation (NIV). A) The curves are compared according to the NIV times (≤6 h, 7–24 h and >24 h) prior to the use of IMV, yielding a log rank p-value of 0.479. B) In the subgroup with pediatric acute respiratory distress syndrome (PARDS), the log rank p-value was 0.830. C) In the subgroup without PARDS, the log rank p-value was 0.086.
We used BiPAP Synchrony (Respironics®, Pittsburg, PA, USA) and Trilogy 202 ventilators (Respironics®, Pittsburg, PA, USA) with an active humidification system (MR 850 Fisher Paykel Healthcare, Auckland, New Zealand). Performax facial and Comfort Full oronasal masks were used (Respironics®, Pittsburg, PA, USA). Appendix B of the supplementary material describes the suggested initial parameters according to the protocol of the Unit. Chloral hydrate and/or midazolam were used as sedatives to secure good adaptation. Brief disconnections (<20 min) were made every 4−6 hours for kinesiological and nursing care, and to decompress pressure points of the interface. Routine arterial puncture to assess oxygenation was not performed; instead, we used the SpO2/FiO2 (SF) index (employing SpO2 values between 88–97%)17; arterial or venous blood gas measurements were reserved for patients with partial response (unsatisfactory reduction of respiratory effort).
Failure of NIVFailure of NIV was defined as the need for intubation – the latter being considered when labored breathing or dyspnea worsens (as evidenced by the modified Woods clinical scale) or in the presence of SpO2 <85% despite high ventilatory support. High assist was considered as an inspiratory positive airway pressure (IPAP) ≥20 cmH2O, continuous positive airway pressure or expiratory positive airway pressure (CPAP/EPAP) ≥10 cmH2O.
Study variablesThe following variables were recorded for all patients included in the study: age, weight, gender and Pediatric Index of Mortality II (PIM2) upon admission18; diagnosis established by the supervising pediatrician; use of antibiotic treatment, indicated by the pediatrician due to bacterial superinfection (suspected or confirmed)14; SF index17; diagnosis of PARDS, based on the criteria of the Pediatric Acute Lung Injury Consensus Conference (PALICC) for patients on NIV using the SF index19; ventilatory strategy (ventilator, interface, mode, maximum expiratory and inspiratory pressure); reason for the failure of NIV, defined as complications related to NIV (intolerance, air leakage, pressure ulcers, etc.), persistent labored breathing (reduction of respiratory frequency and insufficient or inexistent modified Woods score) and/or hypoxemia (SF <150).
For the purpose of analysis, we divided the cohort according to the duration of NIV before intubation, stratifying the patients according to Mayordomo-Colunga et al.5 into ≤6 hours, between 7−24 hours and >24 hours. In relation to monitoring once intubated, we recorded the duration of IMV (in hours), PICU and in-hospital length of stay (in days), and in-hospital mortality.
Data collection was carried out by kinesiologists of the Unit, who were in charge of recruitment and follow-up of the participants that met the screening criteria. Recording was made using an ad hoc form during follow-up and until hospital discharge or death.
Statistical analysisNormal data distribution was checked using the Kolmogorov–Smirnov test. The variables were reported as the median and interquartile range (IQR). Categorical variables were presented as absolute values and percentages. The comparison of continuous variables between groups was carried out using the Kruskal–Wallis test with Bonferroni correction, while categorical variables were compared using the chi-square test (χ2) or Fisher’s exact test (F) in the case of fewer than 5 elements per cell.
In relation to the hours of IMV, time to the event was estimated using the Kaplan–Meier method, taking the hours of IMV as time and extubation as event. Comparison of the time to event curves was made using the log-rank test. The association of risk of increased duration of IMV was evaluated by means of the Cox proportional hazards model, reporting the hazard ratio (HR), p-value and 95% confidence interval (95% CI). The HR was interpreted as the risk of increasing the duration of IMV, and the analysis was adjusted for PARDS. Statistical significance of the log-rank test and HR was considered for p < 0.05.
Considering that there is no evidence on the duration of IMV in patients with ARF presenting failed NIV, the statistical power was calculated on a post hoc basis with an alpha error of 5% for the duration of IMV compared with the duration of IMV in the studies of Payen et al.7 and Morris et al.20
ResultsDescriptive analysisA total of 109 patients with failed NIV were included in the present study, out of a total of 470 subjects (23.2%) that received NIV during the recruitment period. The main cause of ARF was pneumonia (90%), with respiratory syncytial virus (RSV) being the most frequent etiological agent (70.6%). In 89% of the cases use was made of antibiotics in the context of bacterial superinfection (suspected or confirmed). In turn, 25.8% of the patients presented some chronic disease – the most frequent condition being asthma (66.6%). The main reason for starting NIV was increased respiratory effort, in 84.4% of the cases. The median PIM2 was 3.4% (2.8−3.8%). The diagnosis of PARDS was established in 37.6% of the cases. The Trilogy 202 ventilator with full-face mask and bi-level mode was used in 90.5% of the cases. The median maximum inspiratory (IPAP) and expiratory pressures (EPAP) were 14 cmH2O (IQR 14–15) and 7 cmH2O (IQR 6–7), respectively. In 74 cases (67.9%) the failure of NIV was attributed to persistent or increased breathing difficulty. Furthermore, in 32 subjects (28.4%) the decision to intubate was due to mixed factors (hypoxemia and respiratory effort). Regarding the duration of NIV before failure, 89.9% of the patients failed within the first 24 hours of ventilation. Table 1 shows the characteristics of the study sample.
Demographic and clinical characteristics of the patients intubated following noninvasive mechanical ventilation (NIV) failure.
| Variables | Results |
|---|---|
| Age (months) | 7 (3; 14) |
| Male gender | 60 (55.1) |
| Weight (kg) | 8.7 (6.4; 10.5) |
| Without chronic disease | 82 (75.2) |
| Diagnosis | |
| Pneumonia | 98 (89.9) |
| Acute bronchiolitis | 9 (8.3) |
| Asthma | 2 (1.8) |
| Etiology, RSV | 77 (70.6) |
| Use of antibiotics | 97 (89.0) |
| Pediatric Index of Mortality (PIM2) score | 3.4 (2.8−3.8) |
| SatO2/FiO2 index | 235.7 (70.1) |
| PARDS | 41 (37.6) |
| Severity of PARDS | |
| Mild | 18 (43.9) |
| Moderate | 16 (39.0) |
| Severe | 7 (17.1) |
| Reason for failure of NIV | |
| Complications of NIV | 3 (2.8) |
| Increased respiratory effort | 74 (67.9) |
| Increased respiratory effort and hypoxemia | 32 (29.3) |
| Hours of NIV prior to intubation | 9 (6; 15) |
| Time of NIV to intubation | |
| ≤6 h | 41 (37.6) |
| >6 and ≤24 h | 57 (52.3) |
| >24 h | 11 (10.1) |
| Hours of IMV | 105 (84; 142) |
| Days of hospital stay | 13 (11; 16) |
| Days of PICU stay | 6 (5; 8) |
| Mortality in PICU | 1 (0.9) |
PARDS: pediatric acute respiratory distress syndrome; PICU: Pediatric Intensive Care Unit; IMV: invasive mechanical ventilation; NIV: noninvasive ventilation; RSV: respiratory syncytial virus.
The data are reported as n (%) and P50 (P25-P75), as applicable.
The median duration of IMV in the global study sample was 105 hours (84–142). No association was found between the duration of IMV and the duration of NIV before intubation (log-rank test, p = 0.479). Fig. 1A shows the time to event curve for the suspension of IMV, which compares the curves of the three NIV failure times. The presence of PARDS was identified as a risk factor for a longer duration of IMV (Table 2). To avoid possible confusion in the evaluation of the main causal relationship evaluated, we adjusted a multivariate Cox proportional hazards model in which the effect of the time on NIV upon the duration of posterior IMV was controlled for by the presence of PARDS. In this model, the duration of prior NIV showed no statistical significance (Table 3).
Univariate analysis of the risk of increased duration of invasive mechanical ventilation (IMV) following noninvasive mechanical ventilation (NIV) failure.
| Variable | Hazard ratio | p | 95%CI |
|---|---|---|---|
| Male gender | 0.768 | 0.179 | [0.522−1.129] |
| Weight, kg | 0.978 | 0.159 | [0.949−1.009] |
| Age, months | 0.993 | 0.191 | [0.983−1.003] |
| Use of antibiotics | 0.748 | 0.348 | [0.408−1.372] |
| PIM2 score | 0.006 | 0.931 | [0.881−1.148] |
| Bronchiolitis | 0.873 | 0.079 | [0.930−3.774] |
| Pneumonia | 0.706 | 0.284 | [0.374−1.333] |
| Asthma | 0.663 | 0.566 | [0.163−2.703] |
| Presence of PARDS | 0.643 | 0.042 | [0.420−0.985] |
| Mild PARDS | 0.831 | 0.478 | [0.498−1.386] |
| Moderate PARDS | 0.838 | 0.557 | [0.465−1.510] |
| Severe PARDS | 0.536 | 0.120 | [0.244−1.176] |
| Hours of NIV prior to intubation | 0.995 | 0.335 | [0.984−1.006] |
| Time of NIV to intubation | |||
| ≤6 h | Reference | ||
| >6 and ≤24 h | 0.896 | 0.593 | [0.598−1.342] |
| >24 h | 1.382 | 0.396 | [0.655−2.919] |
PIM2: Pediatric Index of Mortality; PARDS: pediatric acute respiratory distress syndrome; PICU: Pediatric Intensive Care Unit NIV: noninvasive ventilation.
Multivariate analysis of the risk of increased duration of invasive mechanical ventilation (IMV) following noninvasive mechanical ventilation (NIV) failure, adjusted for PARDS.
| Variable | Hazard ratio | p | 95%CI |
|---|---|---|---|
| PARDS | 0.605 | 0.024 | [0.392−0.935] |
| ≤ 6 h | Reference | ||
| >6 and ≤24 h | 0.841 | 0.407 | [0.560−1.265] |
| >24 h | 1.510 | 0.285 | [0.709−3.214] |
PARDS: pediatric acute respiratory distress syndrome.
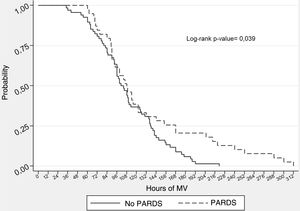
To complement the analysis, we used the presence of PARDS as control variable, considering that it presents a strong correlation to NIV failure8 and that in our sample we identified an association to a longer duration of IMV (Table 2); as a result, the analysis was performed in the PARDS (Fig. 1B) and No PARDS subgroups (Fig. 1C). However, the results showed no association between the duration of IMV and the duration of NIV before intubation in either the PARDS subgroup (log-rank test, p = 0.830) or the No PARDS subgroup (log-rank test, p = 0.086). Nevertheless, as can be seen in Fig. 2, the duration of IMV was greater in the PARDS subgroup than in the No PARDS subgroup (log-rank test, p = 0.0386).
ICU and in-hospital stayThe median UCI stay was 6 days (5–8), while the median in-hospital stay was 13 days (11–16). The stay in both the UCI (p = 0.25) and in hospital (p = 0.67) was not associated to the IMV connection times.
Post hoc power estimateFor the post hoc statistical power estimate, we included the IMV times recorded in this study (118.3 ± 53.97 hours) and those reported in the literature (in two similar studies): 182.3 ± 556.7 hours7 and 4 days (2–7)20, for an alpha error of 5%, yielding a power of 100% and 99.1%, respectively.
DiscussionAccording to our review of the literature, this is the first prospective study in the pediatric setting to explore the possible clinical implications of the duration of NIV before intubation. In our cohort of patients with NIV failure, no significant association was found between the different durations (≤6 hours, 7–24 hours and >24 hours) of NIV before intubation and the duration of IMV, or the PICU and in-hospital length of stay. The results in relation to the duration of IMV (median 105 hours, equivalent to 4.3 days) and PICU stay (median 6 days) are comparable to those published by Morris et al.20, with a median of four days of IMV and 5 days of PICU stay.
The study carried out by Morris et al.20 was notorious due to its large prospective sample and the analysis made. The authors found the use of “initial NIV” (as first-line management of ARF) to be significantly associated with a decrease in the duration of IMV (1.6 days), an increase in mechanical ventilation-free days over 28 days (3.7 days), and a shortening of PICU stay of 2.1 days. However, it must be noted that the NIV group included both NIV success and failure – a condition that limits the analysis of the failure group and its ulterior outcomes. On the other hand, Payen et al.7, in a retrospective study, found the failure of NIV to be associated with a duration of IMV ≥96 hours. However, the characteristics of their study population differed from those of our group. In turn, Ganu et al.6, in retrospective study involving 10 years of follow-up, recorded significant differences in the median duration of IMV between patients with severe acute bronchiolitis subjected to initial IMV (4.41 ± 5.57) and those subjected to initial NIV (5.55 ± 3.63 ). However, no mention was made of the duration of NIV before intubation, and the sample moreover corresponded to a specific disease condition. Given the nature of the latter two studies, it is not possible to determine whether the association between NIV and a greater duration of IMV constitutes a causal relationship. An explanation for the lack of association between the duration of NIV and the analyzed clinical outcomes could be the timely detection of NIV failure. This is a consequence of close and permanent monitoring by the clinical team, together with the adoption of adequate failure criteria — allowing a reduction of the risk associated with delays in intubation.21
In our study, the presence of PARDS was associated with a longer duration of IMV. This is consistent with the data published by Monteverde et al.22, who recorded a significantly greater PARDS rate (definition of Berlin) in the prolonged IMV group versus the non-prolonged IMV group (44% versus 20% respectively, p < 0.01). Furthermore, it has been shown that the degree of hypoxemia is closely correlated to a greater probability of intubation (log-rank, p = 0.0003).8 In this regard, the presence of PARDS has been associated with failure of NIV, a longer duration of IMV, and increased mortality. In this group it is therefore reasonable to insist that the response time should be limited, as recommended by expert consensuses.11,23 However, in our study, on using the variable PARDS as control parameter, the Kaplan–Meier curves (Fig. 1B and C) showed no association between the duration of NIV and the duration of IMV. Likewise, the multivariate Cox analysis adjusted for PARDS detected no statistically significant association between the two variables (Table 3). On examining this lack of an association, it must be taken into account that only 17.1% of the cases of PARDS would be severe. Nevertheless, the consensus established that it is not possible to classify the cases of PARDS by severity in NIV.19
Another relevant aspect is the duration of NIV, which in our study corresponded to a median of 9 hours (range 6–15 hours). This is less than the median duration (13 hours; range 0.5−77 hours) reported by Mayordomo-Colunga et al.5 and the 22 hours (range 1–312 hours) documented by Bernet et al.24. The variability of these ranges suggests that failure of NIV as an event is not exclusive of the first hours, and that assessment of the response therefore should be constant and prolonged.
LimitationsThe present study has limitations. In effect, the results correspond to a single center, although we included a sample composed mainly of previously healthy children with pneumonia and bronchiolitis (98.2%) caused by respiratory syncytial virus – these being characteristics extrapolatable to those of an undifferentiated PICU. On the other hand, the methodology describes the material means employed, and Appendix B Supplementary material summarizes the management recommendations of our Unit. In turn, on interpreting the results, it must be taken into account that the study sample presented a high incidence of pneumonia associated with antibiotic use, which could indicate a strong presence of hypoxemic ARF – a condition that has been shown to be independently correlated to NIV failure10. Likewise, to stratify the sample, we used the NIV duration ranges proposed by Mayordomo-Colunga et al.5 together with time as a continuous variable (without stratification); in this regard, although a different type of stratification of the duration of NIV might have yielded a different result, there is no standard for stratifying the duration of NIV.
ConclusionsIn the present study, the duration of NIV before intubation, classified into three categories (<6 hours, 7−24 hours and >24 hours), was not associated with an increase in the duration of IMV, or to an increase in PICU or hospital length of stay. Although the presence of PARDS was identified as a risk factor for longer IMV when used as control parameter, we likewise observed no significant associations to the analyzed clinical outcomes.
Financial supportThis study has received no financial support of any kind.
Contribution of the authorsFernando Bustos-Gajardo developed the study idea and contributed substantially to its conception and design, global data compilation for analysis and interpretation, and drafting of the article.
Soledad Luarte-Martínez contributed to the study design, analysis and interpretation of the data, and drafted the article, with critical review of its content.
Sebastian Dubo contributed to the study design, analysis and interpretation of the data, and drafted the article, with critical review of its content.
Rodrigo Adasme contributed to the study design, analysis and interpretation of the data, and drafted the article, with critical review of its content.
All the authors approved the final version of the manuscript, assume responsibility for all its aspects, and declare that all issues related to the correctness or integrity of all the parts of the manuscript were adequately investigated and resolved.
Conflict of interestThe authors declare that they have no direct or indirect conflicts of interest in relation to the present study.